Abstract
Proline (Pro) accumulation protects plant cell under abiotic stress. Hydroxyproline (Hyp) as selection agent is a toxic analog of proline and promotes Pro overaccumulation. In this study, Chardonnay calli were firstly irradiated with different dosages of 60Co and then cultured on a Hyp-added medium. Finally, some stable hydroxyproline-resistant (HR) calli were obtained. When calli were cultured on 4 mM Hyp medium for 7 days, intracellular Pro content of the HR calli was five times higher than that detected in the normal calli. The regeneration of HR calli into plantlets was much slower than that of normal ones. When cultured on woody plant medium (WPM) containing 10 mM NaCl for 14 days, HR plantlets still grew well with lower Pro than withered normal plantlets. qRT-PCR results of Pro biosynthesis-related genes in HR plantlets showed that three genes VvP5CS, VvOAT, and VvP5CDH were conducive for Pro accumulation. These results confirmed that HR plantlets acquired salt tolerance ability. We prospect that this procedure to obtain salt-tolerant plants may be valuable to breed programs and improve grapevine genotypes with increased tolerance to salt and other abiotic stresses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Desertification and salinization of cultivated land are serious problems in viticulture, which affects the yield and fruit quality of grapevines. It is important to rapidly develop new grapevine genotypes that are tolerant to various abiotic stresses. Physical and chemical methods have been employed to mutate plant cells, and some Pro analogs are used as selection agents. Some variations show varied degrees of tolerance to abiotic stresses, usually accompanied by the ability to produce excessive Pro (Xin and Browse 1998; Tantau et al. 2004; Yang et al. 2009). Hydroxyproline, a toxic analog of Pro, was used as a selection agent to screen Pro overaccumulation cell lines eventually acquiring heritable and increased tolerance to salt stress (Fuller et al. 2006; Dörffling et al. 2009). By using Hyp as selection agent, Hyp-tolerant cell lines of crops have been obtained, such as Lucerne (Li et al. 2008). These cell lines exhibited improved stress tolerance ability, positively correlated to intracellular Pro content (Tantau and Dörffling 1991; Anjum and Villiers 1998; Tantau et al. 2004). This breeding strategy has not been applied in grapevines, where traditional hybridization breeding is the normal method to introduce new traits or create new germplasms. Therefore, we set to try this breeding approach in grapevines to rapidly select new salt- or drought-tolerant grapevine genotypes.
Several physiological and biochemical changes happen when plants undergo osmotic stress. Some compatible solutes, such as polyols and proline (Pro), are synthesized and accumulated in plant under abiotic stress (Delauney and Verma 2002). Plants respond to abiotic stresses, usually through intracellular Pro accumulation to protect protein structure and activities and prevent cell damage by forming hydrophilic colloids in aqueous media (Goring and Thien 1979; Parvanova et al. 2004a, b). Intracellular Pro can be synthesized from glutamate or ornithine (Orn) in plants (Verbruggen and Hermans 2008; Szabados and Savouré 2010). △1-Pyrroline-5-carboxylate synthase (P5CS, EC1.2.1.41) catalyzes glutamate to form pyrroline-5-carboxylate (P5C) and then reduced by △1-pyrroline-5-carboxylate reductase (P5CR) to form Pro (Hu et al. 1992). P5CS appears to catalyze the rate-limiting step in Pro biosynthesis pathway that the expression of P5CS gene plays a key role in Pro levels. The enzyme orn-δ-amino-transferase (OAT) can catalyze the transamination of Orn to synthesize Pro. Pro degradation is catalyzed by two sequential enzymes: Pro dehydrogenase (ProDH, EC1.5.99.8) that acts on P5C, and P5C dehydrogenase (P5CDH. EC1.5.1.12) catalyzes conversion of P5C to glutamate. Plants under abiotic stress have higher expression levels of P5CS gene while the ProDH expression is inhibited (Yoshiba et al. 1997). P5CDH also plays an important role in preventing cell damages from intermediary metabolites such as P5C (Deuschle et al. 2001; Rizzi et al. 2015). Therefore, Pro level in plants appears to be regulated mainly by P5CS and P5CDH.
