Abstract
Despite their great economic importance, relatively little is known about bamboo sexual reproduction because they usually spread through rhizomes and have long intervals between flowering periods. Bambusa tuldoides is no exception; the intervals between flowering periods are about 23 years and often do not result in successful caryopsis production. The aim of the present work was to characterize Bambusa tuldoides sexual reproduction at three stages of flower development and investigate possible male sterility. Pollen was cultured onto several types of culture medium in order to encourage germination, but not a single of the thousands of observed pollen germinated under any condition. Anthers and microspores were analyzed by scanning electron microscopy, transmission electron microscopy, and optical microscopy techniques. Anther dehiscence appeared to be normal when compared to other species. In contrast, microspores began to develop abnormally starting as early as the first flower development stage: retraction of the cytoplasm and rupture of the nuclear and mitochondria membrane. As the interior machinery of the microspores degenerated, starch accumulated within numerous amyloplasts during stages two to four of flower development. The sporoderms of these microspores were similarly incomplete: though they possessed an exine, they lacked an intine. The results here obtained suggest that the non-viability of these abnormal pollen grains prevents the development of Bambusa tuldoides caryopses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natural bamboo populations (family Poaceae, subfamily Bambusoideae) are present on almost every continent of the globe except Europe and Antarctica (Williams et al. 1994; Scurlock et al. 2000). Among the countries of the Americas, Brazil has the highest diversity, with 33 genera and about 250 species, of which about 160 are endemic, in addition to the presence of more than 20 exotic species (Filgueiras and Goncalves 2004; Filgueiras et al. 2013).
Bamboo is particularly valuable for industry, because the quality and tensile strength of its wood may be used to manufacture high quality goods, such as construction material, furniture, bulk cellulose, and bioenergy and also the meristematic region of young shoots is used as food (Scurlock et al. 2000; Bonilla et al. 2010). Fast-growing bamboo cultivation may indirectly reduce deforestation and soil erosion while preserving plant biodiversity (Embaye 2000). Despite the great economic importance of bamboos, their propagation is mostly based on vegetative methods (Mudoi et al. 2013).
Bambusa tuldoides Munro, a woody bamboo native to China, is widely cultivated in the tropical and subtropical America (Guerreiro and Lizarazu 2010). First introduced by the Portuguese during the colonial period, B. tuldoides has since become one of the most widespread species through Brazil (Azzini et al. 1988). Bambusa tuldoides flowering periods are sporadic with an interval of about 23 years of vegetative development, during which bamboo spread asexually through growing new stems from clumps (Azzini et al. 1988; Guerreiro and Lizarazu 2010). A survey on sporadic flowering of six woody bamboo species introduced in Brazil showed that only four flowering episodes of B. tuldoides were observed in 1954, 1984, 2005, and, most recently, in 2017 in three different locations. However, caryopsis formation occurred only in a single flowering event in a population in Santa Teresinha, Goias, Brazil, and Argentina (Filgueiras and Castro de Silva 2007; Guerreiro and Lizarazu 2010).
The absence of caryopsis formation reported in other bamboo species may be related to male sterility, though many studies only analyzed pollen grains with light microscopy (Koshy and Pushpagadan 1997; Koshy and Jee 2001; Jijeesh et al. 2014; Wang et al. 2015; Das et al. 2017). There are comparatively fewer studies on ultrastructural processes resulting in male sterility, and many of those reports studied cultivated or mutant plants (Sanders et al. 1999; Ku et al. 2003; Zhou et al. 2011) or the formation of unisexual female flowers (Coimbra et al. 2004; Haddad et al. 2018). However in other bamboo species, the absence of caryopsis does not occur, such as in Arundinaria alpina, Oxytenanthera abyssinica, Dendrocalamus membranaceus, D. hamiltonii, D. brandisii, and Bambusa arundinacea (Thapliyal et al. 1991; Rawat and Thapliyal 2003; Warrier et al. 2004; Lakshmi et al. 2014; Bahru et al. 2015; Singh and Richa 2016; Xie et al. 2016).
Knowledge of the aspects related to pollen grain development is of great importance for the propagation and conservation of B. tuldoides because it is intrinsically linked to successful embryo and seed formation (Zhang et al. 2014). Thus, this work aimed at to characterize the anthers and pollen grain of B. tuldoides in three stages of flower development through histochemical and ultrastructural techniques looking at to elucidate aspects related to male sterility.
Material and methods
Vegetable material
Bambusa tuldoides inflorescences were collected from mother plants located in Parque Cidade das Abelhas-UFSC (27 ° 32′20.04” S, 48°30′10.87” W), in Florianópolis, Santa Catarina, Brazil, in June 2017. At the time of collection, the inflorescences were packaged in plastic bags and placed in ice-containing styrofoam boxes to be transported to the Plant Physiology and Developmental Genetics Laboratory (LFDGV) and to the Central Electron Microscopy Laboratory (LCME) of the Federal University of Santa Catarina (UFSC), Florianópolis, Santa Catarina, Brazil. Voucher specimens were deposited in the Herbarium FLOR – UFSC (FLOR 63000).
Identification of flower developmental stages
One hundred inflorescences were analyzed under a stereomicroscope (Olympus, model SZH10), to determine flower development stages (McClure 1966).
