Abstract
Polyamines (PAs) produce H2O2 and nitric oxide (NO) during their normal catabolism and modulate plant growth and development. To explore the biochemical basis of PAs-induced growth inhibition in Triticum aestivum L seedlings, we examined the role of O2·-, H2O2 or NO in shoot and root development. Although all PA treatments resulted in a variable reduction of root and shoot elongation, spermine (Spm) caused the greater inhibition in a similar way to that observed with the NO donor, sodium nitroprusside (SNP). In both cases, O2·- production was completely blocked whereas H2O2 formation was high in the root apex under SNP or Spm treatments. Catalase recovered root and shoot growth in SNP but not in Spm-treated plants, revealing the involvement of H2O2 in SNP-root length reduction. The addition of the NO scavenger, cPTIO, restored root length in SNP- or Spm-treated plants, respectively, and partially recovered O2·- levels, compared to the plants exposed to PAs or SNP without cPTIO. A strong correlation was observed between root growth restoration and O2·- accumulation after treating roots with SNP + aminoguanidine, a diamine oxidase inhibitor, and with SNP + 1,8-diaminoctane, a polyamine oxidase inhibitor, confirming the essential role of O2·- formation for root growth and the importance of the origin and level of H2O2. The differential modulation of wheat growth by PAs through reactive oxygen species or NO is discussed.

Polyamines, nitric oxide and ROS interaction in plants during plant growth
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Putrescine (Put), spermidine (Spd), and spermine (Spm) are aliphatic amines namely polyamines, present in all plant cells (Alcázar et al. 2011; Wojtasik et al. 2015), particularly in actively growing tissues (Takahashi and Kakehi 2010). They have been involved in diverse physiological processes, such as cell division, rhizogenesis, root elongation, senescence, floral development, fruit ripening, and the response to biotic and abiotic stresses, as reported by Alcázar and Tiburcio (2014), Alcázar et al. (2011), Gupta et al. (2013, 2016) and Groppa et al. (2008). Most studies carried out until now have been focused on the beneficial effects of polyamines, pointing out that a correlation exists between stress tolerance and elevated polyamine levels (Minocha et al. 2014). Moreover, some studies have revealed that application of exogenous PAs alleviate abiotic stress symptoms, thus conferring stress tolerance (Gupta et al. 2016 and references therein). However, PAs could also be deleterious to growth and development when they are applied under non-stressful environmental conditions, revealing a new dimension for these nitrogenous compounds which were unexpected earlier. PAs are well known to induce PCD or alter growth or development upon depletion/overproduction with respect to their physiological levels (Alcázar et al. 2005; Gupta et al. 2016). Thus, it appears that the former idea that higher PAs levels are better for stress tolerance cannot be applied under all conditions (Pottosin and Shabala 2014; Alcázar and Tiburcio 2014; Minocha et al. 2014). For instance, regarding root development, de Agazio et al. (1995) have reported that exogenous supply of Spd in Zea mays affected cell elongation and mitotic index, whereas Tisi et al. (2011) have shown that it inhibited root cell elongation, promoted deposition of phenolics in cell walls and resulted in a higher number of cells resting in G1 and G2 phases in the maize primary root apex. Moreover, Alcázar et al. (2005) have addressed that transgenic plants with elevated Put content presented dwarfism and late flowering.
Reactive oxygen species (ROS), like superoxide anion (O2·-) or hydrogen peroxide (H2O2), are by-products constantly formed along all physiological processes, such as photosynthesis or photorespiration, as has been mentioned by Foyer and Noctor (2009). They also function in signaling pathways, regulating plant development in response to physiological and environmental signs (Apel and Hirt 2004; Suzuki et al. 2011). Despite abnormal high level of ROS can be lethal for the plant cell integrity, it was recently demonstrated that ROS are also essential for growth processes when they are present at low levels (Foreman et al. 2003). Apoplastic ROS were suggested to play a key role in cell expansion during morphogenesis of roots and leaves via effects on cell-wall loosening (Carol and Dolan 2006; Francoz et al. 2015).