Vitis vinifera Chardonnay, one of the most popular white wine grape cultivars, is sensitive to abiotic stresses (Cavagnaro et al. 2006; Vincent et al. 2007). The goal of this study was to develop grape plants with improved tolerance to salt stress. In this study, Hyp-resistant calli of Chardonnay were obtained through 60Co irradiation and Hyp selection, which contained higher Pro content than the normal calli. The intracellular Pro content of new plantlets was measured under salt stress. In addition, the expression levels of genes related in Pro biosynthesis pathway during Chardonnay subjected to salt stress was evaluated using qRT-PCR.
Materials and methods
Pre-embryogenic culture and growth conditions
Vitis vinifera Chardonnay pre-embryonic calli (PEs) were obtained from the Center for Viticulture and Small Fruit Research, Florida Agricultural and Mechanical University. The calli were cultured on Murashige and Skoog (MS) basal medium with vitamins (Murashige and Skoog 1962) for long-term maintenance (LTM), which contained 2.5 μM 2,4-D, 5 μM 6-benzylaminopurine, 0.1 mg L−1 inositol, 20 g L−1 sucrose, and 3 g L−1 phytagel. All calli were cultured in 9-cm Petri dishes at 25 °C and 16 h (light intensity 70 μmol s−1 m−2) photoperiod cycle.
Hyp-resistant calli selection
PEs of Chardonnay were proliferated on a liquid maintenance (LM) medium consisting of MS basal medium supplemented with 18 g L−1 maltose, 0.37% (v/v) glycerol, and 5 μM β-naphthoxyacetic acid (pH = 5.8) and incubated in a constant temperature shaker maintained at 25 °C and 150 rpm. PEs were collected and irradiated with 60Co γ-rays. The irradiations were carried out at 10 Gy min−1 dosage rate, with a 2 × 2 cm2 irradiation area and 80 cm source skin distance of 10, 40, 60, 100, and 500 Gy from the cobalt source. After irradiation, PEs were immediately placed on LM medium and incubated for 14 days in darkness. Following this, these PEs were transferred to a semi-solid LTM selection medium with or without 10 mM Hyp (LTM-H) for 2-year recurrent selection. Some Hyp-based resistant (HR) calli were selected and further cultured on LTM medium to recover and grow. HR calli were regularly sub-cultured on LTM-H medium every 28 days. Stable HR calli were then transferred onto somatic embryo multiplication (SEM) medium for further differentiation.
Testing Hyp tolerance
To test HR calli tolerant to Hyp, normal Chardonnay calli and HR calli were both transferred to LTM medium supplemented with 1 or 4 mM Hyp in 50 ml flasks. Ten embryo clusters, with a diameter of 1 cm, were placed in each flask. Three replications of each treatment were maintained at 25 °C in darkness. After 0.5, 1, 1.5, 2, 4, and 7 days of Hyp treatment, 0.3 g fresh calli were sampled and transferred to liquid nitrogen to measure intracellular Pro content. The survival rate (number of viable clusters/total number of clusters × 100%) of embryogenetic calli was calculated after incubating the culture in dark for 28 days.
Regeneration
After culturing on SEM medium for 2 months, Vitis vinifera HR (VvHR) embryogenic calli became granular, and some somatic embryos appeared. For embryo differentiation and maturity, both normal and HR somatic embryos of Chardonnay were transferred onto Lloyd & McCown woody plant medium w/ vitamins (WP medium) supplemented with 600 mg L−1 casein hydrolysate, 100 mg L−1 glutamine, 100 mg L−1 asparagine, 100 mg·L−1 arginine, 0.5 μM NAA, and 2 μM BA (Lloyd and McCown 1981; Agüero et al. 2006). Embryos were cultured at 25 °C, 16 h photoperiod, and with a light intensity of 70 μmol s−1 m−2 and sub-cultured every 28 days. Small plantlets were separated from the embryo clusters and further grown on WP medium to develop into plants.
Salt treatment
Chardonnay normal and HR plantlets were cultured on WP medium supplemented with 5, 7.5, and 10 mM NaCl (salt solution) for 14 days when normal plantlets exhibited some signs of wilting. The aerial parts of plantlets were harvested and placed immediately in liquid nitrogen and stored at − 80 °C for subsequent total RNA isolation and the determination of Pro content.