Bambusa tuldoides inflorescences, also called spikes, are composed of structural units called spikelets and a single bisexual flower. In stages one and two of flower development, the gynoecium and androecium are protected by the lemma. Anthesis begins at stage three with the opening of the palea and lemma and the exteriorization of the anthers. In stage four (anthesis), the anthers are externalized with subsequent dehiscence and pollen release (Table 1). Spikelets lacked nectaries and noticeable odors (Fig. 1).
Developmental stages of Bambusa tuldoides spikelets: (1) Early stage of development: gynoecium and androecium are protected by the lemma; (2) pre-anthesis: lemma begins to open; (3) beginning of the anthesis: lemma and palea are fully open and anthers begin to exteriorize; (4) anthesis: anthers are fully exteriorized and have undergone dehiscence. Scale bar: 5 mm. References: Le, lemma; Pa, palea; Ant, anther; Spk, spike; Spt, spikelet
In vivo pollen viability
Ten flowers were collected at the pre-anthesis stage to determine the viability of pollen grain in vivo. Six anthers were removed from each flower, macerated with one drop (2 μL) of acetic carmine dye on a labeled glass slide and covered by a coverslip. After 5 minutes, the pollen grains were counted under an optical microscope (Olympus, model BX40) under 10x magnification. Pollen grains that stained red were considered viable, and uncolored pollen grains were considered nonviable. The results were expressed as a percentage of viable pollen grains (Jijeesh et al. 2012). A completely randomized design with four replicates was used, each replicate being represented by a slide.
In vitro pollen grain germination
Pollen grain germination viability was determined based on an adapted methodology of Almeida et al. (2011). Three separate experiments, consisting of four replicates for all treatments applied, each conducted to investigate pollen germination on culture medium containing 100 mL of distilled water, 1 g of agar, and the additives listed below:
–Experiment 1: sucrose (10, 15, 30, 45%) and boric acid (0, 80, 160 mg / L)–Experiment 2: sucrose (10, 15, 30, 45%); boric acid (0, 40, 80 mg / L) and 400 mg / L calcium chloride–Experiment 3: calcium chloride (mg / L), sucrose (%) and boric acid (mg / L) – Treatment 1: 1500/10/0; Treatment 2: 500/10/0; Treatment 3: 1500/0/0; Treatment 4: 500/0/0; Treatment 5: 1500/10/300; Treatment 6: 500/10/300
The components were added to a container and heated until dissolved without boiling. After the medium cooled to about 27 °C, pollen collected from flowers in development stage two (pre-anthesis) were added and kept at this temperature for 24 and 48 h for germination. Two-hundred pollen grains were counted per replicate for presence of a clearly visible pollen tube. A completely randomized design was used, with the experiments 1 and 2 in factorial scheme.
Light microscopy analysis
Samples from six anthers at three flower developmental stages (stage one, pre-anthesis and anthesis), were fixed in paraformaldehyde solution (2.5%) in phosphate buffer (0.2 M) pH 7.3 at a ratio of 1:1 for 24 h at 4 °C. After fixation, the material was washed three times in phosphate buffer for 30 min (Bouzon 2006) and dehydrated in a gradual ethanol series (Sanders et al. 1999). Subsequently, the samples were pre-infiltrated in LeicaTM historesin with absolute ethyl alcohol at a ratio of 1:1 for 72 h and infiltrated into LeicaTM historesin for 72 h, according to the manufacturer’s instructions with minor alterations. The samples were sectioned on a rotary microtome (microTec, model CUT 4055) to produce 5-μm thick transverse and longitudinal sections (Nakamura et al. 2010). Sections were stained with toluidine blue (O’Brien et al. 1965) and Lugol histochemical test for starch identification. Sections were placed in either reagent for 10 min and washed in distilled water (Johansen 1940). Observations and records were performed using a light microscope (Leica, model DM5500 B).
Transmission electron microscopy analysis
Samples from six anthers at three stages of flower development (stage one, pre-anthesis stage, and anthesis) were fixed in 2.5% glutaraldehyde solution buffered with 0.1 M cacodylate (pH 7.2) for 24 h. The samples were then washed four times in the same buffer. They were then post-fixed in 1% OsO4 in 0.1 M cacodylate buffer, pH 7.2 (1:1) for 4 h at room temperature (Pueschel 1979). Subsequently, the material was washed twice in 0.1 M cacodylate buffer, pH 7.2 (30 min each), dehydrated in an acetone series (30%, 50%, 70%, 90%, and 100%) for 30 min at each concentration with the 100% acetone stage repeated twice more (adapted from Coimbra et al. 2004). After dehydration, the material was slowly infiltrated with Spurr resin and polymerized in horizontal oven molds at 70 °C for 24 h (Spurr 1969). The material was sectioned with an ultramicrotome (Leica) allocated on copper grids and stained with 5% uranyl acetate and lead citrate (Reynolds 1963). The samples were observed and recorded with a transmission electron microscope (Joel, model JEM 1011).
Scanning Electron microscopy analysis
Samples from six anthers at three flower developmental stages (stage one, pre-anthesis stage, and anthesis) were fixed in 2.5% glutaraldehyde and 0.1 M sodium cacodylate buffer (pH 7.2) with 2 M sucrose for 24 h. Subsequently, the samples were dehydrated with an ethanol series (30%, 50%, 70%, 90%, and 100%) for 30 min at each concentration and then washed twice more with 100% ethanol (adapted from Schmidt et al. 2012) and critical point dried with CO2 (Leica, model EM-CPD-030). The dried samples were covered with 20 nm gold in a metallizer (Baltec, CED 030) (Schmidt et al. 2012). The samples were observed and documented in a scanning electron microscope (SEM) (Jeol, model JSM-6390LV).