Superoxide anions and H2O2 are the most well studied ROS in plant cells (Gapper and Dolan 2006) and can be generated via plasma membrane (PM)-localized NADPH oxidases (NOXs), which are homologous to the catalytic subunit (gp91phox) of mammalian phagocyte NOX (Jones et al. 2000). Superoxide anion signaling has been associated with root hair growth (Foreman et al. 2003) and root gravitropism (Joo et al. 2001) whereas growth of adventitious roots requires the presence of H2O2 (Montiel et al. 2012). The superoxide ion may be converted into H2O2 spontaneously or enzymatically via superoxide dismutase (SOD), and this molecule can further be reduced by peroxidases to generate more reactive free radicals, as hydroxyl radical (•OH) (Liszkay et al. 2004). Verbelen et al. (2006) stated that root growth is brought about by the activity of the subapical meristem and the elongation of the newly formed cells, whereas Dunand et al. (2007) reported that hydroxyl anion is involved in cell elongation by cell wall loosening mechanism in the growing zone of roots.
Polyamines are oxidatively deaminated at their primary or secondary amino-groups by the copper-containing amine oxidases (CuAOs) and the FAD-dependent amine oxidases (PAOs), respectively (Angelini et al. 2010), releasing H2O2 as one of the catabolic products. In this way, CuAOs and PAOs do not only contribute to important physiological processes through the regulation of cellular PA levels with diverse specificities and expression patterns, but they also do so through their reaction products, especially H2O2 (Fincato et al. 2012; Moschou et al. 2008; Planas-Portell et al. 2013; Tavladoraki et al. 2006).
Nitric oxide (NO) is a hydrophobic, highly diffusible gaseous molecule involved in communication from organ to organ or from plant to plant (Beligni and Lamattina 2001). In the last few years, a plethora of data have demonstrated that NO has an outspread spectrum of regulatory functions, including those related to processes like germination, metabolism, flowering, senescence, cell death, ion homeostasis, as well as responses to both biotic and abiotic stresses (Beligni and Lamattina 2001; Correa-Aragunde et al. 2004; Groppa et al. 2008; Moreau et al. 2008), most of them resembling those of PAs. Moreover, PAs and NO are clearly interconnected through a common precursor, L-arginine. This aminoacid is a substrate for the biosynthesis of PAs but it can also be used to form NO by a still unknown pathway (Wimalasekera et al. 2011). Polyamines and NO have common signaling functions, they interact with hormones, perform biological functions including growth, development and stress responses (Lamattina et al. 2003; Takahashi and Kakehi 2010). The possible linkage between PAs and NO metabolism have started to be explored just a few years ago, when Tun et al. (2006) reported that PAs induced rapid NO biosynthesis in specific tissues of Arabidopsis thaliana seedlings, mainly in the elongation zone of root tips and in the veins and trichomes of primary leaves. In this sense, a previous work of our group has demonstrated that exogenous Spm increased endogenous NO content and reduced wheat root growth rates (Groppa et al. 2008; Rosales et al. 2012).
Tissue specific cross-talk exists between ROS/NO producing organelles inside the cell, such us mitochondria, chloroplasts and peroxisomes (Apel and Hirt 2004; Farnese et al. 2016). It is well known that ROS are involved in signal transduction pathways during biotic and abiotic stress protective responses while, at the same time, they could directly damage membranes, metabolites as chlorophylls, or oxidize lipids and proteins (Gapper and Dolan 2006). In this way, PAs have been involved in diverse protective responses in the cell (Groppa et al. 2008; Tanou et al. 2014; Zepeda-Jazo et al. 2011), such as the protection of membranes and other macromolecules, which are the targets of ROS damage. Numerous studies have emphasized the complexity of the interaction between PAs and NO, which sometimes appears to be diametrically opposed, especially when plants are under stress (Pottosin et al. 2014; Velarde-Buendía et al. 2012).
The antioxidant properties of PAs have also been widely studied in the context of their response to several types of abiotic stress (Minocha et al. 2014). Nevertheless, there is few data concerning the effect of PAs on certain aspects of physiological development, like those harmful effects caused by the excess accumulation of PAs as a result of the overexpression of the genes involved in their biosynthesis or to their exogenous addition to plant cells (Tiburcio et al. 2014). Considering that ROS and NO can be produced by PAs, either during normal catabolism (H2O2) or by still unknown mechanisms (NO), we decided to study the involvement of ROS and NO on root elongation in wheat plants using a pharmacological approach by exogenously adding Put, Spd or Spm and in comparison with SNP-treated plants. Looking for the mechanisms involved in such effect, and considering that PAs, NO and ROS are undoubtedly linked and can share a cross-linked pathway in essential developmental processes, the objective of the present work was to study the interplay between PAs-derived ROS and NO and to gain insight into the mechanisms by which PAs affect root growth.