Determination of Pro content
Intracellular content of free Pro was determined by colorimetry according to Bates et al. (Bates et al. 1973). In brief, 300 mg of the material was boiled in 5 ml of 3% sulfosalicylic acid for 10 min, filtered into a clean tube after chilling. The tube contained distilled water, 2 ml glacial acetic acid, and 2 ml 2.5% ninhydrin (acetic acid:water, 60:40, v/v). The mixture was further boiled for 30 min, and the chromophore was extracted with 4 ml toluene by centrifugation. The supernatant was collected and absorbance was measured by spectrophotometry at 520 nm. Commercially available, pure Pro (Sigma-Aldrich, MO, USA) was used as the standard. All the experiments were carried out using three different calli and plantlets.
qRT-PCR analysis
Total RNA of aerial parts of plantlets were extracted using improved CTAB method (Logemann et al. 1987). A UV spectrophotometer was used to determine the RNA content and quality. Samples were treated with DNase using the Turbo DNA-free kit (TAKARA, Japan). DNase-treated total RNA served as the template for the first-strand cDNA synthesis using M-MLV reverse transcriptase (Promega, USA). A control reaction for each sample was performed simultaneously, however, without the reverse transcriptase.
Primer pairs for VvP5CS, VvOAT, and VvP5CDH genes are shown in Table 1 and were used in the RT-PCR. An EF1-α gene cDNA fragment (110 bp) from Pinot Noir was used as the reference. The specificity of PCR products was validated by dissociation curve analysis and agarose gel electrophoresis. Three biological replicates for each sample were used for qRT-PCR analysis, and three technical replicates were analyzed for each biological replicate. Statistical analysis was performed using SPSS version 19 (ANOVA, SNK test). Statistical analysis (P < 0.05) of Pro content and the expression profiles were performed between HR plants and normal plants.
Results
Irradiation and selection of Hyp-resistant PEs
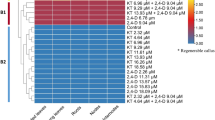
Pre-embryogenic calli of V. vinifera Chardonnay were firstly irradiated with different dosages of 60Co. Following irradiation, these calli were cultured on 10 mM Hyp-added medium for 28 days. We discovered that embryogenic calli irradiated with 100 Gy of 60Co survived on the 10 mM Hyp-added medium. Subsequently, a few HR calli were obtained after extended culture on Hyp-added and Hyp-free medium for 2 years (Fig. 1).
To further verify the tolerance of new HR embryogenic calli to Hyp, both HR and normal embryogenic calli were cultured on 1 and 4 mM Hyp medium for 28 days. The results showed that all HR embryogenic calli (100%) were healthy and survived both on the 1 and 4 mM Hyp medium for 28 days (Fig. 2b, d). However, only 56.67% of normal calli survived on 1 mM Hyp medium (Fig. 2a) and none of them survived on the 4 mM Hyp medium (Fig. 2c). These results indicated that the HR calli exhibited higher Hyp resistance ability.
Effect of Hyp treatment on intracellular Pro content
The intracellular Pro content of both normal and HR calli was investigated after culturing on Hyp-supplemented LTM medium (Fig. 3). When cultured on Hyp-free LTM medium, Pro content in the normal calli was determined to be 7.37 μg g−1 Fw. When normal calli were cultured on 1 mM Hyp medium, Pro content increased rapidly and reached peak levels (sevenfold higher than the original level) within 0.5 day before a drastic reduction to reach the original level at 1.5 days and remained constant until 2 days. At 7 days, we observed a remarkable increase, reaching approximately ninefold higher than the original Pro level. Similarly, when normal calli were instead cultured on 4 mM Hyp medium, Pro content also rose rapidly to peak (twofold higher than the original level) at 1 day and then decreased at 1.5 days. However, it rapidly spiked to reach the second peak level (threefold higher than the original level) at 4 days. Then, it gradually decreased and remained constant twofold higher than the original Pro level at 7 days. At this stage, we observed that some normal calli became brown and infiltrating.
Different patterns of Pro accumulation were observed in HR calli cultured on Hyp-added LTM medium. When HR calli were cultured on Hyp-free LTM medium, Pro content was only 10.31 μg g−1 Fw, slightly higher than that in the normal calli (7.37 μg g−1 Fw). When HR calli were cultured on 1 mM Hyp LTM medium, Pro content increased slowly before 0.5 day, but increased rapidly until 7 days, considerably higher than that in the normal calli. Similarly, when HR calli were cultured on 4 mM Hyp medium, Pro accumulation was at low levels until 1.5 days, after which it increased rapidly to 124.56 μg g−1 Fw at 7 days, which was approximately ninefold higher than that observed in the normal calli. Furthermore, HR calli remained healthy at this stage.
