Abstract
Cuticular wax is a hydrophobic barrier between the plant surface and the environment that effectively reduces the loss of water. The surface of Welsh onion leaves is covered with wax. To explain the relationship between wax composition and water loss, we conducted this experiment. The water permeability and wax composition of leaves were determined by chemical and GC-MS methods. We performed a comparative analysis of the differences between the two cultivars and analyzed the relationship between water permeability and waxy components. Overall, the permeability to water was higher in ‘Zhangqiu’ than in ‘Tenko’. The wax amount of ‘Tenko’ was 1.28-fold higher than that of ‘Zhangqiu’ and was primarily explained by the much larger amounts of ketones and alcohols in the former. Among the waxy components, C29 ketones were most abundant. There were substantial discrepancies in wax composition, total wax content, and water permeability between the two cultivars. The main reason for the discrepancy in water permeability may be the significantly lower aliphatic fraction in ‘Zhangqiu’ than in ‘Tenko’. This study makes a vital contribution to drought resistance research on allium plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The plant cuticle is a chemical and physical structure, and its functional properties largely depend on its chemical composition and structural arrangement (Jetter et al. 2007). The cuticle is impregnated with an insoluble polymer matrix (cutin, dominated by C16 and C18 hydroxyl fatty acids and their derivatives) impregnated by and covered with solvent-soluble lipids, termed ‘waxes’ (Chu et al. 2018). Plant cuticular wax is principally composed of an organic mixture such as a very-long-chain fatty acid and its derivatives. It covers the surface of various tissues and organs of terrestrial plants that are exposed to the air (Yeats and Rose 2013). Numerous studies have shown that plant epidermal wax functions in dealing with external biotic and abiotic stresses and inhibits the nonstomatal loss of water (Bernard and Joubès 2013; Delude et al. 2016), protecting plants from pests and diseases, ultraviolet radiation, and high-temperature radiation (Bueno et al. 2019; Krauss et al. 1997; Long et al. 2003; Olascoaga et al. 2014; Sharma et al. 2019). Especially under drought conditions, the wax on the surface of plants is thickened and increased in a manner that is synergized with the closing of pores, effectively reducing the loss of water in plants (Patwari et al. 2019). The total amount of cutin monomers and waxes varies widely among diverse species (Martin and Rose 2014). These differences explain the difference in barrier properties, especially regarding the transpiration barrier function of the cuticle. Studies have shown that cuticular wax is an important barrier to prevent uncontrolled water loss. The composition and amount of plant cuticular waxes differ significantly among species, tissues, organs, growth stages, and environments, and differences in waxiness can occur within the same species and even the same organ (Huang et al. 2017). Due to the diversity of the waxy composition of the epidermis, the transpiration barrier varies greatly among numerous plants and even diverse cultivars.
Welsh onion (Allium fistulosum L.) has long been cultivated as a vegetable and spice in Asia for its flavor and aroma, its typical features is that leaf surface is covered with cuticular wax (Tendaj and Mysiak 2007). ‘Zhangqiu’ and ‘Tenko’ are two representative cultivars of Welsh onions cultivated in China. ‘Zhangqiu’ Welsh onion was recognized as a geographical indication product by the Chinese Ministry of Agriculture in 2007. It has the characteristics of high, long, crisp, and sweet with pseudostems as food organs. ‘Tenko’, introduced to China in 2003, was selected by the Musashino Seed Co. Ltd., Tokyo, and the main feature is the early maturity and good resistance. Although they have similar genetic lineages, their leaf epidermal waxes vary widely, and this difference is obvious. However, comprehensive experimental studies for understanding the specific differences in leaf epidermal waxes between the two cultivars are lacking. Therefore, the exact experimental analysis of the cuticular wax is significant.
To increase our understanding of the water barrier properties and the diversity of waxy components, this study aimed to compare the leaf water permeability and epidermal wax differences between the two Welsh onion cultivars (‘Zhangqiu’ and ‘Tenko’). The transpiration of Welsh onion leaves through the epidermal wax barrier provides further insight into the chemical composition associated with the epidermal transpiration barrier properties, and a theoretical basis for the breeding of highly drought-resistant Welsh onion cultivars.