Materials and methods
Plant material and treatments
Wheat (Triticum aestivum L. 75 Aniversario) seeds (provided by Buck S.A.) were germinated on wet Whatman paper over a 48-h period. After this time, seedlings were transferred to a hydroponic system with Hoagland (Hoagland and Arnon 1950) solution containing 0.1 mM of the NO-donor SNP, or 1 mM Put, Spd or Spm. As control of CN− release by SNP, a solution of 0.1 mM SNP was left on the bench under light for 24 h and then used. This treatment did not affect growth compared to controls (data is not shown). The NO-trapping agent 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO, 0.3 mM) was added to the treatment solutions to corroborate NO effects. When necessary, the following compounds were used as exogenous pharmacological treatments: 0.1 to 10 mM H2O2; 1000 U ml−1 catalase (CAT) to eliminate H2O2; 0.5 mM aminoguanidine (AG) as a CuAO inhibitor; 0.3 mM 1,8-diaminoctane (1,8-DO) as a PAO inhibitor. Inhibition of NADPH oxidase activity was evaluated by means of DPI (0.1 mM). To study the effect of calcium ions, 2 mM CaCl2 was used. Preliminary assays were performed to establish which concentrations were more adequate for modulating ROS generation and/or root or shoot growth. Plants were grown with a 16/8-h photoperiod at 26/20 °C, under fluorescent white light (photon flux density: 175 μmol m−2 s−1 at plants level) in a controlled environmental growth chamber and were harvested after 5 days of growth on each treatment solution. Roots and leaves were used for determinations.
Root and shoot length
A sample of 10 to 15 plants per treatment was used to record root (the main root) and shoot length at indicated time. Data from four to five independent experiments were pooled for the statistical analysis. Seedlings representative of each treatment were chosen for imaging.
H2O2 determination
Hydrogen peroxide concentration in wheat roots was measured according to Sergiev et al. (1997). Root tissues (200 mg) were ground to powder using liquid N and then homogenized in 5 ml 0.1% (w/v) TCA. The homogenate was centrifuged at 12,000g for 15 min and 0.5 ml of the supernatant was added to 0.5 ml 10 mM potassium phosphate buffer (pH 7.0) and 1 ml of 1 M KI. The absorbance of the supernatant was read at 390 nm. The content of H2O2 was given on a standard curve generated with known concentrations of H2O2.
Root tissue staining of ROS
Superoxide accumulation in tissues was determined using nitroblue tetrazolium (NBT), which reacts with O2·- producing a blue formazan precipitate. Immediately after removal from the plant, 1.5–2 cm of the apical portions of the primary roots were immersed in a 0.05% solution of NBT in 50 mM potassium phosphate buffer (pH 6.4) and vacuum-infiltrated. The vacuum was broken and re-established every 30 s, three successive times. Then, they were incubated in the same solution for 30 min under light. To determine that this staining was attributable to NADPH oxidase dependent O2·− formation, root segments were incubated in 0.1 mM DPI for 45 m previously to NBT staining (Sagi and Fluhr 2001). To visualize the presence of H2O2, the apical portion of the primary root were immersed in an aqueous solution of 1 mg ml−1 3,3-diaminobenzidine (DAB), vacuum infiltrated as describe above, and incubated for 45 min in the dark. As a control for color development, catalase (CAT) was used to dissipate H2O2. On each experiment, 8–10 individuals randomly sampled from two different trays were used. Stained segments were washed 3 times and put in water until images were taken (Thordal-Christensen et al. 1997).
O2 ·- and NO fluorescent imaging
The presence of endogenous O2·- or NO formation were detected by incubating 1.5–2 cm of the apical portions of the primary roots with 10 mM of the positive fluorescent probe dihydroethidium (DHE) or 10 μM DAF-2DA diaminofluorescein diacetate (DAF-2DA) prepared in 10 mM Tris–HCl (pH 7.4) over 30 min, according to Pagnussat et al. (2003) and Rodríguez-Serrano et al. (2009) with some modifications, respectively. Thereafter, roots were washed twice with the above buffer, and fluorescence was examined in a fluorescence microscope (Olympus BX50, Tokyo, Japan) with an excitation filter of 490 nm to detect red fluorescence of DHE (490-nm excitation, 520-nm emission) or green fluorescence of DAF-2DA (485-nm excitation, 530-nm emission). Exposure times were equal for all samples. Experiments were repeated at least five times and similar results were obtained. Photographs are representative of the observations made. All manipulations were performed under dim red light. Autofluorescence was recorded in unstained controls.