Whether 1 or 4 mM Hyp was added to the medium, Pro content in HR calli generally increased and responded quickly by changing concentration and remained at higher levels over time. This contrasts with the accumulation pattern observed in the normal calli that showed an increase in Pro content at first, but then decreased over time, and slowly increased again. Both normal calli and HR calli responded faster on 1 mM Hyp medium than on 4 mM Hyp medium. One possible explanation for this rapid response with low Hyp content is that the calli may need more time to adapt to the stress caused by 4 mM Hyp medium.
Regeneration into plantlets
When Chardonnay normal calli and HR calli were both cultured in the germination medium for differentiation (Fig. 4a, b), normal calli regenerated into small plantlets with two to three leaves at 28 days (Fig. 4c). However, it is approximately 180 days for HR calli to reach the same stage as that of the normal embryogenic calli at 28 days (Fig. 4d), which may be probably due to 60Co irradiation. Similar effects of 60Co irradiation decreasing growth rate have been reported in tall fescue grass (Xiong and Fei 2013). Nevertheless, no obvious morphological differences were observed between plantlets generated from normal embryogenic calli and HR embryogenic calli.
Effect of salt stress on Pro content
To study tolerance ability of HR plantlets to salt stress, Pro content of normal and HR plantlets was determined when plantlets were treated with 5, 7.5, and 10 mM NaCl for 14 days (Fig. 5). Pro content of HR plantlets remained at approximately constant levels when subjected to different NaCl treatments, whereas Pro content of normal plantlets increased with increase in NaCl concentration. Pro content of normal plantlets was found to be 130.87 μg g−1 Fw before NaCl treatment, which decreased to 56.94 μg g−1 Fw after 5 mM NaCl treatment. This increased to 209.43 μg g−1 Fw with 10 mM NaCl treatment. Notably, Pro content of HR plantlets was significantly lower than that of normal plantlets under 10 mM NaCl treatment. Morphologically, normal plantlets displayed wilting symptoms under 10 mM NaCl treatment for 14 days (Fig. 6c), the roots brown and subsequently the whole plantlets dead. HR plantlets remained healthy throughout the treatment period (Fig. 6d).
The morphology of normal and HR plantlets under salt stress. a Normal plantlets cultured on WP medium without NaCl for 14 days. b HR plantlets cultured on WP medium without NaCl for 14 days. c Normal plantlets cultured on WP medium with 10 mM NaCl for 14 days. d HR plantlets cultured on WP medium with 10 mM NaCl for 14 days
Effects of salt stress on the expression of VvP5CS, VvOAT, and VvP5CDH
To determine the relationship between Pro accumulation and transcriptional levels of a few related genes under salt stress, expression levels of VvP5CS, VvOAT, and VvP5CDH genes were assessed by real-time qRT-PCR. As shown in Fig. 7, the expression level of these genes in normal and HR plantlets varied significantly.
When normal plantlets were subjected to varying levels of NaCl treatments, we noticed a positive correlation between VvP5CS upregulated expression levels and NaCl concentration. On the contrary, VvOAT upregulated expression level had a negative correlation with NaCl concentration, indicating that Pro synthesis in normal plantlets was mainly channeled through the OAT pathway under low NaCl content and PSCS pathway under high NaCl concentration. The expression levels of VvP5CDH gene in normal plantlets were upregulated with concomitant increase in NaCl concentration. Combined with the above results on Pro content of normal plantlets under NaCl stress, the results of gene expression reveal that the upregulated expressions of VvP5CS and VvOAT genes promoted Pro accumulation to overcome NaCl stress. Expression of Pro metabolism-related gene VvP5CDH was upregulated and in turn decreased Pro accumulation when Pro was excessive, through the Pro feedback mechanism.
Compared to normal plantlets, the expression levels of VvP5CS and VvOAT genes in HR plantlets were considerably upregulated under 10 mM NaCl at 14 days, whereas they were not significantly affected by low NaCl concentrations (Fig. 7). The expression levels of VvP5CDH in HR plantlets were upregulated under low NaCl contents, whereas the upregulation levels of VvP5CDH expression were inhibited by high NaCl contents. Taken together, these results of Pro content in HR plantlets under NaCl treatment reveal that when Pro accumulation was insufficient to help defense severe NaCl stress, the expression of Pro synthesis-related genes VvP5CS and VvOAT was upregulated and the expression levels of VvP5CDH gene remained unchanged probably to favor Pro accumulation and subsequently increase the stress tolerance levels of HR plantlets under high salt treatment.