Materials and methods
Plant material and cultivation
Two cultivars of Welsh onion (‘Zhangqiu’ and ‘Tenko’) were used for all experiments. The seeds were obtained from a local market. The Welsh onion seeds were rinsed with sterilized distilled water, sown in plugs containing substrate, and grown in a greenhouse with standard irrigation and fertilization. Representative seedlings with four true leaves were transferred and cultured hydroponically in darkened plastic containers (area 0.036 m2, twenty plants per container). Full-strength Hoagland nutrient solutions, which contained 0.5 mM NH4H2PO4, 2.0 mM Ca(NO3)2·4H2O, 3.2 mM KNO3, 1.0 mM MgSO4·7H2O, and full-strength trace elements, were used. The electrical conductivity (EC) and pH of the nutrient solutions were maintained at 2.2–2.5 ms·cm−1 and 6.8–7.0, respectively. The nutrient solutions were aerated every 2 h by air pumps, supplemented to the original volumes every day, and refreshed every 2–3 days. Finally, the plastic containers were transferred to a growth room at 24 °C under a 12-h photoperiod. After the seedlings showed uniform growth, the measurements were performed.
Measurement of the leaf pigment content
For the determination of leaf pigment contents, 0.64 cm2 leaf discs were cut from the leaves. Three discs were immersed in 20-mL tubes with 10 mL of 96% ethanol, incubated in the dark at 25 °C, and then extracted for 24 h. The pigment in the solution was quantified using an ultraviolet-visible spectrophotometer (UV-2450, Shimadzu, Japan) as described in (Zhao et al. 2002).
Measurements of epidermal permeability
The water transpiration of whole leaves was performed to measure water loss over time. Fresh leaves without any defects were selected from each cultivar. Before measurement, the experimental plants were dark-acclimated for at least 4 h, and the leaf sheath was sealed with medical sealant. The entire measurement was carried out in a laboratory where the temperature and humidity were controlled. We recorded the weight loss of the leaves every 15 min using a digital balance with an accuracy of 0.01 g. The transpiration rate and cuticular permeance were calculated according to (Burghardt and Riederer 2003; Schuster et al. 2017).
Besides, the leaf chlorophyll leaching method, modified from (Hiscox and Israelstam 1979), was used to estimate the epidermal permeability. The third leaf from the top was sampled from seedlings with four true leaves, and the seedlings were subjected to a 3-h dark-acclimation period before measurement. Leaves from five seedlings were collected and immersed in 50-mL tubes with 30 mL of 80% ethanol solution, and the tubes were covered with aluminum foil and agitated very gently on a shaker platform. The absorbance values of the leaching solutions at 647 nm and 664 nm were measured by UV spectrophotometer at 15 min, 30 min, 45 min, 60 min, 75 min, 90 min, 105 min, 120 min, 135 min, 150 min, 165 min, 180 min, and 24 h. The percentage of extraction at each time point was converted into a ratio based on the amount extracted at 24 h. Chlorophyll extracted at each time point was expressed as a percentage of the total chlorophyll extracted after 24 h in 80% ethanol.
Extraction and analysis of cuticular wax
Wax extraction and gas chromatography-mass spectrometry (GC-MS) analysis were performed following the protocol by (Haslam et al. 2012; Kolattukudy and Walton 1973) with minor modifications. Briefly, plain leaf discs (with an area of 2.05 cm2) were dipped into glass vials containing 10 ml of chromatography grade n-hexane and gently shaken for 30 s at 65 °C. The extraction was performed twice, and the two extracts were combined and filtered. An internal standard, 20 μL (1 μg·μL−1) n-tetracosane, was added to the filtrate, and the filtrate was evaporated at 50 °C under a stream of nitrogen. The waxes were derivatized by the addition of 100 μL of a pyridine:bis-N, N-(trimethylsilyl) trifluoroacetamide (BSTFA) solution (1:1, v:v) and incubation for 60 min at 80 °C. Then, the remaining BSTFA was evaporated under nitrogen gas and added to the n-hexane.