Results
In vitro pollen grain germination, in vivo pollen viability, anthers, and microspores analysis
By staining with acetic carmine (in vivo viability), we were able to observe that 68.12% of pollen were viable. However, despite many different treatments, pollen germination was not observed. Because of these unexpected results, we used histological analysis to investigate possible reasons for non-germination of pollen grains.
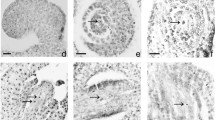
In the stage one, the tetrasporangiate anther had a wall with a unistratified epidermis with juxtaposed cells, followed by a well-structured endothecium, middle layer, and irregularly shaped uni and binucleated tapetum cells (Figs. 2a–d). In the endothecium, starch dispersed in the cytoplasm of cells was evident (Fig. 2d). The tapetum was of a secretory type, as opposed to a plasmodial type, because a layer of cells remained intact around the anther’s locus with uninucleated and occasionally binucleated cells (Fig. 2b and c). The cell walls of the epidermis, endothecium, middle layer, and tapetum were stained blue (toluidine blue) indicating cellulosic compounds. In the parenchymatic tissue between the anther locules, well-developed cells formed a clear connection to the central vascular bundle (stained blue) (Figs. 2b and c). Free microspores had non-polarized nuclei, blue-stained sporoderms, and signs of cytoplasm retraction (Figs. 2b and c). At this stage one inflorescence development, deformed microspores showing cytoplasm poor in organelles (Figs. 2e and f). The free microspores showed signs of a retraction of the cytoplasm (Figs. 3a–c and 3e), with a rupture of the cytoplasmic membrane (Figs. 3b, c, and e). Most mitochondria had membrane disruption (Figs. 3b, d, and e). The microspore sporoderm at this stage was incomplete: the exine (tectum, columella, and endoexine) was formed, but the intine was absent (Fig. 3f). Scanning eletron micrographs showed that the pore and annulus formation in microspores (Fig. 2e).
Scanning and transmission electron micrographs and light microscopy of the Bambusa tuldoides anthers and microspores at stage one of flower development. a, b, d – Cross sections. c – Longitudinal sections. a Epidermis, endothecium, connective tissue and stomium in the anther. Arrowheads indicate microspores. b. Epidermis, endothecium, middle layer and irregularly-shaped tapetum cells. Remaining the parenchyma cells and stomium. In the locule, free microspores with retraction of the cytoplasm (arrowheads) were present. c. Epidermis, endothecium, irregularly-shaped tapetum cells and free microspores with retraction of the cytoplasm (arrowhead). d. Starch deposition in epidermis, endothecium, middle layer. Microspores without starch deposition were present (arrowheads). e. Free microspores with irregular shapes (arrows). Pore and annulus formation in microspores. f. Free microspores with irregular shapes and showing cytoplasm poor. Scale bar: a, b, c, d – 50 μm; e – 20 μm; f – 2 μm. References: Ep, epidermis; Ed, endothecium; T, tapetum; ML, middle layer; St, stomium; PC, parenchyma cells; Cn, connective tissue; An, annulus; Po, pore. (Optical microscopy: b, c – toluidine blue dye; d – Lugol reagent)
Electron transmission micrograph of Bambusa tuldoides microspores at stage one of flower development. a. Irregularly shaped free microspores with retraction of the cytoplasm (arrows). b. Microspore with ruptured plasma membrane (arrowheads), cytoplasm retraction (arrow) and membrane rupture in mitochondria. c. Microspore with rupture (arrowhead) and retraction of the plasma membrane (arrow). Cytoplasmic content with dispersed amyloplasts. d. Microspore with some disruption of mitochondrial membranes. e. Microspore with plasma membrane rupture (arrowheads) and cytoplasm retraction (arrow). Some disruption of mitochondrial membranes close to the nucleus. f. Microspore with sporoderm formed by exine (tectum, endoexine and columella) and starch deposited amyloplast inside. Scale bar: a, b, c, e – 2 μm; d, f – 0.5 μm. References: Nu, nucleolus; N, nucleus; S, starch granule; Mi, mitochondria; Te, tectum; End, endoexine; Co, columellae
In the stage two (pre-anthesis), the epidermis and endothecium cells remained with the same level of blue stain without indication of thickening of the walls of these cells (Fig. 4b). The tapetum cells and the middle layer began to degenerate, and, elsewhere, the connective regions between the anther locules began to break down (Figs. 4a and b). The microspores sporoderm thickness remained unchanged, and the nuclei became hard to visualize (Fig. 4b). At this stage, positive Lugol stains identified the beginning of starch reserve accumulation (Fig. 4c and d).