Discussion
With more and more agricultural land salinization, the development of high-yielding and salt-tolerant cultivars has become an important breeding objective. Mutagenesis can produce new variants with new traits which are not found in the nature and can thus enrich the genetic and breeding resources available for salinization resistance and other desirable traits (Liu and Zhen 2004; Jain 2001; Predieri 2001). 60Co-γ as a mutagen has direct mutagenic effects on DNA, as it can cause DNA breakage at high concentrations and interfere with DNA replication and synthesis at low concentrations by inhibiting DNA polymerases and ligases activity. Owing to its safety and high efficiency, 60Co-γ is being widely used in crop development programs (Weng et al. 2005; Xu et al. 2016). Given these advantages, we selected 60Co-γ to create mutants of Chardonnay pre-embryonic calli for breeding new genotypes with abiotic stress resistance.
To breed new germplasm resources, the combination of in vitro mutagenesis and directed screening for mutants via tissue culture can save labor and other resources (Ahloowalia et al. 1998). The proline analog Hyp has been used in tissue culture to select for stress-tolerant cell lines of many crops (Li et al. 2008; Zhang et al. 2008; Sui et al. 2015). Hydroxyproline-resistant calli could be obtained with higher stress tolerance ability, for example, Hyp was successfully used to select Pro-overproducing lines in potato and spring wheat to improve frost tolerance (Van Swaaij et al. 1986; Tantau and Dörffling 1991). Additionally, researchers used somaclonal variations to screen Hyp-tolerant mutants and finally to obtain regenerated plants with increased cold resistance (Zhang et al. 2008). The use of pingyangmycin-based in vitro mutagenesis in combination with directed screening with Hyp is effective for the creation of potential drought-tolerant mutants of peanut (Sui et al. 2015). In our work, grape somatic embryogenic calli after irradiation with 60Co rays were screened with Hyp, and HR plantlets with higher salt-tolerance ability were obtained.
In a wide range of environmental stresses, free proline accumulates in plant cells. Proline is a highly water soluble amino acid (Manafi et al. 2015) and is involved in imparting sustainable tolerance in plants (Parvanova et al. 2004a). Although Pro accumulation in plants plays an important role in osmotic balance, overaccumulation of Pro could result in slow plant growth and prolonged the florescence (Mattiol et al. 2009). Pro toxicity could cause ultra-structural alterations in chloroplast and mitochondria as well as several features of programmed cell death (Hare et al. 2002; Deuschle et al. 2001; Lehmann et al. 2010). Therefore, intracellular free Pro content can be used as an index to assess the tolerance capacity of plants to abiotic stress. Our results showed that normal calli under Hyp stress and normal plantlets under NaCl treatment were both sensitive to the environment and accumulated Pro at a faster rate, whereas HR calli and plantlets could sustain a stable physiological status under abiotic stresses, better than normal calli and plantlets. These results revealed that it was important for plants to maintain the internal environment homeostasis. The accumulation of Pro may play an important role in adjusting the osmotic potential to tolerate abiotic stress (Ashraf and McNeilly 2004).
Pro content of HR plantlets under NaCl treatments remained relatively stable under low salt stress, whereas Pro content of normal plants was significantly higher than that of HR plantlets under 10 mM salt stress. We speculated the reasons that caused different responses between normal plantlets and HR plantlets. Under salt treatment which simulated the natural environment, plantlets suffered salt stress not only from penetration imbalances but also from toxic effects of Na+/Cl− ions. In a saline medium, cells need to acquire essential nutrients from a milieu with a preponderance of ions that are potentially toxic (Chowdhury et al. 1995). To some extent, Pro ameliorated the deleterious effect of NaCl and favored better growth of plants. Some normal plantlets cultured on NaCl-added medium showed higher intracellular Pro content to maintain the osmotic balance. As HR plantlets may do not have the same sensitivity to salt as the normal plantlets, Pro content in HR plantlets was lower than that in normal plantlets.
