Abstract
Self-incompatibility (SI) is genetically determined reproductive barrier preventing inbreeding and thereby providing the maintenance of plant species diversity. At present, active studies of molecular bases of SI mechanisms are underway. S-RNAse-based SI in Petunia hybrida L. is a self-/non-self recognition system that allows the pistil to reject self pollen and to accept non-self pollen for outcrossing. In the present work, using fluorescent methods including the TUNEL method allowed us to reveal the presence of markers of programmed cell death (PCD), such as DNA fragmentation, in growing in vivo petunia pollen tubes during the passage of the SI reaction. The results of statistical analysis reliably proved that PCD is the factor of S-RNAse-based SI. It was found that preliminary treatment before self-pollination of stigmas of petunia self-incompatible line with aminooxyacetic acid (AOA), inhibitor of ACC synthesis, led to stimulation of pollen tubes growth when the latter did not exhibit any hallmarks of PCD. These data argue in favor of assumption that ethylene controls the passage of PCD in incompatible pollen tubes in the course of S-RNAse-based SI functioning. The involvement of the hormonal regulation in SI mechanism in P. hybrida L. is the finding observed by us for the first time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Self-incompatibility (SI) genetically controlled universal mechanism by means of which pistil accepted non-self pollen and rejected self pollen. This mechanism is inherent in the representatives of 71 families (Takayama and Isogai 2005; Iwano and Takayama 2012). Using genetic, molecular, and biochemical approaches, it was established that flowering plants are equipped with two different systems for pollen recognition, self- and non-self-recognition. Both systems are controlled by pollen-S and pistil-S determinants which are close to each other at the S-locus (Iwano and Takayama 2012).
To date, intensive molecular studies on five families whose representatives possess SI were performed. Based on chemical nature of pollen and pistil S-genes, it is believed that two families, Brassicaceae and Papaveraceae, are characterized by different mechanisms of SI while three from five families, Solanaceae, Rosaceae, and Plantaginaceae, use the same, Solanaceae type, mechanism of SI (Iwano and Takayama 2012).
SI system of self-recognition
In families Brassicaceae and Papaveraceae, SI specificity is controlled by S-pollen and S-pistil determinants from the same S-haplotype.
In Brassicaceae, SI specificity is controlled by the S-locus, which encodes three highly polymorphic proteins, namely SRK (S-locus receptor kinase, S-stigma determinant), SCR (S-locus protein 11, S-pollen determinant), and SLG (S-locus glycoprotein) (Nishio and Hinata 1977; Shimosato et al. 2007; Watanabe et al. 2012; Ma et al. 2016). SP11 is secreted from the anther tapetum (Stephenson et al. 1997; Schopher et al. 1999; Takasaki et al. 2000; Iwano et al. 2003). SRK localizes in PM of papillar cells and is the receptor for SCR, which allows the stigma to discriminate self pollen in the SI response (Takasaki et al. 2000; Takayama et al. 2001; Murase et al. 2004). Upon self-pollination, SP11 molecules penetrate into the papilla cells and interact with SRK which triggers SI responses in the stigmatic cells (Shimosato et al. 2007; Watanabe et al. 2012). At present, the molecular mechanism of SI in Brassicaceae family is under active investigation (Ma et al. 2016).
In the field poppy (Papaver rhoeas L.), SI reaction occurs in the incompatible pollen tubes (Thomas and Franklin-Tong 2004; Thomas et al. 2006; Li et al. 2007; Wu et al. 2011; Wilkins et al. 2011, 2014, 2015). The S-determinants for P. rhoeas are PrsS and PrpS. The female S-determinant, PrsS (P. rhoeas stigma S), is a highly polymorphic small (15 kDa) protein secreted by the stigmatic papilla cells. The male S-determinant, PrpS (P. rhoeas pollen S), is a 20-kDa transmembrane protein (Wheeler et al. 2009, 2010). A direct self-recognition between PrsS and PrpS triggers the SI responses in the pollen tube. Interaction of PrsS with incompatible pollen stimulates increase in cytosolic free Ca2+ and depolymerization of the actin cytoskeleton resulting in pollen tube growth inhibition. The Papaver SI system utilized ROS and nitric oxide (NO) to integrate signaling with programmed cell death (PCD) in incompatible pollen tube (Thomas and Franklin-Tong 2004; Thomas et al. 2006). Downstream targets include also soluble inorganic pyrophosphatase, Pr-p26.1, and a MAP kinase, PrMPK9-1 (Wilkins et al. 2014, 2015).
SI system for non-self-recognition
A completely different SI mechanism, namely the Solanaceae type, is inherent in families Solanaceae, Rosaceae, and Рlantaginaceae. Here, this is regulated by the highly polymorphic S-locus with multiple haplotypes. The results of several research groups led to the conclusion that two closely linked genes on S-locus, (1) gene S-RNAse controlling pistil-S specificity (McClure et al. 1990) and (2) multiple genes S-locus F-box (SLF or SFB) controlling pollen-S specificity (Lai et al. 2002; Kao and Tsukamoto 2004; Sijacic et al. 2004; Hua et al. 2007; Sun et al. 2015; Williams et al. 2014; Wu et al. 2018), are involved in Solanaceae-type SI.
S-RNAse, S-pistil determinant, and proteins SLF (S-locus F-box), S-pollen determinant, interact in the cytoplasm of pollen tube (Liu et al. 2014; Williams et al. 2014; Wu et al. 2018). It was established that the pollen rejection is tightly connected with proteolysis including ubiquitin-proteasome pathway (Entani et al. 2014).
A single female S-determinant in these families is the style glycoprotein of 30-kDa, S-RNAse, possessing ribonuclease activity (Anderson et al. 1989). Two independent research teams dealing with Petunia inflata R.E.Fr. (Lee et al. 1994) and Nicotiana alata Link & Otto (Murfett et al. 1994) showed that S-RNAse is a reasonable condition required for pistil recognizing and rejecting self pollen while non-self pollen tube growth remained to be unaffected. Thus, it has been established that in families Solanaceae, Rosaceae, and Plantaginaceae, S-RNAse-based SI functions. Biochemical mechanism of self-pollen tubes rejection in this case includes RNA degradation in the incompatible pollen tubes, i.e., S-RNAse functions as a toxic ribonuclease.
Most F-box proteins are the component of SCF (SKP1/Cullin1/F-box) ubiquitin-ligase complex which functions as E3 ubiquitin–ligase in the ubiquitin-mediated protein degradation system due to 26S-proteasome (Sun and Kao 2013; Sun et al. 2015). It is believed that SLF proteins function as the module for recognizing substrate of SCF complex which inactivates non-self S-RNAses in ubiquitin-26S-proteasome pathway. From petunia pollen, Entani et al. (2014) isolated components involved in composition of a SCFSLF (SCF = SKP1-CUL1-F-box-RBX1) and showed that the SCFSLF polyubiquitinates the non-self RNAses in vitro and then polyubiquitinated S-RNAses degraded by the 26S-proteasome.
To explain biochemical basic principles of SI, the term “collaborative non-self-recognition” was offered. In this system, many SCFs and each type of SLF possessing own specificity collaboratively polyubiquitinate all non-self S-RNAses mediating their ubiquinating and degradation by 26S-proteasome (Kubo et al. 2010; Hua and Kao 2008). In this system, very SLF positively or negatively charged interacts with subgroup of non-self S-RNAses having opposite charge. According to these interactions, SLF proteins together recognize and detoxify all non-self S-RNAses, with none of SLF proteins can interact with self S-RNAase.