Scanning and transmission electron micrographs and light microscopy of the Bambusa tuldoides anthers and microspores at stage two of flower development: a, b, c – Cross sections. a. Epidermis, endothecium, connective tissue and stomium in the anther. Arrowheads indicate microspores b. Epidermis, endothecium, remaining tapetum cells (arrows) and stomium. Irregularly-shaped micropores (arrowheads). c. Epidermis, endothecium and anther tapetum cell degeneration. The parenchyma cells can be seen between the locules, the connective tissue and the region of the stomium. Microspores (arrows) with starch deposition in the cytoplasm. d. Microspores with starch deposition. Scale bar: a – 100 μm; b, c – 50 μm; d – 2 μm. References: Ep, epidermis; Ed, endothecium; St, stomium; PC, parenchyma cells; Cn, connective tissue; S, starch granule; Sp, sporoderm. (Optical microscopy: b – toluidine blue dye; c – Lugol reagent)
At the stage four (anthesis), the total destruction of the walls between the anther locules and the dissolution of the tapetum cells was observed (Fig. 5a and b). The stomium had ruptured, and few pollen grains were present inside the anther. Microspores were stained in blue, which indicated the deposition of cellulosic compounds on the sporoderm. Starch continued to accumulate according to the intensity of Lugol staining inside the microspores (Figs. 5b and c). The sporoderm was maintained, and the evident retraction of the cytoplasm was clearly visible at this stage with the total destruction of the plasma membrane, organelles, and cytoplasmic content, and neither the nucleus nor the nucleolus could be visualized (Figs. 5c).
Electron transmission micrograph and light microscopy of the Bambusa tuldoides anthers and pollen grains at stage four of flower development: a, b. Cross sections. a. Epidermis, endothecium, tissue rupture in the anther stomium region and the free microspores (arrowheads) with retracted cytoplasm and irregular shapes. b. epidermis, endothecium and tissue rupture in the anther stomium region. Microspores with higher starch deposition in the cytoplasm (arrows). c. Microspores with increased starch deposition and numerous amyloplasts. Clear retraction of the cytoplasm with destruction of the plasma membrane, organelles and cytoplasmic content. The sporoderm remained intact. Scale bar: a, b – 50 μm; c – 2 μm
Discussion
Analysis of anther in different stages of floral development
The anther contains the reproductive and non-reproductive tissues that are responsible for the production and release of pollen grains. During the stage one of B. tuldoides flower development, the tetrasporangiate anther, with the epidermal outer wall, a subepidermal inner layer called the endothecium and the well-structured middle layer, could be easily identified. More internally, we found the secretory tapetum with irregularly shaped cells (Figs. 2a and c). At the stage two, both the tapetum and the middle layer had degenerated further (Figs. 4a–c), thus starting the anther dehiscence process. Sanders et al. (1999) described the main events that occur during pollen grain release in Arabidopsis: the middle layer and tapetum degenerate; the endothelial layer expands; the septum degenerates to form the bilocular anther; and the stomium cells rupture. Similarly, we observed that the destruction of the locules wall led to the generation of the bilocular anther (Fig. 4a). During stage four (anthesis), the stomium ruptured (Figs. 5a) and released pollen grains into the environment. According to Wilson et al. (2011), anther development, coupled with functional pollen release, is fundamental for plant reproduction. However, although B. tuldoides anthers had normal development and dehiscence, pollen grains were not functional at the time of release.
Genetic defects in anther development often cause male sterility through the production of nonfunctional pollen grains (Ku et al. 2003). One cause may be interference with the process of tapetum development or degeneration, which are both tightly regulated (Parish and Li 2010). Premature or late programmed cell death (PCD) in these cells promotes disruption of pollen grain development (Haddad et al. 2018). The tapetum acts as a source of nutrients for developing pollen grains, as well as a source of exine and lipid precursors for their coating and sporophytic recognition proteins (Furness and Rudall 2001; Shi et al. 2015). Removal of this vital source of many compounds could lead to drastic defects in pollen. For example, Sorghum bicolor (L.) Moench strains that had changes in tapetum development (anomalous nuclei and increased vacuolization) produced sterile pollen grains because the flow of nutrition and other compounds was disrupted (Tsvetova and Elkonin 2013). The abnormal expansion of Bambusa sinospinosa tapetum cells caused trinucleated microspores to shrink and develop a deformed sporoderm (Wang et al. 2015).
According to Parish and Li (2010), tapetum development is sensitive to abiotic stress. Indeed, the tapetum cells of the pollen of a thermal-sensitive Oryza sativa L. strain subjected to high temperatures died prematurely (Ku et al. 2003). Nguyen et al. (2009) also observed sterility of Oryza sativa L. japonica cv R31 pollen when subjected to water stress for 3 days. But in Bambusa tuldoides, tapetum degradation begins at stage two, and the in vivo viability showed that 68.12% of pollen were viable (Fig. 2b and c). So this is not the cause of the unviability of pollen grains.
Analysis of pollen grain at different stages of floral development
After anther tapetum cell degeneration, the microspore is expected to undergo mitosis and starch accumulation, culminating in the release of mature and viable pollen (Bedinger 1992; Datta et al. 2002). However, in B. tuldoides, pollen grains are unviable.
In B. tuldoides, the first indication of microspore degeneration is the retraction and disruption of the plasma membrane (Figs. 3a–c and 3e). Similarly, the plasma membrane of Actinidia deliciosa microspores showed signs of retractions, but all other cellular organelles, including mitochondria, had well-preserved structures (Coimbra et al. 2004). In contrast, B. tuldoides microspores showed ruptured mitochondria membranes (Figs. 3b, d, and e). The presence of functional mitochondria is important in the development of microspores, as these provide the vast majority of ATP needed to support an active cellular metabolism (Regan and Moffatt 1990; Logan 2006).