Most of the research work concerning Pro metabolism in plants has been focused on its accumulation in vegetative tissues in response to abiotic stresses such as drought and salinity. Stress-induced Pro accumulation occurs predominantly through enhancing its biosynthesis from glutamate via the pathway catalyzed by enzymes P5CS and P5CR (Boggess et al. 1976; Rhodes and Bressan 1986). In our study, under low contents of NaCl, the expression levels of both VvP5CS and VvOAT genes in HR plantlets were maintained at constant levels, whereas the expression of VvP5CDH was upregulated, which showed that the increase in Pro degradation was more prominent in HR plantlets, maybe to maintain the stabilization of internal environment under low salt conditions. However, under high salt conditions, the stabilization of internal environment in HR plantlets was achieved through the upregulation of Pro synthesis genes VvP5CS and VvOAT and the inhibition of VvP5CDH upregulation expression. When normal plantlets accumulated sufficient amounts of Pro under low NaCl conditions for 14 days, the expression of VvP5CDH increased to trigger Pro degradation, through Pro feedback inhibition mechanism. This phenomenon was slow or delayed under high salt conditions, which illustrates that normal plantlets are sensitive to salt stress. High NaCl content causes plant etiolation and death. It is possible that Pro degradation pathway could be suppressed when homeostasis of Pro cycle is disturbed, leading to a distorted physiological state and damage to tissues.
Conclusion
Selection for Hyp resistance has been investigated as a possible alternative strategy for developing salt tolerance in plants (Dix et al. 2013). HR plantlets resistant to 10 mM Hyp showed improved resistance to NaCl. Proline accumulation as a basis for their Hyp resistance is yet to be confirmed. Tolerance to proline analog associated with proline accumulation is one possible approach but could be associated with agronomically undesirable traits such as loss of vigor (Hanson et al. 1979) and attraction of pests (Haglund 1980). However, the latter possibility has been excluded in proline-accumulating barley mutants (Bright et al. 1982).
In summary, we obtained several Hyp-resistant grape plants which could accumulate Pro without feedback inhibition. We observed strong correlation between Pro accumulation and VvP5CDH gene upregulation under salt stress. In addition, Pro could cause native effects on growth and regeneration of cells, though playing a critical role in protecting physiological activity during stress. Finally, 60Co irradiated HR plants showed higher tolerance to abiotic stress than normal plants. Further research is necessary to fully understand the stress tolerance mechanism in HR plantlets and the effects of Pro on plant growth at a molecular level.
Abbreviations
- Hyp:
-
Hydroxyproline
- HR:
-
Hydroxyproline-resistant
- OAT:
-
Orn-δ-amino-transferase
- Pro:
-
Proline
- P5C:
-
△1-Pyrroline-5-carboxylate
- P5CS:
-
△1-Pyrroline-5-carboxylate synthase
- P5CR:
-
△1-Pyrroline-5-carboxylate reductase
- ProDH:
-
Pro dehydrogenase
- P5CDH:
-
P5C dehydrogenase
References
Agüero CB, Meredith CP, Dandekar AM (2006) Genetic transformation of Vitis vinifera L.cv. Thompson seedless and Chardonnay with the pear PGIP and GFP encoding genes. Vitis 45(1):1–8
Ahloowalia BS, Maluszynski M, Nichterlein K, van Zanten L, Weck E (1998) Induced mutations and in vitro culture techniques for the improvement of horticultural plants. In: Chopra VL, Singh RB, Varma A (eds) crop productivity and sustainability: shaping the future. Oxford & IBH, New Delhi, pp 405–412
Anjum MA, Villiers TA (1998) Selection of hydroxyproline-resistant cell lines from Solanum tuberosum L. callus. I. Stability and frost tolerance. J Genet Breed 53:113–117
Ashraf M, McNeilly T (2004) Salinity tolerance in Brassica oilseeds. Crit Rev Plant Sci 23:157–174
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Boggess SF, Stewart CR, Aspinall D, Paleg LG (1976) Effect of water stress on proline synthesis from radioactive precursors. Plant Physiol 58:398–401