Wax analyses were performed on the GC-MS QP-2010 (Shimadzu, Japan) equipped with an auto-sampler (AOC-5000) and an RTx-5MS column (60 m, 0.25 mm ID, and 0.25 μm df, Rastek) with helium as the carrier gas. GC was carried out with temperature-programmed on-column injection and oven temperature set at 70 °C for 1 min, and then raised by 10 °C min−1 to 200 °C, held for 1 min, increased by 4 °C min−1 to 300 °C, and held for 20 min. One microliter of each sample was injected. At the end of the program run, a scan peak map of each sample was obtained. The component mass spectrometry data was automatically retrieved, and the machine test results were checked against the standard spectrum and related reference literature. The position was compared with that of the internal standard 24-carbon alkane peak map, and the fatty acid composition was determined according to the peak concerning the peak of the standard. Area, the amount of the substance of each component, is determined, and the content of each component is calculated by the area, and the wax content is expressed by μg·cm−2.
Statistical analysis
All data were analyzed by ANOVA using the DPS software package (DPS for Windows, 2009). Duncan’s multiple range test was performed to compare the differences among treatments. Differences in the means among treatments were considered statistically significant when P < 0.05.
Results
Phenotype of cuticular wax of two Welsh onions cultivars
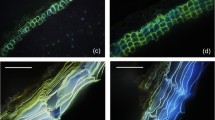
Figure 1 shows that ‘Zhangqiu’ had light-green leaves compared with those of ‘Tenko’. ‘Tenko’ had a visible wax layer on the leaf surface, especially the functional leaves. Because the leaf pigment content affects leaf color, we first determined the leaf pigment content of the two cultivars. No significant difference in chlorophyll content or carotenoid content was observed between the two cultivars. We speculated that the discrepancy in leaf color visible to the naked eye was caused by the difference in the amount of the wax covering the surface of the leaf.
Leaf permeability
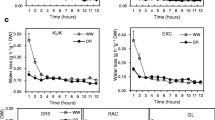
The water permeability was determined for the leaves of the two cultivars (Fig. 2). We usually use ‘cuticular permeance’ (P) to describe the water permeability. However, pores occur on the leaf epidermis, so epidermal transpiration means the lowest stomatal conductance. In determining the water permeability of the leaves, we used darkness to protect the leaf pores. The water permeability of ‘Zhangqiu’ was 1.58-fold higher than that of ‘Tenko’. The chlorophyll leaching rate was much higher in ‘Zhangqiu’. After exposure to 80% ethanol for 3 h, 52.8% of the total chlorophyll was leached from ‘Zhangqiu’ leaves, whereas the chlorophyll leaching from the ‘Tenko’ leaves was 42.1%. ‘Zhangqiu’ lost chlorophyll 0.25-fold more quickly than did ‘Tenko’, an indication that ‘Zhangqiu’ had the most permeable cuticle. As mentioned above, water loss was much more severe in ‘Zhangqiu’.
Leaf permeability of two Welsh onion cultivars. a Water permeance of the surface of Welsh onion leaves. b Chlorophyll leaching rates were expressed as a percentage of the total chlorophyll extracted by n-hexane. Data are given as the means ± standard deviation (n = 10). The asterisks indicate significant differences between ‘Zhangqiu’ and ‘Tenko’ Welsh onions (*P < 0.05, **P < 0.01; Student’s t test)
Wax coverage and chemical composition
The chemical constituents of the epidermal wax of the leaves of the two cultivars were determined by GC-MS and compared. To perform a comprehensive analysis of the cuticular wax mixture, we determined the absolute amount of the total mixture and the compound classes. Overall, the coverage wax load in ‘Tenko’ leaves was 104.92 μg cm−2, and only 46.09 μg·cm−2 in ‘Zhangqiu’ leaves, a significantly lower wax coverage by comparison. Here, seven classes of compounds were identified in the wax mixtures covering the leaves of Welsh onion, including alcohols, aldehydes, alkanes, esters, fatty acids, ketones, and β-sitosterol (Fig. 3). These compounds were detected in all two cultivars. Less than 3% of the wax mixtures from the leaves remained unidentified. Total surface wax amounts per area were 1.28-fold higher in ‘Tenko’ than those in ‘Zhangqiu’, and this was principally explained by the much higher amounts of ketones and alcohols. Total ketone, alcohol, and fatty acid amounts in ‘Tenko’ were all significantly higher than those in ‘Zhangqiu’, by 1.21-, 2.00-, and 0.61-fold, respectively.