In the following stages of pre-anthesis and anthesis, these organelles were no longer visible, leaving only starch grains and the incomplete sporoderm presenting the exine without the formation of the intine (Figs. 3f, 4d, and 5c). In the free microspore stage, intine formation begins and ends during pollen grain maturation (Blackmore et al. 2007). The main components of intine are pectin, cellulose, hemicellulose, hydrolytic enzymes, and hydrophobic proteins. These are necessary for maintaining structural integrity, as well as playing a role in germination and growth of the pollen grain pollen tube (Shi et al. 2015).
Starch deposition in microspores is one of the main processes that must occur before pollen grains are released from anthers (Blackmore et al. 2007). Starch biosynthesis during the late stages of pollen maturation is critical for the pollen of many types of plants, not only because starch is a source of energy and reserves for pollen germination but also serves as a checkpoint for pollen maturity. Therefore, pollen infeasibility may be associated with starch deficiency (Datta et al. 2002). In the case of B. tuldoides microspores, starch was continually deposited even with cytoplasm and nucleus degeneration until anthesis. The first signs of starch deposition occurred at stage one (Figs. 3c and 3f), and starch continued to accumulate from pre-anthesis to anthesis until microspores were filled with amyloplasts (Figs. 4c–d and 5b–c). In Lilium hybrida cv. Citronella, a similar process was observed, in which the cytoplasm progressively degenerated after microspore mitosis, but the cytoplasm of the pollen grains contained a large amount of starch (Varnier et al. 2005).
Carbohydrates produced by the sporophyte and dispersed in the locular fluid are absorbed for microspore development (Clement and Audran 1995) and can have different destinations: consumed immediately; transformed into other molecules; polymerized to form the intine; or stored as starch grains (Pacini and Franchi 1983). This event could explain the continuity of starch deposition in the B. tuldoides microspore cytoplasm even with the degeneration of cytoplasmic structures.
Pollen grain unviability and genetic diversity
Plants may reproduce sexually or asexually, and each method is controlled by distinct mechanisms with contrasting genetic consequences. The most common form of asexual reproduction is clonal growth, in which vegetative modules (ramets) are produced by the parental genotype (genet) (Vallejo-Marín et al. 2010). In bamboos, due to the long period of vegetative growth, many species rely little on sexual reproduction. These reproduce clonally through a complex system of underground rhizomes from which the roots and stalks emerge, allowing them to spread over large areas (Pohl 1991; Guilherme et al. 2017). However, as asexual propagation can only produce genetically identical offspring, it is unable to introduce new genotypes into the population. Thus, dependence on vegetative regeneration can lead to a genetically homogeneous population (Kitamura and Kawahara 2011).
Sexual reproduction is essential in the long term to ensure the sustainability of species populations as it provides an independent dispersal phase, greater genetic diversity, and potential for adaptation to new environments (Wilcock and Neiland 2002; Ramawat et al. 2014). Sexual reproduction in bamboos, therefore, is necessary for population regeneration (Huang et al. 2002). A study of Dendrocalamus giganteus populations located in China showed that there is low genetic variation within the populations of this species. The authors attributed the low genetic variation to its long vegetative phase and widely separated flowering/seed episodes during the species life cycle (Tian et al. 2012). These are typical features of various woody bamboo species (Janzen 1976). Bambusa tuldoides, likewise, have long vegetative periods between flowering events, but, beyond that, the scarce-to-absent production of caryopses may be related to pollen unviability, leading to a potential loss of genetic diversity due to inefficient sexual reproduction.
The unviability of pollen grain and the consequent non-production of caryopses has also been reported in B. striata, B. vulgaris, B. sinospinosa, D. giganteus, and B. balcooa (Koshy and Pushpagadan 1997; Koshy and Jee 2001; Jijeesh et al. 2014; Wang et al. 2015; Das et al. 2017). In B. striata, the non-production of caryopses and flowering after a long growing season was the focus of several studies leading researchers to speculate about the possible risk of extinction of the species (John and Nadgauda 1997; Koshy and Pushpagadan 1997; Koshy and Jee 2001). The absence of B. balcooa caryopses led Das et al. (2017) to state that effective conservation strategies would require careful planning. Despite the knowledge about the lack of caryopsis production in several bamboo species, these works do not generally address how this fact can influence the genetic variability and conservation of these species.
Loss of genetic variation may reduce population viability due to inbreeding depression, reduced adaptability to new environments or, in the case of plants, genetic self-incompatibility systems, and a subsequent reduced availability of compatible partners (Ellstrand and Elam 1993).
Conclusion
During development, pollen grains showed accumulation of amyloplasts, a seeming loss of organelles, signs of cytoplasm retraction, rupture of the cytoplasmic membrane, and an incomplete sporoderm. These pollen grains were released into the atmosphere but were unviable and may be responsible for the non-production of caryopses. Considering that caryopses have not been observed in this study (and in others also), B. tuldoides may maintain their populations mainly, or, perhaps, solely by means of vegetative propagation.