Thiobarbituric acid reactive substances (TBARS) determination
Lipid peroxidation was determined as the amount of TBARS, measured by the TBA reaction (Heath and Packer 1968) with minor modifications. Fresh roots or leaves (300 mg FW) were homogenized in 3 ml of 20% (w/v) TCA. The homogenate was centrifuged at 10,000g for 20 min. To 1 ml aliquot of the supernatant, 1 ml of 20% (w/v) TCA containing 0.5% (w/v) TBA and 100 μl 4% (w/v) butylated hydroxytoluene (BHT) in ethanol were added. The mixture was heated at 95 °C for 30 min and then quickly cooled on ice. The contents were centrifuged at 1000g for 3 min and the absorbance was measured at 532 nm. The value for non-specific absorption at 600 nm was subtracted. The concentration of TBARS was calculated using an extinction coefficient of 155 mM−1 cm−1.
NADPH-dependent O2 ·- generation
Root samples (100 mg) were homogenized under ice cold conditions in 0.7 ml of 50 mM Hepes buffer (pH 7.8) containing 250 mM sucrose, 10 mM cysteine, 3 mM ethylenediaminetetraacetic acid (EDTA), 1 mM 1,4-dithiothreitol (DTT), 1 mM MgCl2 0.6% p/v polyvinylpyrrolidone (PVP) and 0.5 mM phenylmethylsulfonyl fluoride (PMSF) and the homogenate was centrifuged at 27,000g for 20 min. NADPH-dependent O2·- generation was measured using the tetrazolium dye NBT as an electron acceptor, as described by Van Gestelen et al. (1997). Samples were pre-incubated for 1 min at 30 °C and the reaction was initiated by the addition of 100 μM NADPH, monitoring NBT reduction by NADPH at 530 nm for 3 min using 200 μl of the extract in a final volume of 1 ml. NADPH oxidation activity was calculated using an extinction coefficient of 12.8 mM−1 cm−1. Diphenyl iodonium (DPI) was used to inhibit NADPH oxidase-like enzyme activity and NaN3 was used to block peroxidase activity. NADH was used alternative electron donor instead of NADPH.
Antioxidant enzymes
Extracts for determination of guaiacol peroxidase (GPOX), catalase (CAT), and superoxide dismutase (SOD) activities were prepared from root samples (100 mg in 1 ml), homogenized under ice cold conditions in 50 mM phosphate buffer (pH 7.4) containing EDTA (1 mM), PVP (0.6% p/v) and Triton X-100 (0.5% v/v). The homogenates were centrifuged at 27,000g for 20 min and the supernatant fraction was used for the assays. CAT activity (EC 1.11.1.6) was assayed based on the decrease in the absorbance at 240 nm due to the degradation of H2O2, as described by Chance et al. (1979). Total SOD (EC 1.15.1.1) activity was assayed by the inhibition of the photochemical reduction of NBT, as described by Becana et al. (1986), in a reaction mixture containing the O2·- generating solution (14.3 mM methionine, 82.5 μM NBT, and 2.2 μM riboflavin) and 5–25 μl of plant extract. The reduction in NBT was followed by obtaining the A560 values. One unit of SOD was defined as the amount of enzyme required to cause a 50% inhibition of NBT reduction, under the assay conditions. GPOX activity (EC 1.11.1.7) was determined in a reaction mixture containing 50 mM phosphate buffer (pH 7.4), 1 mM guaiacol, 0.1 mM H2O2 and 100 μl of crude extract in a final volume of 1 ml. Activity was measured by following the increase in absorbance at 470 nm due to the formation of tetraguaiacol (ε 26.6 mM/cm) (Maehly and Chance 1954).
Statistics
All data presented are the mean values of three to ten independent set of experiments. Each value was presented as means ± standard errors of the mean (SE), with a minimum of five replicates. Statistical analysis was carried out by one-way ANOVA using the Tukey test to evaluate whether the means were significantly different, taking p < 0.05 as significant.
Results
Exogenous PAs and SNP reduced plant growth
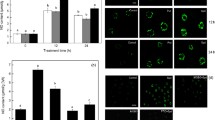
Polyamines and SNP treatments resulted in a reduction in root and shoot elongation to different extents (Fig. 1). The tetraamine Spm was which most affected growth, reducing root length to 32% and shoot elongation to 70% of the control (Table 1), a similar value that the one produced by NO released from SNP (28 and 72% of the C for root and shoot length, respectively). Putrescine and Spd, the diamine and triamine respectively, inhibited root elongation to 63 and 43% of the control, respectively, whereas shoot elongation remained close to control values in both treatments (Table 1).