To date, it has been established that Petunia hybrida L. is characterized by a Solanaceae type SI in which self pollen is rejected by S-RNAse being expressed by pistil as result of its cytotoxic function. Numerous male SI determinants, proteins SLF (S-locus F-box), collaboratively detoxify non-self S-RNAses. In order to achieve compatible pollination, complete set of SLF proteins is required for detoxification of all non-self S-RNAses. At present, active search of other factors participating in self/non-self recognition is conducted. In particular, two key pollen SI factors, PhSSKI (Petunia hybrida SLF-interacting Skp1-like1) and PhCUL1-P were isolated from petunia pollen and their relation to the mechanism of RNAse-based SI (Kubo et al. 2016) was established. It was shown that PhSSKI is a specific component of SCF (Skp1-Cullin1-F-box)SLF system essential for detoxification of non-self S-RNases (Zhao et al. 2010) while PhCUL1-P is the primary component of SCFslf complex involved in SI (Kubo et al. 2015, 2016). The results of the experiments in vitro provided evidence that PhSSK1 forms specific complex with PhCUL1-P in compatible pollen tubes, with removing protein PhCUL1-P suppressed the mechanism of non-self S-RNAes detoxification.
Li et al. (2017) showed that electrostatic potentials are the main physical force providing the interaction between proteins SLF localized in the cytosol and RNAse in P. hybrida L. They found that substitution of one amino acid for the other leads to the change in surface electrical potential of SLF and thereby pollen S-specificity. In the case, if S-RNAses are recognized by their related SLF, electrostatic repulsion of them is generated, and, as a result, the domain responsible for S-RNAse recognizing should not be bound with SLF domain. Thus, the ubiquitinating in such manner of own S-RNAse by SCFSLF complex should be prevented. In contrary, if S-RNAses are recognized by non-self SLF, their electrostatic attraction should be generated leading to binding of S-RNAse recognizing domain with SLF domain and further ubiquinating and degradation of non-self S-RNAses. This model of the interactions supports model of S-RNAse degradation and the system of collaborative recognizing non-self RNAses (Kubo et al. 2010).
Despite numerous attempts to understand the mechanism of S-RNAse-based SI, it remains just incompletely studied. Therefore, the studies concerning a signaling pathway underlying the interaction S-RNAase with SLF proteins are so far at the level of hypotheses. Here, we have demonstrated the presence of PCD markers, such as DNA fragmentation, in growing in vivo petunia pollen tubes during operation of the S-RNAse-based SI mechanism as well as possible involvement of hormonal regulation in the action of this mechanism.
Materials and methods
Plant material
Сlonally propagated microshoots of petunia plants (P. hybrida L.) of two lines, self-incompatible and self-compatible, from laboratory collection were adapted to the soil conditions and grown in the boxes with soil under natural illumination in the greenhouse. Micropropagation was performed in tubes on agar Murashige and Skoog medium in the climatic chamber at 26 °C and 16-h light day.
The experiments of the present work were performed on petunia pollen tubes growing in vivo in self-incompatible petunia pistil tissues after self-incompatible pollination, i.e., self-pollination of self-incompatible line, and after cross-compatible pollination, i.e., pollination of self-incompatible line with pollen of self-compatible line.
Phytohormone treatments
On the eve the experiments planed pollen was harvested from the plants of self-incompatible line while non-pollinated flowers were emasculated. On next day, pistils of emasculated flowers were treated with solutions of phytohormones and other compounds, such as ethylene (to a final dilution of 1:10,000), ABA (10 mM), АОА (inhibitor of ACC (ethylene precursor) synthesis, 10 mM), and fluridone, inhibitor of ABA synthesis (10 mM). Concentrations of AOA and ethrel were selected on the base of our own published data (Kovaleva and Zakharova 2003; Kovaleva et al. 2011) while those of fluridone and AOA were applied taking into account the results of our preliminary experiments. To this end on stigma of very pistil 5 μl solution of every compound was put with pipette; thereafter, stigma was closed by insulator. Pollination with self-pollen was performed in 2 or 6 h after the treatment. In these experiments, self-incompatible line petunia pistils (stigmas and styles) on which prior self-pollination a drop of distillated water was applied served as the control. Collection and fixation of the material (pollinated pistils) were carried out in 8, 12, and 24 h after self-incompatible and cross-compatible pollinations.
Visualization of pollen tubes growing in vivo in pistil tissues
Aniline blue staining
This method is based on specific ability of fluorochrome aniline blue to be bound with callose being a part of pollen tube envelope and to form callose plugs. In the experiments, pollinated pistils fixed in acetic alcohol (90% solution of ethanol and glacial acetic acid in relationship 3:1) were used. Maceration was performed in 20% alcohol solution of KOH during 20–40 min; thereafter, pistils were twice washed with distillated water and poured on with 0.01% solution of aniline blue for 30–40 min. Stained pistils were transferred on slide in a drop of glycerin mixed with water (1:1), closed with cover glass, lightly crushed and examined under fluorescent microscope Zeiss Axioplan (Carl Zeiss, Germany). The number of analyzed pollen tubes in each variant of the experiment was not less than 200.
Trypan blue staining
Trypan blue staining is standard method allowing researches to reveal cell PM damage since the stain is bound with proteins of damaged cell. Pistils were fixed in the solution of acetic alcohol (96% alcohol and glacial acetic acid in relationship 3:1), stained for 1 min with boiling alcohol lactophenol containing 0.1 mg/ml trypan blue, and kept in 2.5 mg/ml chloral hydrate solution for 12–24 h at room temperature (Serrano et al. 2010). Crushed preparations were examined under microscope Zeiss Axioplan (Carl Zeiss, Germany). The number of analyzed pollen tubes in each variant of the experiment was not less than 500.
TEM
Styles (the upper part of pistil, 1.5 cm from the surface of stigma) of petunia were fixed in 2.5% solution of glutaraldehyde (Merck, Germany) in 0.1 M Sorensen phosphate buffer (pH 7.2) supplemented with 1.5% sucrose. The plant material was washed out from the fixing mixture and postfixed with 1% ОsО4 (Sigma, USA), dehydrated in ethanol with rising concentrations (30, 50, 70, 96, and 100%) in propylene oxide (Fluka, Germany), and embedded in the mixture of epon araltide epoxy resins. Ultrathin sections were made with an ultramicrotome LKBV (LKB, Sweden). Sections were contrasted with uranyl acetate and lead citrate according to the standard procedure (Kuo 2007). Sections were studied under H500 electron microscopes (Hitachi, Japan) at an accelerating voltage of 75 kV. The photographs obtained were digitized (scanned with an Epson Perfection 3170) with a resolution of 600 dpi. Processing and arrangement of images were performed in Microsoft Photo Editor and Corel DRAW.
Detection of PCD
For detection of PCD markers two methods were used, namely terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) detection of nuclear fragmentation (TUNEL method) and DNA isolation and agarose gel electrophoresis.
TUNEL detection of nuclear fragmentation
TUNEL assay detects DNA strand breaks using terminal deoxynucleotidyl transferase catalyzing attachment of modified deoxynucleotides on the DNA strand breaks. The technique for visualization of PCD localization site in the pollen-pistil system according to TUNEL method (nuclear DNA degradation) was designed by us with the use of the data represented earlier by Wang et al. (2010) but with some modifications. Experimental procedure here used was the following. In 8–9 h after pollination (self-pollination and cross-pollination) of preliminary emasculated petunia flowers pistils were harvested, fixed in the solution containing 30% formaldehyde, glacial acetic acid, and 50% ethanol in the relationship 5:5:9 and kept at 4 °C. Pistils were washed under running water and incubated in 1 M NaOH for 2 h for softening the tissues, twice washed with distillated water, incubated in 0.01% solution of water-soluble aniline blue for 2 h at room temperature in the dark. Then, the material obtained was washed with citrate buffer (pH 4.1) and stained according to TUNEL method with the use of APO-BrdU™ TUNEL Assay Kit (Thermo Fisher, USA); thereafter, it was additionally stained with specific nuclear dye DAPI (4′,6-diamidino-2-fenilindol, 0.05 mg/ml) often used for revealing nuclear DNA. Crushed samples were prepared on glass slide in glycerin and examined under fluorescent microscope Zeiss Axioplan (Carl Zeiss, Germany). The number of analyzed pollen tubes in each variant of the experiment was not less than 200.