References
Almeida C, Amaral AL, Barbosa Neto JF, Sereno MJCM (2011) Conservação e germinação in vitro de pólen de milho (Zea mays subsp. mays). Rev Bras Bot 34:493–497. https://doi.org/10.1590/S0100-84042011000400003
Azzini A, Ciaramello D, Salgado ALB, Tomazello Filho M (1988) Densidade básica do colmo e fibras celulósicas em progénies de Bambusa tuldoides Munro. Bragantia 47:239–246. https://doi.org/10.1590/S0006-87051988000200008
Bahru T, Mulatu Y, Kidane B (2015) Germination Ecology of Arundinaria alpina (K. Schum.) and Oxytenanthera abyssinica (A. Rich.) Munro Seeds: Indigenous Bamboo Species in Ethiopia. Int J Biodivers:1–8. https://doi.org/10.1155/2015/323128
Bedinger P (1992) The remarkable biology of pollen. Plant Cell 4:879–887. https://doi.org/10.1105/tpc.4.8.879
Blackmore S, Wortley AH, Skvarla JJ, Rowley JR (2007) Pollen wall development in flowering plants. New Phytol 174:483–498. https://doi.org/10.1111/j.1469-8137.2007.02060.x
Bonilla SH, Guarnetti RL, Almeida CMVB, Giannetti BF (2010) Sustainability assessment of a giant bamboo plantation in Brazil: exploring the influence of labour, time and space. J Clean Prod 18:83–91. https://doi.org/10.1016/j.jclepro.2009.07.012
Bouzon ZL (2006) Histoquímica e ultra-estrutura da ontogênese dos tetrasporângios de Hypnea musciformis (Wulfen) J. V. Lamour. (Gigartinales, Rhodophyta). Rev Bras Bot 29:229–238. https://doi.org/10.1590/S0100-84042006000200004
Clement C, Audran JC (1995) Anther wall layers control pollen sugar nutrition in Lilium. Protoplasma 187:172–181. https://doi.org/10.1007/BF01280246
Coimbra S, Torrão L, Abreu I (2004) Programmed cell death induces male sterility in Actinidia deliciosa female flowers. Plant Physiol Biochem 42:537–541. https://doi.org/10.1016/j.plaphy.2004.05.004
Das MC, Gopakumar B, Shameena MK, Jero-Mathu A, Nath AJ, Das AK (2017) Floral biology and pollen sterility in relation to seed set in Bambusa balcooa. J Trop For Sci 29(4):504–508. https://doi.org/10.26525/jtfs2017.29.4.504508
Datta R, Chamusco KC, Chourey PS (2002) Starch biosynthesis during pollen maturation is associated with altered patterns of gene expression in maize. Plant Physiol 130:1645–1656. https://doi.org/10.1104/pp.006908
Ellstrand NC, Elam DR (1993) Population genetic consequences of small population size: implications for plant conservation. Annu Rev Ecol Syst 24:217–242. https://doi.org/10.1146/annurev.es.24.110193.001245
Embaye K (2000) The indigenous bamboo forests of Ethiopia: an overview. Ambio. 29:518–521. https://doi.org/10.1579/0044-7447-29.8.518
Filgueiras TS, Castro de Silva RM (2007) Sporadic flowering in six introduced woody bamboos (Poaceae: Bambusoideae) in Brazil. Bamboo Sci Cult 20:11–14
Filgueiras TS, Goncalves APS (2004) A checklist of the basal grasses and bamboos in Brazil. Bamboo Sci Cult 18:7–18
Filgueiras TS, Longhi-Wagner HM, Viana PL, Zanin A, Oliveira RC, Canto-Dorow TS, Shirasuna RT, Valls JFM, Oliveira RP, Rodrigues RS, Santos-Gonçalves AP, Welker CAD (2013) Poaceae In: Lista de Espécies da Flora do Brasil. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB102232. Accessed 22 September 2018
Furness CA, Rudall PJ (2001) Pollen and anther characters in monocot systematics. Grana 40:17–25. https://doi.org/10.1080/00173130152591840
Guerreiro CI, Lizarazu MA (2010) Flowering of Bambusa tuldoides (Poaceae, Bambusoideae, Bambuseae) in southern South America. Darwiniana 48:25–31
Guilherme DO, Ribeiro NP, Cereda MP (2017) Cultivo, manejo e colheita do bambu. In: Drumond P, Wiedman G (Org) Bambus no Brasil: da biologia à tecnologia. Instituto Ciência Hoje, Rio de Janeiro, pp 28–41
Haddad IVN, Santiago-Fernandes LDR, Machado SR (2018) Autophagy is associated with male sterility in pistillate flowers of Maytenus obtusifolia (Celastraceae). Aust J Bot 66:108–115. https://doi.org/10.1071/BT17174
Huang S-Q, Yang C-F, Lu B, Takahashi Y (2002) Honeybee-assisted wind pollination in bamboo Phyllostachys nidularia (Bambusoideae: Poaceae)? Bot J Linn Soc 138:1–7. https://doi.org/10.1046/j.1095-8339.2002.00001.x
Janzen DH (1976) Why bamboos wait so long to flower. Annu Rev Ecol Syst 7:347–391. https://doi.org/10.1146/annurev.es.07.110176.002023
Jijeesh CM, Seethalakshmi KK, Raveendran VP (2012) Flowering, reproductive biology and post flowering behaviour of Dendrocalamus sikkimensis gamble, in Kerala, India. J Am Bamboo Soc 25:36–42