Effect of exogenous SNP or PAs addition on wheat root and shoot length. Wheat seeds were germinated on moistened paper for 48 h. After this time, seedlings were transferred for 5 days to a hydroponic system with Hoagland solution containing 0.1 mM SNP, 1 mM Put, Spd or Spm, as described in “Materials and methods.” The figure is representative of the growth obtained from eight different experiments with 15 replicated measurements
PAs treatment affected O2 ·-, H2O2 and NO levels in 7-day-old wheat roots
Looking for the reasons implicated in root growth reduction after PAs or SNP exogenous addition, O2·– or H2O2 formation in 1–1.5-cm-root segments, including the root tip, were measured. After 5 days of treatment, the blue formazan deposition denoting the presence of O2·– appeared rather widespread in the main root segment of C, Put or Spd treatments (Fig. 2). The development of the blue color was almost completely inhibited in SNP- or Spm-treated-roots compared to control roots, suggesting a severe reduction of the O2·- levels (Fig. 2). Superoxide anion production was also detected using the fluorescent probe DHE. Red fluorescence due to O2·--dependent DHE oxidation was evidenced in root segments in a comparable pattern to that of NBT staining. Superoxide anion formation was highest in C roots, and a significant reduction in fluorescence was detected in SNP or Spm-treated roots (Fig. 3a).
O2·--NBT staining of wheat root segments after 5 d in the respective treatment as it is indicated in the picture: 0.1 mM SNP; 1 mM Put, Spd or Spm, with the addition of ROS, NO or PAs modulators: 1000 U ml-1 CAT; 200 μM cPTIO; 0.5 mM AG; 0.3 mM 1,8 OD; 2 mM CaCl2. The figure is representative of four different experiments with twelve root segments stained in each experiment
Histochemical detection of a O2·- production in the growing meristematic zone of 7-day-old wheat roots. O2·- production was visualized by incubating a root segment from an intact seedling in 10 mM DHE fluorescent probe for 30 min. b NO accumulation in wheat roots using 10 μM DAF-2DA as a fluorescent probe, with or without 200 μM cPTIO, as described in Materials and methods. Apical portions of the roots (1.0–1.5 cm) were incubated with the probes and were observed under fluorescence microscopy. The figure is representative of five different experiments with 20 replicated measurements
As shown in Fig. 4a, SNP, Spd, and Spm induced H2O2 accumulation (160% for SNP and Spd and 148% for Spm over than those values observed in untreated C roots, respectively) in roots after 5 days of treatment, whereas no change in H2O2 formation was observed in Put treated roots. Almost identical results were obtained for H2O2 detection by staining roots segments with DAB, a histochemical reagent that polymerizes and turns brown in the presence of H2O2. At day 5, SNP, Spd, and Spm markedly increased H2O2 levels compared to the control (Fig. 4b). However, SNP or Spm clearly inhibited O2·- formation. No correlation was neither observed between H2O2 and O2·- levels in control and Put-treated roots, that showed a pale brown color developed after H2O2 reaction with DAB and did not increase quantitatively the H2O2 content compared to C, despite the high O2·- levels observed after NBT staining in these treatments (Fig. 4a, b). Only Spd increased H2O2 levels, accompanying the increase in O2·- content observed with NBT or DHE staining (Fig. 4a, b).
a Quantitative determination of H2O2. Values in columns are the mean of three independent experiments with six replicates. Significant differences (P ≤ 0.05) are indicated with different letters. b H2O2-DAB staining. Measurements were done in wheat root segments after 5 days in the respective treatment as it is indicated in the picture: 0.1 mM SNP; 1 mM Put, Spd or Spm, with the addition of ROS, NO or PAs modulators: 1000 U ml-1 CAT; 200 μM cPTIO; 0.5 mM AG; 0.3 mM 1,8 OD; 2 mM CaCl2. The figure is representative of four different experiments with 20 root segments stained in each experiment
SNP- or PA-induced NO formation was visualized by fluorescence microscopy using the probe DAF-2DA after 5 days of SNP or PA treatments. As shown in Fig. 3b, the three PAs as well as SNP increased NO content to rather similar extents. The addition of the NO-trapping compound (cPTIO) together with SNP or PAs in the incubation medium partially reduced NO formation thus confirming at least in part that the observed fluorescence under PAs or SNP treatments corresponded to NO (Fig. 3b).