DNA isolation and agarose gel electrophoresis
PCD markers were revealed by DNA degradation using electrophoretic analysis of DNA degradation as analytical method for separation of DNA fragments. The material collected was placed into liquid nitrogen. Isolation of DNA from pistils or its parts (stigmas and styles) was carried out according to the standard technique of Bernatsky (1986). DNA was separated in 1.0% agarose gel 1× in Tris-borate buffer.
Results
Morphology and ultrastructure of pollen tube cell in pistil tissues
Visualization of petunia pollen tubes growing in transmitting pistil tissues with aniline blue staining and transmission electron microscopy (TEM) showed that after both cross-compatible and self-incompatible pollinations, almost all pollen grains appeared to be germinated and pollen tubes grew in stigma and style tissues (Figs. 1 and 2).
Transmission electron microphotographs of petunia style tissues with pollen tubes growing in them. a The cells of style tissue before pollination. Here, it can be seen compact cells, the cytoplasm enriched by organelles, numerous starch grains in chloroplasts, and cell with large vacuole. b, d, f Style tissue and pollen tubes cells after self-incompatible pollination. Here, it can be seen pollen tube inner structure destruction, vacuole absence, turgor disturbance, and separation of cell plasma membrane from the cell wall. c, e, g The cells of style tissue and pollen tubes after cross-compatible pollination. Here, well-developed vacuoles and other cell components are observed; bar = 1 μm. b–e Cross sections of the pistil. a, f, g Pistil longitudinal sections. N nucleus, pt pollen tube, v vacuole, ch chloroplast, s starch grain, m mucus. The SI response in petunia pollen tubes causes destruction of their inner structure
In cells of transmitting unpollinated pistil tissues, it could be seen compact cells, the cytoplasm enriched by organelles, numerous starch grains in chloroplasts, and the cell with large vacuole (Fig. 2a).
As shown in Fig. 2, pollen tube growth is accompanied by changes in transmitting style tissue. The data represented in Fig. 2b, d, f show that in the course of incompatible pollen tube growth, sharp morphological changes took place in both in transmitting style tissue and the very incompatible pollen tubes. These were reflected in the absence of vacuoles and other cell components in style tissues, disturbance of turgor in them, and backing of the cell membrane from the cell wall. Growth of incompatible pollen tubes was accompanied by their inner structure disintegration. After self-incompatible pollination, pollen tube growth stopped as a result of occurring RNase-based SI.
In the case of cross-compatible pollination pollen tubes achieved ovary where fertilization occurred. In cells of pistil tissue, after cross-compatible pollination, it could be observed homogenous cytoplasm density, well developed vacuoles and other cell components (endoplasmic reticulum, ribosomes) (Fig. 2c, e, g).
Trypan Blue staining is a standard method allowing researches to reveal the PM damage of the cell because the dye is bound with intracellular proteins of damaged cell. In unpollinated stigmas harvested from isolated preliminary emasculated flowers, none was found of staining papilla cells (Fig. 3a). In the case of pollinated stigmas, it could be observed trypan blue staining pollen grains and pollen tubes (Fig. 3c, d). Differences between a number of the pollen tubes stained after self-incompatible and cross-compatible pollination appeared to be significant (Fig. 3b). Thus, according to the results of this experiment, with application of trypan blue, it was calculated that after self-incompatible pollination, 70% of visual pollen tubes appeared to be stained while after cross-compatible pollination, only unit pollen tubes exhibited their staining, although in some samples, a number of stained pollen tubes accounted 17%.
Trypan blue staining (TBS) of petunia pollen tubes growing in vivo in pistil tissues. a Papilla cells of unpollinated stigma in self-incompatible line petunia (TBS −); bar = 100 μm; b Dead pollen tube percentage: in 12 h after pollination. Data points are the means and their standard errors. c Cross-compatible pollination: pollen tubes in 12 h after pollination (TBS −); bar = 100 μm. d Self-incompatible pollination: pollen tubes in 12 h after pollination (TBS +); bar = 100 μm
PCD as a factor of S-RNAse-based SI in petunia and its hallmarks
To identify hallmarks of PCD, two methods were used: TUNEL method and DNA electrophoresis.
The fact of the presence of PCD hallmarks in incompatible pollen tubes was established by positive TUNEL test exhibited by their nuclei (Fig. 4). Superposition of TUNEL staining pollen tubes and that with aniline blue allowed us to visualize them separately from pistil cells to make sure that luminous nuclei really belong to pollen tubes rather than surrounding cells of transmitting pistil tissue (Fig. 4a). In Fig. 4с, it can be seen that after cross-compatible pollination, TUNEL staining pollen tubes nuclei was not observed while after self-incompatible pollination nuclei of pollen tubes exhibited positive TUNEL response (Fig. 4d). Staining pollen tubes with DAPI showed that TUNEL-positive signal corresponds to nuclear DNA.
Detection of SI-induced nuclear DNA fragmentation in the incompatible petunia pollen tubes with TUNEL method on the crushed samples stained by aniline blue. a The pollen tubes stained with aniline blue and nuclear DNA with DAPI (arrows). b The percentage of TUNEL-positive nuclei in self-incompatible or cross-compatible pollination. Data points are the means and their standard errors. c Eight hours after cross-compatible pollination (TUNEL-negative signal (arrows). d Eight hours after self-incompatible pollination (TUNEL-positive signal (arrows). DAPI staining showed that the TUNEL-positive signal corresponds to nuclear DNA. In every treatment, at least 200 pollen tubes were counted and the experiment was repeated three times; bar = 50 μm
Upon self-incompatible pollination 68.5% of visual nuclei displayed positive TUNEL signal while upon cross-compatible pollination, this value achieved only 16.5% (Fig. 4b). Percent of nuclei exhibiting positive TUNEL signal in relation to visual nuclei was calculated in every experiment (10 pistils in the experiment repeated three times). These findings confirm the idea that PCD is a result of S-RNAse-based SI in petunia.
Hallmarks of PCD by DNA degradation were revealed in pistil tissues of petunia self-incompatible line with growing in its incompatible pollen tubes using electrophoretic analysis of DNA degradation as analytic method of DNA fragment separation (Fig. 5a). From Fig. 5b, it can be seen that DNA degradation is observed only in the styles after self-incompatible pollination.
Electrophoresis of DNA from pistils (stigma and style) of self-incompatible line petunia in 8 h after cross-compatible and self-incompatible pollinations. a Electrophoresis of DNA from 1, pistils after cross-compatible pollination (− DNA degradation); 2, pistils after self-incompatible pollination (+ DNA degradation); 3, developing petunia anthers (positive control); 4, non-pollinated pistils of self-incompatible line petunia (negative control); М, molecular marker 100 bp DNA Ladder (Evrogen, Russia). b Electrophoresis of DNA from stigmas and styles of self-incompatible line petunia in 8 h after cross-compatible and self-incompatible pollinations: М, molecular marker 1 kb DNA Ladder (Evrogen, Russia); 1, stigmas after cross-compatible pollination (− DNA degradation); 2, styles after cross-compatible pollination (− DNA degradation); 3, stigmas after self-incompatible pollination (− DNA degradation); 4, styles after self-incompatible pollination (+ DNA degradation)
Thus, testing a validity of the hypothesis on participation of PCD in the mechanism of RNAse-based SI in petunia (P. hybrida L.) with the use of applied methods allowed us to identify the presence of PCD markers in self-incompatible pollen tubes. Since the positive response related to DNA degradation was observed in styles of self-incompatible petunia in 8 h after self-pollination (Fig. 5), this can be considered as evidence for PCD hallmarks in style tissues observed during passage SI reaction. Thus, the results here obtained lead to statistically reliable conclusion that PCD is an important determinant of RNAse-based SI mechanism in petunia.