Jijeesh CM, Seethalakshmi KK, Beena VB, Raveendran VP (2014) Sporadic flowering of Bambusa striata Lodd. Ex Lindl. An ornamental sympodial bamboo in Kottayam Distirct, Kerala, India. Int Res J Biol Sci 3(12):37–41
Johansen DA (1940) Plant microtechnique. McGraw-Hill Books, New York
John CK, Nadgauda RS (1997) Flowering in Bambusa vulgaris var. vittata. Curr Sci 73(8):641–643
Kitamura K, Kawahara K (2011) Estimation of outcrossing rates at small-scale flowering sites of the dwarf bamboo species, Sasa cernua. J Plant Res 124:683–688. https://doi.org/10.1007/s10265-010-0398-2
Koshy KC, Jee G (2001) Studies on the absence of seed set in Bambusa vulgaris. Curr Sci 81(4):375–378
Koshy KC, Pushpagadan P (1997) Bambusa vulgaris blooms, a leap towards extinction. Curr Sci 72(9):622–624
Ku S, Yoon H, Suh HS, Chung Y-Y (2003) Male-sterility of thermosensitive genic male-sterile rice is associated with premature programmed cell death of the tapetum. Planta 217:559–565. https://doi.org/10.1007/s00425-003-1030-7
Lakshmi CJ, Seethalakshmi KK, Chandrasekhara Pillai PK, Raveendran VP (2014) Seed storage behaviour of the edible bamboo Dendrocalamus brandisii (Munro) kurz. Int J Sci Environ Technol 3(2):571–576
Logan DC (2006) The mitochondrial compartment. J Exp Bot 57:1225–1243. https://doi.org/10.1093/jxb/erj151
McClure F (1966) The bamboos. A fresh perspective. Harvard Univ. Press, Cambridge
Mudoi KD, Saikia SP, Goswami A, Gogoi A, Bora D, Borthakur M (2013) Micropropagation of important bamboos: a review. Afr J Biotechnol 12:2770–2785. https://doi.org/10.5897/AJB12.2122
Nakamura AT, Longhi-Wagner HM, Scatena VL (2010) Anther and pollen development in some species of Poaceae (Poales). Braz J Biol 70:351–360 https://doi.org/10.1590/S1519-69842010005000005
Nguyen GN, Hailstones DL, Wilkes M, Sutton BG (2009) Drought-induced oxidative conditions in rice anthers leading to a programmed cell death and pollen abortion. J Agron Crop Sci 195:157–164. https://doi.org/10.1111/j.1439-037X.2008.00357.x
O’Brien TP, Feder N, Mccully ME (1965) Polychromatic staining of plant cell walls by toluidine blue. Protoplasma 59:368–373. https://doi.org/10.1007/BF01248568
Pacini E, Franchi GG (1983) Pollen grain development in Smilax aspera L. and possible function of the loculus. In: Mulcahy DL, Ottaviano E (eds) Pollen biology and implications in plant breeding. Elsevier, New York, pp 183–190
Parish PW, Li SF (2010) Death of a tapetum: a programme of developmental altruism. Plant Sci 178:73–89. https://doi.org/10.1016/j.plantsci.2009.11.001
Pohl RW (1991) Blooming history of the Costa Rica bamboos. Rev Biol Trop 39:111–124
Pueschel C (1979) Ultrastructure of tetrasporogenesis in Palmaria palmata (Rhodophyta). J Phycol 15:409–424. https://doi.org/10.1111/j.1529-8817.1979.tb00713.x
Ramawat KG, Mérillon J-M, Shivanna KR (2014) Reproductive biology of plants. CRC Press, New York
Rawat MMS, Thapliyal RC (2003) Storage behaviour of bamboo (Dendrocalamus membranaceus) seeds. Seed Sci Technol 31:397–403. https://doi.org/10.15258/sst.2003.31.2.16
Regan SM, Moffatt BA (1990) Cytochemical analysis of pollen development in wild type Arabidopsis and a male-sterile mutant. Plant Cell 2:877–889. https://doi.org/10.1105/tpc.2.9.877
Reynolds ES (1963) The use of lead citrate at light pH as an electron opaque stain in electron microscopy. J Cell Biol 17:208–212. https://doi.org/10.1083/jcb.17.1.208
Sanders PM, Bui AQ, Weterings K, McIntire KN, Hsu Y-C, Lee PY, Truong MT, Beals TP, Goldberg RB (1999) Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex Plant Reprod 11:297–322. https://doi.org/10.1007/s004970050
Schmidt ÉC, Pereira B, Santos R, Pontes CLM, Scherner F, Horta PA, Paula MR, Latini A, Ramlov F, Maraschin M, Bouzon ZL (2012) Alterations in architecture and metabolism induced by ultraviolet radiation-B in the carragenophyte Chondracanthus teedei (Rhodophyta, Gigartinales). Protoplasma 249:353–367. https://doi.org/10.1007/s00709-011-0286-1
Scurlock JMO, Daytonb DC, Hamesb B (2000) Bamboo: an overlooked biomass resource? Biomass Bioenergy 19:229–244. https://doi.org/10.1016/S0961-9534(00)00038-6
Shi J, Cui M, Yang L, Kim Y-J, Zhang D (2015) Genetic and biochemical mechanisms of Pollen Wall development. Trends Plant Sci 20:741–753. https://doi.org/10.1016/j.tplants.2015.07.010
Singh G, Richa (2016) Viability loss of Dendrocalamus hamiltonii seeds with storage associated with membrane phase behaviour and hormonal analysis. Int J Life Sci 4(4):547–553