a Comparison of total cuticular wax loads from the leaves of two cultivars. b Comparison of leaf cuticular components of two cultivars. c Percentage of wax composition in leaves of Welsh onions. Values represent the leaf area ± SD (n = 3) in μg·cm−2 for each wax component. The asterisks indicate significant differences between ‘Zhangqiu’ and ‘Tenko’ Welsh onions (*P < 0.05, **P < 0.01; Student’s t test)
A high abundance of ketones was very evident in two cultivars. The percentage of ketone in the wax mixture significantly differed, ‘Zhangqiu’ was larger. Alcohols were the second dominant compound class, accounting for 24.23 and 31.94% of the wax mixtures in ‘Zhangqiu’ and ‘Tenko’, respectively. Only trace amounts of sterols were detected. Through PCA analysis, samples of the two cultivars were distinguished according to their extent of wax coverage (Fig. 4). Principal component 1 separated the two species well. ‘Zhangqiu’ was in the negative direction of the first component, and ‘Tenko’ was in the positive direction of the first component. Most wax classifications were directly proportional to that of the ‘Tenko’ leaves.
Chain length distributions of within-compound classes of Welsh onion leaf wax mixtures
Next, the chain length profile within these wax mixtures was further analyzed (Fig. 5). The leaf wax ketones had odd-numbered chain lengths and were present as C11, C13, C27, and C29 homologs, with C29 being the most abundant in ‘Tenko’. However, the ketones were present in significantly lower abundances in ‘Zhangqiu’. Alcohols occurred in fairly broad chain lengths (C9 to C33) and were predominated by C31 in both cultivars. The fatty acid fractions of the leaf wax ranged from C15 to C29 in ‘Zhangqiu’ and were predominated by C19, whereas they ranged from C11 to C29, with a dominant abundance in C19, in ‘Tenko’ leaf waxes. The wax alkanes had even-numbered chain lengths from C32 to C60, with C60 being the most abundant. These wax alkanes were not different between the two cultivars, except C40. Aldehyde (C21 to C29) and ester (C8 to C30) homologs were also observed. In contrast, only trace amounts of sterols were detected, with the only constituent being β-sitosterol in both cultivars.
Wax chemical profiles, presented as chemical classes, on the leaves of Welsh onion. Carbon chain lengths of individual constituents are shown for each wax chemical class in μg·cm−2. The asterisks indicate significant differences between ‘Zhangqiu’ and ‘Tenko’ Welsh onions (*P < 0.05, **P < 0.01; Student’s t test)
In addition, the ratio of aliphatics was different between the two Welsh onion cultivars (Table 1). Overall, the deposition of cuticular waxes appears to vary in a species-specific manner; therefore, the contribution of each component to the barrier properties may be subject to various factors.
Discussion
This study provides a detailed description of the water permeability characteristics of the leaves of Welsh onion and comprehensive qualitative and quantitative analyses of the cuticular wax of two cultivars. This is the first comprehensive description of the leaf cuticular wax characteristics of Welsh onion.
Under conditions of severe drought, minimizing the water loss of leaves, which represent the main interface between the plant and the atmosphere, is of decisive importance (Caird et al. 2007). Previous studies have shown that leaves in Prunus laurocerasus prevent 96% of water from diffusing, and, in some species, even up to 99.5%, which indicates that the cuticular wax forms an almost complete barrier against the diffusion of the water across the cuticle. Significant differences in cuticular permeability were obtained from water permeability and chlorophyll leaching data. The leaf permeability of most species is less than 2.0 × 10−4 m s−1(Schuster et al. 2017). The leaf water permeability of both cultivars was much lower than that of most species, which may increase the drought resistance of Welsh onion. Compared with ‘Zhangqiu’, ‘Tenko’ has a less water-permeable cuticle, which may result from defects in cutin or wax deposition in the former.