AOA suppresses SI-induced PCD in self-incompatible petunia pollen tubes
The data earlier obtained by us (Kovaleva and Zakharova 2003; Kovaleva et al. 2005, 2011, 2013) allowed us to conclude that ethylene is the factor required for pollen tube growth and successful fertilization. Therefore, there are good reasons to believe that this is the regulator of gametophyte–sporophyte interaction in the course of the progamic phase of fertilization. In turn, this proposes the presence of extremely complex interplay of ethylene signaling pathways with those of other phytohormones, in particular ABA, and common signaling system of germinating male gametophyte. According to our hypothesis, ethylene and/or ABA can participate in triggering/passing PCD in self-incompatible pollen tubes in the course of functioning of RNAse-based SI.
To test this hypothesis, emasculated flower stigmas of self-incompatible line petunia were treated with solutions of phytohormones, such as ethylene and ABA, as well as inhibitors of their synthesis, AOA and fluridone. Pollination was performed in 2 and 6 h after the treatment. Time of the latter was determined by the results of preliminary experiments in which the treatment of petunia stigmas of self-incompatible line with ethylene (ethrel), ABA, and inhibitors of their synthesis led to different responses, stimulation, or inhibition of pollen tube growth dependently on duration of their action. Harvesting and fixation of plant material (pollinated pistils) were performed in 24 h after self-incompatible pollination. In these experiments, the length of pollen tubes and impact of phytohormones and inhibitors of their synthesis on pollen tube growth and PCD passage (by DNA degradation intensity in styles) were determined. The results obtained are represented in Table 1.
In control variants of the experiments, in 24 h, after self-incompatible pollination, pollen tube length was 7891 ± 406 μm, i.e., only 27% of style length.
Preliminary treatment of petunia self-incompatible stigmas with ethrel before 2 h to pollination led to the increase in pollen tube length to 15089 ± 506 μm (57.7% of style length) while before 6 h to pollination, this accounted only 12397 ± 391 μm (42.8% style length).
Preliminary treatment of stigmas before 2 h to pollination with AOA, a known inhibitor of ACC synthesis, ethylene precursor stimulated pollen tube growth even to greater extent; their length achieved 12168 ± 178 μm (44.4% of style length) while before 6 h to pollination, this value achieved 24609 ± 183 μm (89.3% of style length). In the latter case, pollen tubes almost achieved ovary.
Preliminary treatment of stigmas with ABA before 2 h to pollination inhibited pollen tube growth that was reflected in the decrease in their length to only 5127 ± 153 μm (15.5% of style length), while the same treatment before 6 h to pollination did not exert any effect on their length which was only 8221 ± 185 μm (25.4% style length).
Preliminary treatment of stigmas with fluridone, inhibitor of ABA synthesis, before 6 h to pollination, contrary, exerted opposite, inhibitor effect on pollen tubes growth that was expressed in the decrease in their length to 4007 ± 129 μm (13.9% of style length), while the same treatment before 2 h to pollination stimulated their growth and their length achieved 13293 ± 588 μm, i.e., 48.1% of style length.
Thus, preliminary treatment of self-incompatible line petunia stigmas before pollination with ethylene and ABA as well as inhibitors of their synthesis impact to different extent on incompatible line pollen tube growth, dependently on duration of this procedure. AOA, inhibitor of ethylene synthesis, exhibited the most stimulatory effect after the treatment before 6 h to pollination when pollen tubes almost achieved ovary.
DNA degradation was revealed in petunia styles after self-incompatible pollination in the following two variants of the experiments: in control variant, i.e., without treatment, and after preliminary (before 2 and 6 h to pollination) treatment of stigmas with ethylene (ethrel) and ABA (Figs. 6 and 7). Although preliminary treatment of stigmas with fluridone stimulated pollen tube growth to the middle style length, DNA degradation in them was observed as well. Almost complete absence of the degradation in styles was observed only in the case of preliminary treatment of stigmas with AOA, inhibitor of ACC, ethylene precursor, before 2 and 6 h to pollination, with to the greater extent after the pretreatment to 6 h to pollination (Figs. 6 and 8).
Electrophoresis of DNA isolated from stigmas and styles of self-incompatible line petunia after preliminary treatment of them with АОА and ethrel before self-pollination. М, molecular marker 1 kb DNA Ladder (Evrogen, Russia); 1, stigmas (AOA treatment before 2 h to self-pollination); 2, styles (AOA treatment before 2 h to self-pollination) (− DNA degradation); 3, stigmas (AOA treatment before 6 h to self-pollination); 4, styles (АОА treatment before 6 h to self-pollination) (− DNA degradation); 5, stigmas (ethrel treatment before 2 h to self-pollination); 6, styles (ethrel treatment before 2 h to self-pollination) (+ DNA degradation); 7, stigmas (ethrel treatment before 6 h to self-pollination); 8, styles (ethrel treatment before 6 h to self-pollination) (+DNA degradation)
Electrophoresis of DNA isolated from stigmas and styles of self-incompatible line petunia after preliminary treatment of them with fluridone and ABA before self-pollination. М, molecular marker 1 kb DNA Ladder (Evrogen, Russia); 1, stigmas (fluridone treatment before 2 h to self-pollination); 2, styles (fluridone treatment before 2 h to self-pollination) (+ DNA degradation); 3, stigmas (fluridone treatment before 6 h to self-pollination); 4, styles (fluridone treatment before 6 h to self-pollination) (+ DNA degradation); 5, stigmas (ABA treatment before 2 h to self-pollination); 6, styles (ABA treatment before 2 h to self-pollination (+ DNA degradation); 7, stigmas (ABA treatment before 6 h to self-pollination; 8, styles (ABA treatment before 6 h to self-pollination) (+ DNA degradation)
In summary, these findings, taken together, support a validity of our hypothesis that ethylene and ABA participate in the role of PCD determinant in the course of functioning the mechanism responsible for S-RNAse-based SI in petunia. The results obtained provide evidence that ethylene controls PCD passage at the level of DNA degradation in self-incompatible pollen tubes in the course of SI mechanism functioning. Thus, for the first time, the fact of the involvement of the hormonal regulation in the mechanism responsible for S-RNAse-based SI has been established.
Discussion
PCD in the SI systems related to incompatible pollen-pistil interactions
PCD as an active and genetically controlled form of cell death is a fundamental cellular process occurring throughout plant life and essential for both normal development and responses to biotic and abiotic stresses (Rogers 2006). Although understanding of plant PCD regulation at the molecular level is still unsettled problem, morphological criteria such as altered nuclear morphology; swelling of vacuoles, mitochondria, and endoplasmic reticulum; protoplast shrinkage; and cytoskeleton reorganization are often used for identification of this process. Other non-morphological criteria used to define plant PCD type are DNA fragmentation, caspase-like activity, and ROS and reactive nitrogen species (RNS) accumulation (Wilkins et al. 2011). It is known that one of key events of genetically determined cell death is cell nucleus degradation while internucleosome fragmentation of nuclear DNA represents itself one of the main biochemical markers of PCD.
To date, a number of studies carried out in different plant species exhibiting SI have shown that PCD is triggered in self-incompatible pollen after pollen–pistil interactions. PCD hallmarks have been found in pollination assays carried out in vitro as well as in vivo on distantly related species, such as poppy (P. rhoeas L.), pear (Pyrus pyrifolia L.), and olive (Olea europaea L.) (Bosch and Franklin-Tong 2007; Serrano et al. 2010; Wang et al. 2010).