Bright SWJ, Lea PJ, Kueh JSH, Woodcock C, Hollomon DW, Scott GC (1982) Proline content does not influence pest and disease susceptibility of barley. Nature 295:592–593
Cavagnaro JB, Ponce MT, Guzmán J, Cirrincione MA (2006) Argentinean cultivars of Vitis vinifera grow better than European ones when cultured in vitro under salinity. Biocell 30(1):1–7
Chowdhury MAM, Moseki B, Bowling DJF (1995) A method for screening rice plants for salt tolerance. Plant Soil 171(2):317–322
Delauney AJ, Verma DPS (2002) Proline biosynthesis and osmoregulation in plants. Plant J 4:215–223
Deuschle K, Funck D, Hellmann H, Däschner K, Binder S, Frommer WB (2001) A nuclear gene encoding mitochondrial △’-pyrroline-5-carboxylate dehydrogenase and its potential role in protection from proline toxicity. Plant J 27:345–356
Dix P, Mclysaght U, Plunkett A (2013) Plant tissue culture and its agricultural applications: Proceedings of Previous Easter Schools in Agricultural Science, Published by Butterworths, London, Department of Biology, St. Patrick’s College, Maynooth, Co. Kildare, Ireland: 469
Dörffling K, Dörffling H, Luck E (2009) Improved frost tolerance and winter hardiness in proline over-accumulating winter wheat mutants obtained by in vitro-selection is associated with increased carbohydrate, soluble protein and abscisic acid (ABA) levels. Euphytica 165:545–556
Fuller MP, Metwali EMR, Eed MH, Jellings AJ (2006) Evaluation of abiotic stress resistance in mutated populations of cauliflower (Brassica oleracea var. botrytis). Plant Cell Tiss Org 86:239–248
Goring H, Thien BH (1979) Influence of nutrient deficiency on proline accumulation in the cytoplasm of Zea mays seedlings. Biochem Physiol Pflanz 174:9–16
Haglund BM (1980) Proline and valine - cues which stimulate grasshopper herbivory during drought stress? Nature 288(5792):697–698
Hanson AD, Nelsen CE, Pedersen AR, Everson EH (1979) Capacity for proline accumulation during water stress in barley and its implications for breeding for drought resistance. Crop Sci 19:489–493
Hare PD, Cress WA, Van Staden J (2002) Disruptive effects of exogenous proline on chloroplast and mitochondrial ultra-structure in Arabidopsis leaves. S Afr J Bot 68:393–396
Hu CA, Delauney AJ, Verma DP (1992) A bifunctional enzyme (△’-pyrroline-5-carboxylate synthetase) catalyzes the first two steps in proline biosynthesis in plants. Proc Natl Acad Sci USA 89:9354–9358
Jain SM (2001) Tissue culture-derived variation in crop improvement. Euphytica 118:153–166
Lehmann S, Funck D, Szabados L, Rentsch D (2010) Proline metabolism and transport in plant development. Amino Acides 39:949–962
Li H, Li B, Wang LL, Yang WG (2008) Screening and characterizing of L-hydroxyoroline resistant variants in Medicago sativa. J Pratacul Sci 25:29–33
Liu JP, Zhen CM (2004) Induced mutation in connection with in vitro culture for crop breeding. Acta Agric Shanghai 20:19–22
Lloyd G, McCown BH (1981) Commercially-feasible micropropagation of Mountain Laurel, Kalmia latifolia, by shoot tip culture. Comb Proc Int Plant Propag Soc 30:421–427
Logemann J, Schell J, Willmitzer L (1987) Improved method for the isolation of RNA from plant tissues. Anal Biochem 163(1):16–20
Manafi E, Modarres Sanavy SAM, Aghaalikhani M, Dolatabadian A (2015) Exogenous 5-aminolevulenic acid promotes antioxidative defence system, photosynthesis and growth in soybean against cold stress. Not Sci Biol 7(4):486–494
Mattiol R, Falasca G, Sabatini S, Maddalena Altamura M, Costantino P, Trovato M (2009) The proline biosynthetic genes P5CS1 and P5CS2 play overlapping roles in Arabidopsis flower transition but not in embryo development. Physiol Plantarum 137:72–85
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plantarum 15:473–497
Parvanova D, Ivanov S, Konstantinova T, Karanov E, Atanassov A, Tsvetkon T, Alexieva V, Djilianov D (2004a) Transgenic tabacco plants accumulating osmolytes show reduced oxidative damage under freezing stress. Plant Physiol Biochem 42:57–63