The total wax load between the two cultivars varied greatly. Total surface wax amounts in ‘Zhangqiu’ leaves were much lower than those of ‘Tenko’ leaves. The total wax load for leaves of the Welsh onion was consistent with the water permeability trends. The difference in water permeability may be caused by the discrepancy in the wax load of two cultivars. However, some reports suggest that the plant’s cuticle is a persistent structure that is not considered to be subject to major short-term regulatory adjustments (Johnson et al. 1983; Olascoaga et al. 2014). We speculate that the role of wax in preventing excessive water loss may be mainly related to waxy components.
Typical cuticular wax components include very-long-chain aliphatic compounds and cyclic molecules. Wax composition varied definitely among plants (Busta et al. 2017). For allium plants, leaf waxes are formed from VLCFAs and their derivatives such as ketones, alkanes, and aldehydes (Damon et al. 2014; Lokesh et al. 2018; Rhee et al. 1998). The VLCFAs are closely packed and form a barrier, a water-impermeable sheet, in the cuticle. In this study, both cultivars primarily contained VLCFA derivatives. These VLCFAs are the main element in the formation of a transpiration barrier. In ‘Tenko’, leaf wax mixture was mainly composed of three components: ketones, alcohols, and aldehydes. The predominant chain length was C29 for ketones, C31 for alcohols, and C29 for aldehydes. These components, which can assemble the crystalline of cuticle, were arranged in the leaf epidermis to form a water-impermeable region. The impermeability forces water to propagate, and an extended route of transmission increases the resistance of the cuticle to the water that passes through its (Beisson et al. 2012). The number of prominent components in the ‘Zhangqiu’ leaf waxes was much lower than that in the ‘Tenko’ leaf waxes, so the crystalline structure of ‘Tenko’ was tighter.
To date, most studies have concluded that the fatty compounds in wax form a barrier (Go et al. 2014; Lee et al. 2016). The pentacyclic triterpenoid in the plant epidermis wax, mainly forming an amorphous matrix, and its contribution seem to be small or even negligible (Jetter et al. 2007; Schuster et al. 2016). The cyclic compounds were detected in both cultivars, and ‘Zhangqiu’ had a higher proportion of cyclic compounds than did ‘Tenko’. The aliphatic fraction in the ‘Tenko’ leaves was significantly higher than that in the ‘Zhangqiu’ leaves, indicating that more aliphatic substances were arranged together to make the leaf epidermal wax crystals a veritable hydrophobic barrier in ‘Tenko’ than in ‘Zhangqiu’, which ultimately affected the difference in the permeability of ‘Zhangqiu’ leaves. The epidermal barrier properties are primarily attributed to the formation of a long-chain aliphatic wax compound of crystalline structure that is impermeable to water molecules (Beisson et al. 2012; Gorb et al. 2005). Besides, in the leaf epidermal wax of plants, a phenomenon of spontaneous recombination occurs and the protamine of the epidermal wax is not arranged in a complete single layer but forms a multicomponent semicrystalline wax (Chambers et al. 1976). The increase in the proportion of aliphatic fraction provides sufficient raw materials for the reorganization of the epidermal wax. This may also be the cause of the water permeability of ‘Tenko’ being lower than that of ‘Zhangqiu’.
Conclusion
The ‘Tenko’ blades had visible wax deposits, and the wax load was higher than in ‘Zhangqiu’. In Welsh onion, the wax mixtures contained seven classes of compounds: ketones, alcohols, alkanes, aldehydes, fatty acids, esters, and β-sitosterol. The predominant component in Welsh onion was the C29 ketones, and the amount of β-sitosterol was negligibly low. The abundance of each class differed between the two cultivars, so the water permeability of the leaves was significantly distinct. In addition, the greater the wax coverage was, the more raw materials were used to build cuticle barrier. As a consequence, the lower aliphatic fraction in ‘Zhangqiu’, compared with that in ‘Tenko’, could be a prominent factor leading to a higher cuticular permeability. In summary, the results presented elucidate the leaf wax coverage and composition in allium, providing a further link between the chemical composition of the epidermis and the physiological blocking of water transpiration. The evidence provides a reference for further exploration of the wax composition of allium plants.