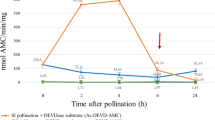
In particular, in P. rhoeas, PCD as a mechanism to prevent pollination and fertilization by incompatible pollen was established by Thomas and Franklin-Tong (2004). Subsequent studies showed various links between SI-induced PCD and actin dynamics (Thomas et al. 2006; Poulter et al. 2010). It was demonstrated that in order to trigger PCD, 50% depolymerization of actin cytoskeleton is required, and namely actin dynamics is a key connecting link to signaling to initiate PCD in Papaver pollen. In particular, it was shown that actin depolymerization/polymerization is sufficient for caspase-3-like induction in the incompatible pollen. Besides, the data indicating cytosolic and nuclear localization of SI-activated DEVDase were obtained (Bosch and Franklin-Tong 2007). Also, there is evidence that ROS and NO mediate actin reorganization and PCD in SI signaling in Papaver pollen (Wilkins et al. 2011). With the use of whole-cell patch clamp technique in Papaver pollen protoplasts, the nature of the SI signaling with respect to ion channel activity in pollen plasma membrane was investigated, and it was found that SI stimulates S-specific activation of Ca 2+- and K+-permeable channel conductance (Wu et al. 2011). SI-induced PCD in poppy pollen involves the decrease in pHcyt of incompatible pollen tube cytosol (Wilkins et al. 2015). The results of the experiments in vivo showed that pHcyt during the first 10 min achieved the value of 6.4 after SI induction and further remained to be stable at the level 5.5. It was proved that cytosol acidification represents itself an essential event in PCD being necessary and sufficient for DEVDase/caspase-3-like activity stimulation and formation of punctate actin foci. Cyclase-associated protein and actin-depolymerizing factor were identified here as key targets. Chai et al. (2017) showed that MAP kinase PrMPK9-1 is a key regulator for SI in P. rhoeas pollen and acts upstream of PCD involving actin cytoskeleton reorganization and activation of DEVDase.
Serrano et al. (2010, 2012, 2015) established that PCD was involved in pollen–pistil interactions in olive (O. europaea L.). Here, this process was revealed in pistils excised from freely pollinated flowers at different stages before and during the progamic phase of fertilization using different cytochemical techniques of PCD detection including trypan blue staining, TUNEL assay, DNA degradation analysis, and detection of caspase-3-like activity. Although the molecular SI mechanism and SI determinants have not been identified in olive, authors detected RNAse activity in pollinated pistils and in vitro germinated pollen and came to conclusion that RNAses may be involved in pollen rejection in olive. PCD hallmarks were revealed in papillar cells and self-incompatible pollen tubes. The results obtained showed that ROS and NO are key components in pollen–stigma interactions leading to PCD in papillar cells and self-incompatible pollen.
A long-standing research of author team (Hiratsuka et al. 2001; Liu et al. 2007) conducted on in vitro system represented by the pear (P. pyrifolia L., family Rosaceae) showed that pistil RNAse participates in self pollen tube growth arrest. Authors identified S-RNAse from pear style that inhibits self pollen germination and pollen tube growth (Hiratsuka et al. 2001). The results here obtained allowed to conclude that in P. pyrifolia L.,S-RNAse-type is responsible for SI mechanism (Wang et al. 2009, 2010; 2011). Further studies provided evidence for PCD hallmarks in SI response of P. pyrifolia L. including the depolymerization of actin cytoskeleton (Liu et al. 2007), functional changes of mitochondria, and nuclear DNA degradation in self-incompatible pollen tubes (Wang et al. 2009). The data here obtained were considered as preliminary results, indicating that PCD may occur in pear SI. The nuclear DNA of incompatible pollen tube was found to be degraded under the action of SI mechanism in vitro or in vivo (Wang et al. 2009, 2010). This implies that this degradation is really a reason rather than a result of pollen tube growth arrest. By authors’ opinion (Wang et al. 2010), RNA degradation may represent itself only the beginning of the SI response but not its end. Although Wang and Zhang (2011) obtained some preliminary evidence in favor of PCD including mitochondrial membrane potential collapse, cytochrome c release from mitochondria into the cytosol, mitochondrial cristae reduction, and nuclear DNA degradation, these findings by their opinion are still not a good reason for passage of the given process in the course of SI in P. pyrifolia L. Recently, Qu et al. (2017) showed that phospholipase C (PLC-IP3) is involved in SI response in Pyrus species and self-S-RNAse inhibits Ca2+-permeable channel activity at pollen tube apices and selectively decreases PLC activity at P. pyrifolia L. pollen tubes PM.
Alterations in the integrity of F-actin cytoskeleton capable of trigging PCD have been described in self-incompatible pollen in Nicotiana alata L. (Roldan et al. 2012). These results indicate that during the SI response, disruption of the F-actin cytoskeleton precedes vacuolar membrane breakdown.
Investigations performed in the present work and directed to any particularities of self-incompatible petunia pollen grain germination and pollen tube growth showed that PCD is an essential factor of S-RNAse-based SI in Petunia hybrida L. Complex methods used by us allowed to detect PCD markers at several levels. Morphology pattern of apoptosis includes cell volume lowering and cytoplasm membrane wrinkling. All these phenomena as well as protoplast contracting and absence of organelles were observed in the incompatible pollen tubes (Fig. 2). Disturbances of their cytoplasm membrane are detected by trypan blue staining (Fig. 3). To identify hallmarks of PCD, two corresponding methods were used, such as TUNEL method and DNA electrophoresis. The fact of the presence of PCD hallmarks in incompatible pollen tubes was established by positive TUNEL test exhibited by their nuclei (Fig. 4). Staining pollen tubes with DAPI showed that TUNEL-positive signal corresponds to nuclear DNA. Hallmarks of PCD by DNA degradation were revealed in style tissues during incompatible pollen tube growth in them by using electrophoretic analysis of DNA separation (Fig. 5). These results correlate with the data that S-RNAse, pistil SI determinant in P. pyrifolia L., participates in rejecting own pollen triggering DNA degradation in self-incompatible pollen tubes (Wang et al. 2010). Since the positive response related to DNA degradation was observed in styles of self-incompatible petunia in 8 h after self-pollination (Fig. 5), it can be concluded that PCD revealed in style tissues occur really due to SI reaction passage. The above findings allow us to conclude that PCD is the important determinant of RNAse-based mechanism SI in P. hybrida L. In perspective, it needs to study biochemical aspects of the given process, in particular to identify the enzymes behaving as apoptosis markers associated with the caspase-like activity. At the given step, it cannot be to even suggest to what type of PCD S-RNAse-based SI may be belonged but taking into account the cytological data, it can be believed that most probably we deal here with vacuolar type of PCD. The complex mechanisms of S-RNAse-based SI-induced PCD must be to be intensively studied in the future at biochemical, molecular, and genome levels.
Ethylene and ABA in the regulation of pollen–pistil interactions in petunia
A gaseous plant hormone ethylene is involved in diverse physiological processes throughout the plant life cycle from seed germination to senescence. Ethylene signaling is a part of complex network in plant cross-talk with internal and external cues.
Ethylene plays a key role in plant development, growth, and senescence triggering network of signaling pathways through cross-talk with other phytohormones (Iqbal et al. 2017). It promotes or inhibits growth and senescence processes depending on its concentration, timing of application, and plant species.
It was established that ethylene participate in the mechanism of perforation formation in the lace plant Aponogeton madagascariensis (Mirb.) H. Bruggen, which occurs through developmentally regulated PCD (Rantong et al. 2015). Since transcript levels of ethylene receptors AmERS1a and AmERS1c declined during senescence related to PCD, authors suggested that during leaf senescence, cell fate is determined by a combination of ethylene and their receptor levels. Two of the best known examples of PCD passage are xylogenesis (Bollhoner et al. 2012) and formation of aerenchima in semiaquatic species (Mühlenbock et al. 2007). It is shown that active phase of xylogenesis and airenchima formation are regulated by ethylene.
Ethylene and other phytohormones may converge on the induction of ethylene-responsive genes in flowers. Thus, Liu et al. (2011) found that in petunia petals, expression of some members of the ERF gene family, the largest transcription factor gene family in plants, was affected by ABA in a gene-specific manner, thereby indicating cross-talk between ethylene and other phytohormones in petal senescence.
It was shown that transcription factors are involved in signaling pathways during plant responses under abiotic and biotic stresses (Müller and Munné-Bosch 2015). Intensive studies allowed the authors to conclude that ERFs are a key regulatory system in hormonal and stress signaling (Müller and Munné-Bosch 2015). Cao et al. (2018) investigated a role of ERF8 (a member of the APETALA2/ETHYLENE RESPONSE FACTOR (AP2/ERF)) superfamily and led to the conclusion that it has dual functions in ABA signaling and bacterial immunity, with overexpression of the ERF8 resulted in PCD.