Parvanova D, Popova A, Zaharieva I, Lambrev P, Konstantinova T, Taneva S, Atanassov A, Goltsev V, Djilianov D (2004b) Low temperature tolerance of tobacco plants transformed to accumulate proline, fructans, or glycine betaine. Variable chlorophyll fluorescence evidence. Photosynthetica 42(2):179–185
Predieri S (2001) Mutation induction and tissue culture in improving fruits. Plant Cell Tissue Organ Cult 64:185–210
Rhodes DH, Bressan RA (1986) Metabolic changes associated with adaptation of plant cells to water stress. Plant Physiol 82:890–903
Rizzi YS, Monteoliva MI, Fabro G, Grosso CL, Laróvere LE, Alvarez ME (2015) P5cdh affects the pathways contributing to Pro synthesis after ProDH activation by biotic and abiotic stress conditions. Front Plant Sci 6:572
Sui J, Wang Y, Wang P, Qiao L, Sun S, Hu X, Chen J, Wang J (2015) Generation of peanut drought tolerant plants by pingyangmycin-mediated in vitro mutagenesis and hydroxyproline-resistance screening. PLoS One 10(3):e0119240
Szabados L, Savouré A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15(2):89–97
Tantau H, Dörffling K (1991) In vitro-selection of hydroxyproline-resistant cell lines of wheat (Triticum aestivum): accumulation of proline, decrease in osmotic potential, and increase in frost tolerance. Physiol Plantarum 82:243–248
Tantau H, Balko C, Brettschneider B, Melz G, Dröffling K (2004) Improved frost tolerance and winter survival in winter barley (Hordeum vulgare L.) by in vitro selection of proline over-accumulating lines. Euphytica 139:19–32
Van Swaaij AC, Jacobsen E, Kiel JAKW, Feenstra WJ (1986) Selection, characterization, and regeneration of hydroxyproline-resistant cell lines of Solanum tuberosum: tolerance to NaCl and freezing stress. Physiol Plantarum 68:359–366
Verbruggen N, Hermans C (2008) Proline accumulation in plants: a review. Amino Acids 35(4):753–759
Vincent D, Ergül A, Bohlman MC, Tattersall EAR, Tillett RL, Wheatley MD, Woolsey R, Quilici DR, Joets J, Schlauch K, Schooley DA, Cushman JC, Cramer GR (2007) Proteomic analysis reveals differences between Vitis vinifera L. cv. Chardonnay and cv. Cabernet Sauvignon and their responses to water deficit and salinity. J Exp Bot 58(7):1873–1892
Weng B, Xu G, Zheng X, Ying ZY, Huang YB (2005) Study on several characters of Chamaecrista spp. irradiated by 60Co-γ. Sci Agric Sin 38(12):2566–2570
Xin Z, Browse J (1998) Eskimo1 mutants of Arabidopsis are constitutively freezing-tolerant. Plant Biol 95:7799–7804
Xiong ZB, Fei YJ (2013) Effects of 60Co-γ radiation on plant growth of second generation tall fescue. J Yangtze Univ (Natural Science Edition) 11:008
Xu Z, Lan X, Yang M, Xiao B, Fei Y (2016) Effects of 60Co-γ radiation on the chromosome karyotypes of two Festuca arundinace cultivars. J Northeast Agric Sci 41(3):18–24
Yang SL, Lan SS, Gong M (2009) Hydrogen peroxide-induced proline and metabolic pathway of its accumulation in maize seedlings. J Plant Physiol 166:1694–1699
Yoshiba Y, Kiyosue T, Nakashima K, Yamaguchi-Shinozaki K, Shinozaki K (1997) Regulation of levels of proline as an osmolyte in plants under water stress. Plant Cell Physiol 38(10):1095–1102
Zhang DL, Xu YJ, Chen JJ (2008) Primary study on in vitro selection of hydroxyproline-resistant mutants of eggplant. J Hubei Agric Sci 47(7):805–809
Acknowledgments
We are grateful to the following for providing Vitis vinifera Chardonnay calli for this study: X. Xia at Center for Viticulture and Small Fruit Research, Florida A&M University.
Funding
This study was supported by the earmarked fund for China Agriculture Research System (Grant No. CARS-29-yc-2) and Guangxi Bagui Scholar Fund (2013-3).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Hanns H. Kassemeyer
Rights and permissions
About this article
Cite this article
Wang, C., He, R., Lu, J. et al. Selection and regeneration of Vitis vinifera Chardonnay hydroxyproline-resistant calli. Protoplasma 255, 1413–1422 (2018). https://doi.org/10.1007/s00709-018-1240-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-018-1240-2