References
Beisson F, Li-Beisson Y, Pollard M (2012) Solving the puzzles of cutin and suberin polymer biosynthesis. Curr Opin Plant Biol 15(3):329–337
Bernard A, Joubès J (2013) Arabidopsis cuticular waxes: advances in synthesis, export and regulation. Prog Lipid Res 52(1):110–129
Bueno A, Alfarhan A, Arand K, Burghardt M, Deininger A-C, Hedrich R, Leide J, Seufert P, Staiger S, Riederer M (2019) Effects of temperature on the cuticular transpiration barrier of two desert plants with water-spender and water-saver strategies. J Exp Bot 70(5):1613–1625
Burghardt M, Riederer M (2003) Ecophysiological relevance of cuticular transpiration of deciduous and evergreen plants in relation to stomatal closure and leaf water potential. J Exp Bot 54(389):1941–1949
Busta L, Hegebarth D, Kroc E, Jetter R (2017) Changes in cuticular wax coverage and composition on developing Arabidopsis leaves are influenced by wax biosynthesis gene expression levels and trichome density. Planta 245(2):297–311
Caird MA, Richards JH, Donovan LA (2007) Nighttime stomatal conductance and transpiration in C3 and C4 plants. Plant Physiol 143(1):4–10
Chambers TC, Ritchie IM, Booth MA (1976) Chemical models for plant wax morphogenesis. New Phytol 77(1):43–49
Chu W, Gao H, Chen H, Fang X, Zheng Y (2018) Effects of cuticular wax on the postharvest quality of blueberry fruit. Food Chem 239:68–74
Damon SJ, Groves RL, Havey MJ (2014) Variation for epicuticular waxes on onion foliage and impacts on numbers of onion thrips. J Am Soc Hortic Sci 139(4):495–501
Delude C, Fouillen L, Bhar P, Cardinal M-J, Pascal S, Santos P, Kosma DK, Joubès J, Rowland O, Domergue F (2016) Primary fatty alcohols are major components of suberized root tissues of arabidopsis in the form of alkyl hydroxycinnamates. Plant Physiol 171(3):1934–1950
Go YS, Kim H, Kim HJ, Suh MC (2014) Arabidopsis cuticular wax biosynthesis is negatively regulated by the DEWAX gene encoding an AP2/ERF-type transcription factor. Plant Cell 26(4):1666–1680
Gorb E, Haas K, Henrich A, Enders S, Barbakadze N, Gorb S (2005) Composite structure of the crystalline epicuticular wax layer of the slippery zone in the pitchers of the carnivorous plant Nepenthes alata and its effect on insect attachment. J Exp Biol 208(24):4651–4662
Haslam TM, Mañas-Fernández A, Zhao L, Kunst L (2012) Arabidopsis ECERIFERUM2 is a component of the fatty acid elongation machinery required for fatty acid extension to exceptional lengths. Plant Physiol 160(3):1164–1174
Hiscox JD, Israelstam GF (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57(12):1332–1334
Huang D, Feurtado JA, Smith MA, Flatman LK, Koh C, Cutler AJ (2017) Long noncoding miRNA gene represses wheat β-diketone waxes. Proc Natl Acad Sci 114(15):E3149–E3158
Jetter R, Kunst L, Samuels AL (2007) Composition of plant cuticular waxes. In: Annual Plant Reviews, Biology of the plant cuticle, vol 23. https://doi.org/10.1002/9780470988718.ch4
Johnson DA, Richards RA, Turner NC (1983) Yield, water relations, gas exchange, and surface reflectances of near-isogenic wheat lines differing in Glaucousness. Crop Sci 23(2):318–325
Kolattukudy PE, Walton TJ (1973) The biochemistry of plant cuticular lipids. Prog Chem Fats Other Lipids 13:119–175
Krauss P, Markstadter C, Riederer M (1997) Attenuation of UV radiation by plant cuticles from woody species. Plant Cell Environ 20(8):1079–1085
Lee SB, Kim HU, Suh MC (2016) MYB94 and MYB96 additively activate cuticular wax biosynthesis in Arabidopsis. Plant Cell Physiol 57(11):2300–2311