The purpose of the present study was to explore the role of phytohormones, ethylene and ABA, in the regulation of P. hybrida L. male gametophyte growth including the mechanism of S-RNA-based SI whose functioning leads to inhibition of self-incompatible pollen tube growth in style tissues. The following facts earlier established by us served as a stimulus for conducting these studies. First of all, we took into account that in many plants, pollination is known to initiate cascade of the events involving induction of ethylene formation capable of coordinating further flower development (O’Neill et al. 1993). The results of investigations performed on petunia provided evidence that ethylene production and its evolution by pistil tissues is a factor required for pollen tube growth and successful fertilization (Singh et al. 1992; Holden et al. 2007; Kovaleva and Zakharova 2003; Kovaleva et al. 2005, 2011, 2013). The growth of self (incompatible) pollen tubes in vivo in pistils after self-incompatible pollination was accompanied by higher level of ethylene as compared to that in the case of non-self pollen tube growth after cross-compatible pollination which is characterized by higher ACC content as well (Kovaleva et al. 2011). The data obtained during study of ethylene role in the processes of germination, development, and growth of petunia male gametophyte, taken together, allowed us to suggest that this phytohormone is a regulator of gametophyte-sporophyte interactions in the progamic phase of fertilization.
ABA is known to be also involved in the control of senescence and, much like to ethylene, behaves as a positive regulator of the senescence process (Iqbal et al. 2017). As follows from our results (Kovaleva et al. 2011, 2013), petunia male gametophyte germination and growth after their self-compatible pollination were accompanied by high ethylene and ABA production in the pollen–pistil system. However, even greater levels of these hormones, 2–2.5 times exceeding their levels after cross-compatible pollination, were observed by us in the case of male gametophyte growth inhibition after self-incompatible pollination.
In addition to the above results, the findings of the present work allow us to conclude that the main, if not key, target of exogenous plant hormones is represented by pollen PM H+-ATPase playing a crucial role in hormone-induced pollen tube germination and growth as well as the related processes based on the activation of ion and water transport across the PM (Kovaleva et al. 2016).
Recently, we have obtained the results demonstrating the involvement of ABA and ethylene in germination and growth of petunia male gametophyte in vitro (Kovaleva et al. 2017). Two potential targets of ABA action in a pollen tube were identified. These are represented by (1) plasma membrane (PM) H+-ATPase, electrogenic proton pump participating in PM polarization, and (2) Ca2+-dependent K+-channels localized in the same membrane. It was established that a stimulatory effect of ABA on electrogenic activity of H+-ATPase is mediated by the increase in free Ca2+ level in pollen tube cytosol and ROS generation. Based on the results obtained concerning the role of K+ ions in the hormonal control of water transport-driving forces in a pollen tube, the hypothesis suggesting stimulation of pollen grain germination and pollen tube growth by ABA via activation of K+-channels in their PM was put forward.
Besides, we have found that disturbance in ethylene and ABA functioning with the use of inhibitors of their action (1-MCP) or synthesis (AOA and fluridone, respectively) leads to complete or partial suppression of pollen tube germination and growth. Exogenous ABA abolished an inhibitor effect on petunia pollen tube growth in vitro like fluridone (inhibitor of ABA synthesis), 1-МСР (ethylene reception blocker), and АОA (aminooxyacetic acid, inhibitor of ACC synthesis). In the pollen–pistil system in vivo (with stigmas pretreatment with ABA and AOA before pollination), ABA suppresses inhibitory effect of AOA (Kovaleva et al. 2017).
In addition to the above data, evidence allowing us to consider ABA as a potential signal to male sterility in petunia and a possible determinant of PCD in tapetum (Kovaleva et al. 2018) was obtained.
In particular, it was found that preliminary treatment of petunia self-incompatible line stigmas before pollination with ethylene and ABA as well as inhibitors of their synthesis impact to a different extent on incompatible pollen tube growth dependently of the treatment duration used (Table 1; Figs. 6, 7, and 8). AOA, inhibitor of the synthesis of ACC, ethylene precursor, exhibited the most stimulatory effect in this system after the treatment of stigmas before 6 h to pollination when pollen tubes almost achieved ovary (Table 1). Almost complete absence of DNA degradation and fragmentation in styles we observed only in the case of preliminary treatment of stigmas with AOA before 2 and 6 h to pollination, with to the greater extent after the pretreatment before 6 h to pollination (Table 1; Figs. 7 and 8).
To date, enough data allowing to say that ethylene is one of the positive regulators of PCD during development under normal (Mühlenbock et al. 2007; Bollhoner et al. 2012; Rantong et al. 2015) and stress (Müller and Munné-Bosch 2015) conditions has been received. In ethylene-dependent petal senescence in Japanese morning glory, transcription factor genes have revealed the involvement of two proteins in the transcriptional regulation of the ethylene biosynthesis pathway (Shibuya et al. 2016). In particular, recently, it has been shown that ethylene and salinity antagonistically modulate the salt-induced PCD via controlling the transcripts of BAG family genes which can play a key role by inhibiting the salt-induced PCD as well as expression of ethylene- and senescence-related genes in Arabidopsis (Pan et al. 2016). Here, authors have provided evidence that exogenous АСС in the saline solution could reactivate expression of Bag6 and BAG7 able to play a key role in inhibiting the salt-induced PCD.
All the above findings suggest the existence of extremely complex interactions of ethylene and ABA signaling pathways with common signaling system operating during male gametophyte germination. According to our hypothesis, balance of phytohormones and/or their interactions can modulate time of triggering PCD passage in the pollen–pistil system. In other words, ethylene and ABA exerting antagonistic/synergetic effects possibly control PCD passage at the level of DNA degradation in self-incompatible pollen tubes in the course of SI mechanism functioning. Thus, the data of the present work argue in favor of our ideas that ACC may be one of signaling molecules during RNAse-based SI mechanism functioning in the progamic phase of fertilization in petunia.