NADPH-dependent O2 ·- generation
To evaluate if O2·- formation was related to a NADPH oxidation activity, a quantitative measurement of NADPH-dependent NBT reduction was estimated (Table 2). In accordance to the results obtained regarding O2·- staining with NBT or DHE, a similar degree of NADPH-dependent NBT reduction activity was observed in C, Put or Spd-treated roots, while a reduction of 44 and 32% respect to the C was measured in NBT-reduction rate in SNP or Spm-treated roots, respectively (Table 2). Almost no NBT-reduction activity was observed when NADH was used instead of NADPH (data not shown). The NADPH oxidation was completely inhibited by 10 μM diphenylene iodonium added to the reaction mixture whereas azide did not modify NADPH-dependent NBT reduction (data not shown).
O2 ·-, H2O2, and NO are involved in SNP or PA-induced root and shoot growth inhibition
With the aim to verify if H2O2 was directly involved in the growth inhibition observed in SNP- or Spm-treated wheat roots, CAT was used to eliminate H2O2. Possible due to the crucial role that ROS play in normal root growth (Foreman et al. 2003), CAT by itself reduced growth of control roots to 52% of the growth without the scavenger (Table 1). However, exogenously added CAT recovered root and shoot growth in SNP-treated plants (Table 1), restoring 60% of root length and 95% of shoot elongation respect to the C treatment devoid of CAT. The almost complete inhibition of O2·- production in SNP or Spm-treated roots was evidently reversed when CAT was added simultaneously with the respective treatment, suggesting a direct engagement of H2O2 in the inhibition of O2·- formation, possible due to a direct effect on superoxide forming enzyme (Fig. 2). In addition, CAT partially reduced DAB staining derived from H2O2 formation under SNP treatment (Fig. 4b), where plant growth was strikingly recovered. In contrast, in Spm-treated roots, CAT did not revert the effect produced by the polyamine on H2O2 formation and did not restore root elongation (Table 1), although it recovered superoxide anion production. These results could suggest that in Spm treatment, plant growth remained inhibited due to the high levels of H2O2 still present in these roots or due to the additional NO effect on root growth inhibition (Figs. 2 and 4a, b).
To evaluate if NO was involved in root-inhibited elongation upon SNP or PA treatments, we tested the simultaneous addition of SNP, Put, Spd, or Spm with cPTIO (Table 1). The recovery of root length was 64, 26, 23, and 54%, in SNP-, Put-, Spd-, or Spm-treated plants, respectively, compared to the plants exposed to PAs or SNP without cPTIO. Shoot growth was restored 41% in SNP + cPTIO treated plants respect to SNP without the scavenger (Table 1), whereas in all PA treatments with cPTIO, shoot growth was not modified compared to the respective treatments without cPTIO.
In roots treated with SNP or Spm plus cPTIO, the increase in NO formation was visible reduced (Fig. 3b), O2·- formation revealed with NBT staining was evidently restored, and H2O2 formation diminished, accompanying the growth recovery observed under those treatments. Plants treated with Put or Spd plus cPTIO showed a similar result in growth restoration (Table 1), suggesting that NO was engaged in both O2·- and H2O2 generation and consequently, in root elongation (Figs. 2 and 4a, b).
Effect of PAO and DAO modulators on root and shoot elongation and ROS formation
With the aim to confirm if the level of ROS linked to the reduction of root elongation were at least in part, a result of PA catabolism, we checked the effect of DAO and PAO modulators on root and shoot elongation by adding AG (an amino oxidase inhibitor) or 1,8-DO (a PAO inhibitor), to the growth medium simultaneously with SNP or PAs treatments (Table 1). In the absence of exogenous PAs or SNP addition, both DAO and PAO inhibitors slightly increased root or shoot length, as compared to the control (Table 1). When AG or 1,8-DO were used with SNP, root elongation was recovered to 86 and 53% of the control, respectively, whereas shoot growth was restored to equal or more than 100%, compared to control seedlings (grown without inhibitors). Both AG and 1,8-DO recovered Put-treated roots length to 78 and 85% of the control values, whereas the inhibition of root elongation was not reversed when plants were treated simultaneously with the specific PAO inhibitor and 1 mM Spm (Table 1).
A strong correlation was observed between root growth restoration and development of NBT staining after treating roots with SNP + AG or SNP + 1,8-DO (Table 1, Fig. 3). Seedlings treated with AG + Put showed growth recovery and maintained NBT staining to similar intensity than Put-treated plants, but 1,8-diaminoctane and AG did not exert any visible effect when they were added together with Spd or Spm. However, roots treated with Spm plus the inhibitors showed a visible blue color compared to the lack of blue staining in Spm-treated roots. The reduction of the brown staining due to H2O2-derived DAB deposition in AG-treated roots could be related to the inhibition of DAO and the consequent decay in H2O2 formation in PAs-treated roots (Fig. 4b).