Spurr AR (1969) A low viscosity epoxy resin-embedding medium for electron microscopy. J Ultrasctrut Res 26:31–43. https://doi.org/10.1016/S0022-5320(69)90033-1
Thapliyal RC, Sood OP, Rawat MMS (1991) Effect of moisture content and storage temperature on the viability of Bambusa tulda seed. Int Tree Crops J 7:67–75. https://doi.org/10.1080/01435698.1991.9752903
Tian B, Yang H-Q, Wong K-M, Liu A-Z, Ruan Z-Y (2012) ISSR analysis shows low genetic diversity versus high genetic differentiation for giant bamboo, Dendrocalamus giganteus (Poaceae: Bambusoideae), in China populations. Genet Resour Crop Evol 59:901–908. https://doi.org/10.1007/s10722-011-9732-3
Tsvetova MI, Elkonin LA (2013) Cytological investigation of pollen development in sorghum line with male sterility induced by sodium ascorbate in tissue culture. Am J Plant Sci 4:11–18. https://doi.org/10.4236/ajps.2013.47A1002
Vallejo-Marín M, Dorken ME, Barrett SCH (2010) The ecological and evolutionary consequences of clonality for plant mating. Annu Rev Ecol Evol Syst 41:193–213. https://doi.org/10.1146/annurev.ecolsys.110308.120258
Varnier A-L, Mazeyrat-Gourbeyre F, Sangwan RS, Clément C (2005) Programmed cell death progressively models the development of anther sporophytic tissues from the tapetum and is triggered in pollen grains during maturation. J Struct Biol 152:118–128. https://doi.org/10.1016/j.jsb.2005.07.011
Wang S-G, Pu X-L, Lin S-Y, Ding Y-L (2015) Reproductive characteristics of three bamboo species. Pak J Bot 47(6):2301–2308
Warrier RR, Sivakumar V, Anandalakshmi R, Vijayachandran SN, Mahadevan NP, Singh BG (2004) Improving storability of Bambusa arundinacea (Retz.) Willd. seeds. J Bamboo Rattan 3(4):375–382. https://doi.org/10.1163/1569159042464707
Wilcock C, Neiland R (2002) Pollination failure in plants: why it happens and when it matters. Trends Plant Sci 7(6):270–277. https://doi.org/10.1016/S1360-1385(02)02258-6
Williams JT, Dransfield J, Ganapathy PM, Liese W, Nor SM, Sastry CB (1994) Research needs for bamboo and rattan to the year 2000. INBAR, Beijing
Wilson ZA, Song J, Taylor B, Yang C (2011) The final split: the regulation of anther dehiscence. J Exp Bot 62:1633–1649. https://doi.org/10.1093/jxb/err014
Xie N, Chen LN, Wong KM, Cui YZ, Yang HQ (2016) Seed set and natural regeneration of Dendrocalamus membranaceus Munro after mass and sporadic flowering in Yunnan, China. PLoS One 11(4):1–7. https://doi.org/10.1371/journal.pone.0153845
Zhang D, Liu D, Lv X, Wang Y, Xun Z, Liu Z, Li F, Lu H (2014) The cysteine protease CEP1, a key executor involved in Tapetal programmed cell death, regulates pollen development in Arabidopsis. Plant Cell 26:2939–2961. https://doi.org/10.1105/tpc.114.127282
Zhou S, Wang Y, Li W, Zhao Z, Ren Y, Wang Y, Gu S, Lin Q, Wang D, Jiang L, Su N, Zhang X, Liu L, Cheng Z, Lei C, Wang J, Guo X, Wu F, Ikehashi H, Wang H, Wan J (2011) Pollen Semi-Sterility1 encodes a Kinesin-1-like protein important for male meiosis, anther dehiscence, and fertility in rice. Plant Cell 23:111–129. https://doi.org/10.1105/tpc.109.073692
Funding
This study was supported by the National Council for Scientific and Technological Development (CNPq, Proc. 457726/2013-0), and RP (302105/2017-4) and MPG (302798/2018-8) productivity scholarships. The authors also thank the Coordination for the Improvement of Higher Education Personnel (CAPES) for the doctoral level scholarship to the first author.
Author information
Authors and Affiliations
Contributions
This work was developed during the PhD of the first author, Priscila Fernandes de Souza, under the supervision of Rosete Pescador and co-supervision of Cristina M. Ribas dos Santos. The study conception and design was carried out together by Priscila Fernandes de Souza, Rosete Pescador, Cristina M. Ribas dos Santos, and Miguel P. Guerra. Material preparation, data collection, and analysis were performed by Priscila Fernandes de Souza and Joseph Ree. The first draft of the manuscript was written by Priscila Fernandes de Souza, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Handling Editor: Benedikt Kost
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Souza, P.F., dos Santos, C.M.R., Ree, J. et al. Male sterility in Bambusa tuldoides Munro. Protoplasma 257, 911–920 (2020). https://doi.org/10.1007/s00709-019-01479-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-019-01479-8