References
Anderson MA, McFadden GI, Bernatzky R, Atkinson A, Orpin T, Dedman H et al (1989) Sequence variability of three alleles of the self-incompatibility gene of Nicotiana alata. Plant Cell 1:483–491
Bernatsky R. (1986) Toward a saturated linkage map in tomato based on isozymes and random cDNA sequences/R. Bernatsky. S.D. Tanksley // Genetics 112: 887–898
Bollhoner B, Prestele J, Tuominen (2012) Xylem cell death: emerging understanding of regulation and function. J Exp Bot 63(3):1081–1094
Bosch M, Franklin-Tong V (2007) Temporal and spatial activation of caspase-like enzymes induced by self-incompatibility in Papaver pollen. Proc Natl Acad Sci U S A 104:18327–18332
Cao FY, DeFalco TA, Moeder W, Li B, Gong Y, Liu XM, Taniguchi M, Lumba S et al (2018) Arabidopsis ETHYLENE RESPONSE FACTOR 8 (ERF8) has dual functions in ABA signaling and immunity. BMC Plant Biol 18(1):211–227
Chai L, Tudor RL, Poulter NS, Wilkins KA, Eaves DJ, Franklin FCH, Franklin-Tong VE (2017) MAP kinase PrMPK9-1 contributes to the self-incompatibility response. Plant Physiol 174(2):1226–1237
Entani T, Kubo K, Isogai S, Fukao Y, Masahiro M, Isogai A, Takayama S (2014) Ubiquitin–proteasome-mediated degradation of S-RNase in a solanaceous cross-compatibility reaction. Plant J 78:1014–1021
Hiratsuka S, Zhang SL, Nakagava E, Kawai Y (2001) Selective inhibition of the growth of incompatible pollen tubes by S-protein in the Japanese pear. Sex Plant Reprod 13:209–215
Holden MJ, Marty JA, Singh-Cundy A (2007) Pollination-induced ethylene promotes the early phase of pollen tube growth in Petunia inflata. J Plant Physiol 160(3):261–269
Hua Z, Kao T-h (2008) Identification of major lysine residues of S (3)-RNAse of Petunia inflata involved in ubiquitin-26Sproteasome-mediated degradation in vitro. Plant J 54:1094–1104
Hua Z, Meng X, Kao T-H (2007) Comparison of Petunia inflata S-Locus F-box protein (Pi SLF) with Pi SLF-like proteins reveals its unique function in S-RNase-based self- incompatibility. Plant Cell 19:3593–3609
Iqbal N, Khan NA, Ferrante A, Trivellini A, Francini A, Khan MIR (2017) Ethylene role in plant growth, development and senescence: interaction with other phytohormones. Front Plant Sci 8:475
Iwano M, Takayama S (2012) Self/non-self discrimination in angiosperm self-incompatibility. Curr Opin Plant Biol 15:78–83
Iwano M, Shiba H, Funato M, Shimosato H, Takayama S, Che FS, Kai N, Watanabe M, Suzuki G, Hinata K, Isogai A (2003) The pollen determinant of self-incompatibility in Brassica campestris. Proc Cell Physiol 44:428–436
Kao T-H, Tsukamoto T (2004) The molecular and genetic bases of S-RNase-based self-incompatibility. Plant Cell 16:72–83
Kovaleva L, Zakharova E (2003) Hormonal status of the pollen-pistil system at the progamic phase of fertilization after compatible and incompatible pollination in Petunia hybrida L. Sex Plant Reprod 16:191–196
Kovaleva LV, Zakharova EV, Minkina YV, Timofeeva GV, Andreev IM (2005) Gernination and growth in vitro of petunia male gametophyte are sensitive to the action of exogenous hormones and are accompanied by a change in the endogenous level of the plant hormones. Russ J Plant Physiol 52:584–590
Kovaleva LV, Dobrovolskaya A, Voronkov A, Rakitin V (2011) Ethylene is involved in the control of male gametophyte development and germination in Petunia. J Plant Growth Regul 30:64–73
Kovaleva LV, Timofeeva GV, Rodionova GB, Zakharova EV, Rakitin V, Yu (2013) Role of ethylene in the regulation of gametophyte-sporophyte interactions during progamic phase of fertilization in the petunia (Petunia hybrida L). Russ J Dev Biol 44(2):91–100
Kovaleva L, Voronkov A, Zakharova E, Minkina Y, Timofeeva G, Andreev I (2016) Regulation of petunia pollen tube growth by phytohormones: identification of their potential targets. J Agric Sci Technol A6:239–254
Kovaleva LV, Zakharova EV, Voronkov AC, Timofeeva GV, Andreev IM (2017) Role of abscisic acid and ethylene in the control of water transport-driving forces in germinating petunia male gametophyte. Russ J Plant Physiol 64(5):782–793
Kovaleva LV, Voronkov AS, Zakharova EV, Andreev IM (2018) ABA and IAA control microsporogenesis in Petunia hybrida L. Protoplasma 255(3):751–759
Kubo KI, Entani T, Takara A, Wang N, Fields AM, Hua Z, Toyoda M, Kawashima SI, Ando T, Isogai A, Kao TH, Takayama S (2010) Collaborative non-self recognition system in S-RNase-based self-incompatibility. Science 330:796–799
Kubo K, Paape T, Hatakeyama M, Entani T, Takara A, Kajihara K et al (2015) Gene duplication and genetic exchange drive the evolution of S-RNAse-based self-incompatibility in Petunia. Nat Plants 1:1400S
Kubo K, Tsukahara M, Fujii S, Murase K, Wada Y, Entani T, Iwano M, Takayama S (2016) Cullin1-P is an essential component of non-self recognition system in self-incompatibility in Petunia. Plant Cell Physiol 57:2403–2416
Kuo J. (2007) Electron microscopy. Methods and protocols. Humana press: 625 S
Lai Z, Ma W, Han B, Liang L, Zhang Y, Hong G, Hue Y (2002) An F-box gene linked to the self-incompatibility (S) locus of Antirrhinum is expressed specifically in pollen and tapetum. Plant Mol Biol 50:29–42
Lee HS, Huang SS, Kao TH (1994) S proteins control rejection of incompatible pollen in Petunia inflata. Nature 367:560–563
Li S, Samaj J, Franklin-Tong VE (2007) A mitogen-activated protein kinase signals to programmed cell death induced by self-incompatibility in Papaver pollen. Plant Physiol 145:236–245
Li J, Zhang Y, Song Y, Zhang H, Fan J, Li Q, Zhang D, Xue Y (2017) Electrostatic potentials of the S-locus F-box proteins contribute to the pollen S specificity in self-incompatibility in Petunia hybrida. Plant J 89: 45–57
Liu ZQ, Xu GH, Zhang SL (2007) Pyrus pyrifolia stylar S-RNase induces alterations in the actin cytoskeleton in self-pollen and tubes in vitro. Protoplasma 232(1–2):61–67
Liu J, Li J, Wang H, Fu Z, Liu J, Yu Y (2011) Identification and expression analysis of ERF transcription factor genes in petunia during flower senescence and in response to hormone treatments. J Exp Bot 62(2):825–840
Liu W, Fan J, Li J, Song Y, Li Q, Zhang Y, Xue Y (2014) SCF SLF-mediated cytosolic degradation of S-RNase is required for cross-pollen compatibility in S-RNase-based self-incompatibility in Petunia hybrid. Front Genet 5:228
Ma R, Han Z, Hu Z, Lin G, Gong X, Zhang H, Nasrallah JB, Chai J (2016) Structural basis for specific self-incompatibility response in Brassica. Cell Res 26:1320–1329
McClure BA, Gray JE, Anderson MA, Clarke AE (1990) Self-incompatibility in Nicotiana alata involves degradation of pollen rRNA. Nature 347:757–760
Mühlenbock P, Plaszczyca M, Plaszczyca M, Mellerowicz E, Karpinski S (2007) Lysigenous aerenchyma formation in Arabidopsis is controlled by LESION SIMULATING DISEASE1. Plant Cell 19(11):3819–3830
Müller M, Munné-Bosch S (2015) Ethylene response factors: a key regulatory hub in hormone and stress signaling. Plant Physiol 169:32–41
Murase K, Shiba H, Iwano M, Che F-S, Watanabe M, Isogai A, Takayama S (2004) A membrane-anchored protein kinase involved in Brassica self-incompatibility signaling. Science 303:1516–1519
Murfett J, Atherton TL, Mou B, Gasser C, McClure B (1994) S-RNase expressed in transgenic Nicotiana causes S-allele-specific pollen rejection. Nature 367:563–566
Nishio T, Hinata K (1977) Analysis of S-specific proteins in stigmas of Brassica oleracea L. by isoelectric focusing. Heredity 38:391–396
O’Neill SD, Nadeau JA, Zhang XS, Bui AQ, Halevy AH (1993) Interorgan regulation of ethylene biosynthetic genes by pollination. Plant Cell 5(4):419–432