Calcium was involved in Spm-inhibited O2 ·- formation
In several plant systems, O2·- generation by a NADPH oxidation activity was shown to be stimulated by Ca2+ availability, so calcium was supplied to the medium together with SNP or Spm to analyze if this inhibition was due to a SNP- or PA-induced decrease in Ca2+ availability (Table 1). The addition of 2 mM CaCl2 partially restored root growth inhibition produced by Spm treatment (to 43% of the C values and almost 50% over the root length compared to Spm-treated roots) (Table 1) as well as NBT staining (Fig. 2), revealing that Ca2+ accessibility was essential for NADPH-dependent superoxide anion formation and root growth. Moreover, DAB intensity was reduced in Spm + CaCl2 compared to Spm-treated roots, denoting a lower H2O2 formation. A similar, though less pronounced result, was observed in Put- or Spd-treated roots respect to root growth recovery and DAB staining (Table 1, Fig. 4). However, neither root elongation nor NBT or DAB staining was modified in SNP + CaCl2-treated roots compared to SNP-treated plants. Root length was increased by almost 30% upon CaCl2 addition in untreated C plants (Table 1).
Hydrogen peroxide and SNP inhibited seedlings growth in a dose-dependent manner
Because H2O2 as well as NO were undoubtedly involved in the restriction of root and shoot elongation in wheat seedlings treated with the NO donor SNP or PAs, we analyzed if this result could be simulated using a pharmacological modulation of seedlings growth through the exogenous addition of increasing concentrations of H2O2 or SNP to 2-day-old wheat seedlings (Fig. 5a). Hydrogen peroxide did not produce any effect added at 0.5 mM but reduced 20% the length of shoots when was supplied at 1 mM, without changes in root growth as compared to the control (Fig. 5a). However, from concentrations higher than 1 mM, a notable reduction both in root or shoot elongation compared to the controls was observed, indicating that root growth inhibition was a direct consequence of H2O2 toxicity. The highest H2O2 concentrations used (5 and 10 mM) produced a comparable degree of inhibition to that observed in SNP- or Spm-treated plants (Fig. 5a). A similar result was observed using SNP as NO donor in a concentration-dependent manner (Fig. 5b). SNP reduced root elongation from 20% with 1 μM SNP to 70% with 100 μM SNP compared to the control without SNP, and inhibited shoot growth only with 50 and 100 μM SNP (24 and 40%, respectively) (Fig. 5b). Seedlings treated with an SNP solution that was left open under light for 72 h were used as control of NO treatment and did not show differences compared to control seedlings (data not shown).
Effect of exogenous addition of increasing a H2O2 or b SNP concentrations on wheat root and shoot length. Wheat seeds were germinated on wet paper for 48 h. After this time, seedlings were transferred for 5 days to a hydroponic system with Hoagland solution containing increasing H2O2 or SNP concentrations, as indicated in the figure. The figure is representative of three different experiments with five replicated measurements. c NBT and DAB staining of roots segments from seedlings grown at increasing H2O2 or d SNP concentrations
Superoxide and H2O2 formation, visualized using NBT and DAB, respectively, also revealed an increasing H2O2 and decreasing O2·- formation in root tissues, as H2O2 (Fig. 5c) or SNP (Fig. 5d) concentrations raised in the incubation medium, suggesting that the observed seedling growth inhibition was concomitant with a H2O2-dependent inhibition of a NOX type-dependent O2·- formation as well as to NO levels.
Antioxidant enzyme activity and lipid peroxidation
Superoxide dismutase activity, an important source of superoxide anion-derived H2O2 in plant cells, did not show changes compared to C roots under SNP, Put or Spm exposure and was slightly increased (20%) in Spd-treated roots (Table 2). Analysis of CAT showed that the enzyme activity was 54 and 43% reduced in Spd- and Spm-treated roots, respectively, whereas GPOX activity was severely decreased by SNP (to 72% below the control) and 47% reduced compared to C in roots treated with Spm (Table 2). To check if root growth reduction was related to oxidative damage produced to membrane lipids, TBARS content was measured (Table 2). The magnitude of the oxidative damage was variable, depending on the treatment and organ. Spermine and SNP did not affect lipid peroxidation in roots compared to C, though they produced a dramatic root growth inhibition and high H2O2 levels (Table 2). However, Put, which was the PAs that less affected growth, raised lipid peroxidation by 32% in roots, whereas Spd increased TBARS by 49% over the C (Table 2).