Lokesh U, Venkatesh B, Kiranmai K, Nareshkumar A, Amarnathareddy V, Rao GL, Anthony Johnson AM, Pandurangaiah M, Sudhakar C (2018) Overexpression of β-ketoacyl co-a synthase1 gene improves tolerance of drought susceptible groundnut (Arachis hypogaea L.) cultivar K-6 by increased leaf epicuticular wax accumulation. Front Plant Sci 9:1869
Long LM, Patel HP, Cory WC, Stapleton AE (2003) The maize epicuticular wax layer provides UV protection. Funct Plant Biol 30(1):75–81
Martin LBB, Rose JKC (2014) There's more than one way to skin a fruit: formation and functions of fruit cuticles. J Exp Bot 65(16):4639–4651
Olascoaga B, Juurola E, Pinho P, Lukeš P, Halonen L, Nikinmaa E, Bäck J, Porcar-Castell A (2014) Seasonal variation in the reflectance of photosynthetically active radiation from epicuticular waxes of Scots pine (Pinus sylvestris) needles. Boreal Environ Res 19(supplement B):132–141
Patwari P, Salewski V, Gutbrod K, Kreszies T, Dresen-Scholz B, Peisker H, Steiner U, Meyer AJ, Schreiber L, Dörmann P (2019) Surface wax esters contribute to drought tolerance in Arabidopsis. Plant J 98(4):727–744
Rhee Y, Hlousek-Radojcic A, Ponsamuel J, Liu D, Post-Beittenmiller D (1998) Epicuticular wax accumulation and fatty acid elongation activities are induced during leaf development of leeks. Plant Physiol 116(3):901–911
Schuster A-C, Burghardt M, Riederer M (2017) The ecophysiology of leaf cuticular transpiration: are cuticular water permeabilities adapted to ecological conditions? J Exp Bot 68(19):5271–5279
Schuster AC, Burghardt M, Alfarhan A, Bueno A, Hedrich R, Leide J, Thomas J, Riederer M (2016) Effectiveness of cuticular transpiration barriers in a desert plant at controlling water loss at high temperatures. AoB Plants 8:plw027
Sharma P, Madhyastha H, Madhyastha R, Nakajima Y, Maruyama M, Verma KS, Verma S, Prasad J, Kothari SL, Gour VS (2019) An appraisal of cuticular wax of Calotropis procera (Ait.) R. Br.: extraction, chemical composition, biosafety and application. J Hazard Mater 368:397–403
Tendaj M, Mysiak B (2007) Usefulness of Japanese bunching onion (Allium fistulosum L.) for forcing in greenhouse. Acta Agrobot 60(1):143–146
Yeats TH, Rose JK (2013) The formation and function of plant cuticles. Plant Physiol 163(1):5–20
Zhao S, Shi GA, Dong XC (2002) Plant physiology experiment report. Chinese Agriculture Science & Technology Press, Beijing
Funding
This study was supported by the National Characteristic Vegetable Industry Technology System Project (Grant No. CARS-24-A-09), the Double First-Class Discipline Construction Project of Shandong Province (No. SYL2017YSTD06), and the Agricultural Variety Project of the Science and Technology Department of Shandong Province (No. 2016LZGC015).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Kun Xu and Xuena Liu designed the research. Xuena Liu, Song Gao, and Ying Liu performed the experiments. Xuena Liu, Bili Cao, and Zijing Chen analyzed the data. Xuena Liu wrote the manuscript, and Kun Xu revised the intellectual content of this manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Peter Nick
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, X., Gao, S., Liu, Y. et al. Comparative analysis of the chemical composition and water permeability of the cuticular wax barrier in Welsh onion (Allium fistulosum L.). Protoplasma 257, 833–840 (2020). https://doi.org/10.1007/s00709-019-01470-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-019-01470-3