Pan Y-J, Liu L, Lin Y-C, Zu Y-G, Li L-P, Tang Z-H (2016) Ethylene antagonizes salt-induced growth retardation and cell death process via transcriptional controlling of ethylene-, BAG- and senescence-associated genes in Arabidopsis. Front Plant Sci 7:696. https://doi.org/10.3389/fpls.2016.00696
Poulter NS, Staiger CJ, Rappoport JZ, Franklin-Tong VE (2010) Actin-binding proteins implicated in the formation of the punctate actin foci stimulated by the self-incompatibility response in Papaver. Plant Physiol 152:1274–1283
Qu H, Guan Y, Wang Y, Zhang S (2017) PLC-mediated signaling pathway in pollen tubes regulates the gametophytic self-incompatibility of Pyrus species. Front Plant Sci 8:1164
Rantong G, Evans R, Gunawardena A (2015) A lace plant ethylene receptors, AmERS1a and AmERS1c, regulate ethylene-induced programmed cell death during leaf morphogenesis. Plant Mol Biol 89(3):215–227
Rogers HJ (2006) Programmed Cell Death in floral organs: how and why do flowers die? Ann Bot 97(3):309–315
Roldan JA, Rojas H, Goldraij A (2012) Disorganization of F-actin cytoskeleton precedes vacuolar disruption in pollen tubes during the in vivo self-incompatibility response in Nicotiana alata. Ann Bot 110:787–795
Schopher CR, Nasrallah ME, Nasrallah JB (1999) The male determinant of self-incompatibility in Brassica. Science 286:1697–1700
Serrano I, Pelliccione S, Olmedilla A (2010) Programmed-cell-death hallmarks in incompatible pollen and papillar stigma cells of Olea europaea L. under free pollination. Plant Cell Rep 29(6):561–572
Serrano I, Romero-Puertas MC, Rodríguez-Serrano M, Sandalio LM, Olmedilla A (2012) Peroxynitrite mediates programmed cell death both in papillar cells and in self-incompatible pollen in the olive (Olea europaea L.). J Exp Bot 63(3):1479–1493
Serrano I, Romero-Puertas MC, Sandalio LM, Olmedilla A (2015) The role of reactive oxygen species and nitric oxide in programmed cell death associated with self-incompatibility. J Exp Bot 66(10):2869–2876
Shibuya K, Tetsuya Y, Kazuo I (2016) Morphological changes in senescing petal cells and the regulatory mechanism of petal senescence. J Exp Bot 67(20):5909–5918
Shimosato H, Yokota N, Shiba H, Iwano M, Entani T, Che FS, Watanabe M, Isogai A, Takayama S (2007) Characterization of the SP11/SCR high-affinity binding site involved in self/nonself recognition in Brassica self-incompatibility. Plant Cell 19:107–117
Sijacic P, Wang X, Skirpan A, Wang Y, Dowd P, McCubbin AG et al (2004) Identification of the pollen determinant of S-RNAse –mediated self-incompatibility. Nature 429:302–305
Singh A, Evensen KB, Kao T-H (1992) Ethylene synthesis and floral senescence following compatible and incompatible pollinations in Petunia inflata. Plant Physiol 9(1):38–45
Stephenson AG, Doughty J, Dixon S, Elleman C, Hiscock S, Dickinson HG (1997) The male determinant of self-incompatibility in Brassica oleracea is located in the pollen coating. Plant J 12:1351–1359
Sun P, Kao T-H (2013) Self-incompatibility in Petunia inflata: the relationship between a self-incompatibility locus F-box protein and its non-self S-RNases. Plant Cell 25:470–485
Sun P, Li S, Lu D, Williams JS, Kao T-h (2015) Pollen S-locus F-box proteins of Petunia involved in S-RNase-based self-incompatibility are themselves subject to ubiquitin-mediated degradation. Plant J 83:213–223
Takasaki T, Hatakeyama K, Suzuki G, Watanabe M, Isogai A, Hinata K (2000) The S receptor kinase determines self-incompatibility in Brassica stigma. Nature 403:913–916
Takayama S, Isogai A (2005) Self-incompatibility in plants. Annu Rev Plant Biol 56:467–489
Takayama S, Shimosato H, Shiba H, Funato M, Che FS, Watanabe M, Isogai A (2001) Direct ligand-receptor complex interaction controls Brassica self-incompatibility. Nature 413:534–538
Thomas SG, Franklin-Tong VE (2004) Self-incompatibility triggers programmed cell death in Papaver pollen. Nature 20:305–309
Thomas SG, Huang S, Li S, Staiger CJ, Franklin-Tong VE (2006) Actin depolymerization is sufficient to induce programmed cell death in self-incompatible pollen. J Cell Biol 174:221–229
Wang CL, Zhang SL (2011) A cascade signal pathway occurs in self-incompatibility of Pyrus pyrifolia. Plant Signal Behav 6(3):420–421
Wang CL, Xu GH, Jiang XT, Chen G, Wu J, Wu HQ, Zhang SL (2009) S-RNase triggers mitochondrial alteration and DNA degradation in the incompatible pollen tube of Pyrus pyrifolia in vitro. Plant J 57(2):220–229
Wang CL, Wu J, Xu GH, Gao YB, Chen G, Wu JY, Wu HQ, Zhang SL (2010) S-RNase disrupts tip-localized reactive oxygen species and induces nuclear DNA degradation in incompatible pollen tubes of Pyrus pyrifolia. J Cell Sci 123(Pt 24):4301–4309
Watanabe M, Suwabe K, Susuki G (2012) Molecular genetics, physiology and biology of self-incompatibility in Brassicaceae. Proc Jpn Acad Ser B Phys Biol Sci 88:519–535
Wheeler MJ, de Graaf BH, Hadjiosif N, Perry RM, Pouter NS, Osman K, Vatovec S, Harper A, Franklin FC, Franklin-Tong VE (2009) Identification of the pollen self-incompatibility determinant in Papaver rhoeas. Nature 459:992–995
Wheeler MJ, Vatovec S, Franklin-Tong VE (2010) The pollen S-determinant in Papaver: comparisons with known plant receptors and protein ligand partners. J Exp Bot 61:2015–2025
Wilkins KA, Bancroft J, Bosch M, Ings J, Smirnoff N, Franklin-Tong VE (2011) Reactive oxygen species and nitric oxide mediate actin reorganization and programmed cell death in the self-incompatibility response of Papaver. Plant Physiol 156(1):404–416
Wilkins KA, Poulter NS, Franklin-Tong VE (2014) Taking one for the team: self-recognition and cell suicide in pollen. J Exp Bot 65:1331–1342
Wilkins KA, Bosch M, Hague T, Teng N, Poulter NA, Franklin-Tong VE (2015) Self-incompatibility–induced programmed cell death in field poppy pollen involves dramatic acidification of the incompatible pollen tube cytosol. Plant Physiol 167:766–779
Williams JS, Natale CA, Wang N, Brubaker TR, Sun P, Kao T-H (2014) Four previously identified Petunia inflata S-locus F-box genes are involved in pollen specificity in self-incompatibility. Mol Plant 7:567–569
Wu J, Wang S, Gu Y, Zhang S, Publicover SJ, Franklin-Tong VE (2011) Self-incompatibility in Papaver rhoeas activates nonspecific cation conductance permeable to Ca2+ and K+. Plant Physiol 155:963–973
Wu L, Williams JS, Wang N, Khatri WA, San Román D, T-h K (2018) Use of domain-swapping to identify candidate amino acids involved in differential interactions between two allelic variants of Type-1 S-Locus F-Box Protein and S3-RNase in Petunia inflate. Plant Cell Physiol 59(2):234–247
Zhao L, Huang J, Zhao Z, Li Q, Sims TL, Xue Y (2010) The Skkp1-like protein SSK1 is required for cross-pollen compatibility in S-RNAse-based self-incompatibility. Plant J 62(1):52–63
Funding
This work was supported by grant from the Russian Foundation for Basic Research (RFBR, no. 17-04-00153).
Author information
Authors and Affiliations
Contributions
L. Kovaleva and E. Zakharova wrote the manuscript; E. Zakharova, G. Timofeeva, and Ya. Golivanov performed the experiments; E. Baranova and L. B Bogoutdinova conducted imaging using TEM; I. Andreev and M. Khaliluev helped in manuscript preparation.
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Benedikt Kost
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kovaleva, L.V., Zakharova, E.V., Timofeeva, G.V. et al. Aminooxyacetic acid (АОА), inhibitor of 1-aminocyclopropane-1-carboxilic acid (AСС) synthesis, suppresses self-incompatibility-induced programmed cell death in self-incompatible Petunia hybrida L. pollen tubes. Protoplasma 257, 213–227 (2020). https://doi.org/10.1007/s00709-019-01430-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-019-01430-x