Discussion
The last years, several works have reported the central role of PAs in stress tolerance. However, despite high concentration of these molecules either endogenously elevated by a stress condition or exogenously added, can seriously modify plant growth, there is scarce information regarding the mechanisms associated to PAs functions. In the present work, PA treatments caused a variable reduction of root and shoot elongation in wheat plants, being Spm the one which produced the most dramatic effects. These results corroborated previous reports from our group (Groppa et al. 2008) and are in accordance with those reported by de Agazio et al. (1995), who showed that exogenously applied Spd elicited several physiological, histochemical and biochemical effects in roots of young maize seedlings, including the decrease in growth rate of primary roots and the increased activities of PAO and peroxidase. Tisi et al. (2011) has also documented that Spd, in a range from 10 μM to 1 mM, strongly inhibited root growth by the modulation of cell elongation in 5-day-old plants of maize, whereas in Arabidopsis plants under non-stressed conditions, 1 mM PAs including Spm did not have any effects on growth of WT or Spm-deficient mutant plants (Yamaguchi-Shinozaki and Shinozaki 2006). This picture of variable results that come from different plants species treated with PAs led us to focus the attention in ROS or NO as the main signaling molecules involved in growth reduction after PAs treatments, especially in roots. In this scenario, the results obtained in PAs-treated plants were compared with those observed in SNP- treated plants, in view that PAs produce NO by still unknown mechanisms.
There is evidence indicating that NOX activity is required for cell expansion and elongation during shoot or root morphogenesis (Liszkay et al. 2004), and are essential in plant development, considering that mutants in various Atrboh genes are affected in growth and development (Jones et al. 2007). It has been reported that Spd and Spm inhibit NADPH oxidase and activity in human neutrophils (Ogata et al. 1996), and Spd also completely inhibits the activity of microsomal NADPH oxidase during chilling injury of cucumber (Shen et al. 2000). Because O2·- generation seems to be a critical process for root growth, the widely used histochemical specific probes NBT and DAB were used to reveal the appearance of O2·- and H2O2, respectively, in the root growing zone. The severe growth inhibition caused by SNP and Spm treatment was accompanied by a clear attenuation of NBT staining and DHE oxidation, suggesting that growth reduction was, at least in part, directly related to O2·- formation. The roots of Arabidopsis mutant plants impaired in rhd2 function have decreased levels of ROS and are 20% shorter than the wild type, indicating that cell expansion is defective in these plants (Foreman et al. 2003).
In several other species (e.g., Lycopersicon esculentum, Glycine max, Arabidopsis), histochemical staining confirmed that apoplastic ROS production in the growing zone of roots represents a common feature of seed plants (Liszkay et al. 2004). Surprisingly, there was no correlation between the high H2O2 accumulation observed in SNP- or Spm-treated wheat roots and the severe inhibition of O2·- production, indicating that H2O2 did not come directly from a NADPH oxidation activity. On the other hand, though NBT staining revealed high levels of O2·- anions in roots treated with Put or in control plants, H2O2 accumulation was not detectable (Fig. 4a, b). This lack of correspondence in the way leading from O2·- to H2O2 could be attributed, on one hand, to the crucial ROS balance that modulates growth and is regulated through the concerted activity of the antioxidant system, but on other hand to the presence of other ROS sources different from NADPH oxidase or SOD, located in other subcellular compartments inside the cell. Among others, PAs catabolic enzymes CuAOs or PAOs in the apoplast could be contributing to this result. The pharmacological assay applying increasing concentrations of H2O2 or the NO donor SNP to 2 day-old wheat seedlings produced a concomitant and dose-dependent reduction of root elongation, implying that both molecules were involved in PAs-related growth inhibition.
In tobacco leaves, NADPH oxidase activity was strongly inhibited by Put, Spd, and Spm, in a concentration-dependent manner and at concentrations considerably higher than those used in this work (2.5 or 5 mM) (Papadakis and Roubelakis-Angelakis 2005). A similar result was demonstrated by Iannone et al. (2013) in tobacco leaf discs, where the activity of the enzyme was strongly inhibited by the three PAs, in a concentration-dependent manner. Conversely, Spd led to a significant increase of O2·− via the activation of the NADPH oxidase in Arabidopsis plants, providing strong evidence for the participation of the NADPH-oxidase in the Spd-induced ROS accumulation (Andronis et al. 2014). In wheat roots, plants treated with Spd showed a clear correlation between O2·- and H2O2 levels, as was indicated using NBT or DAB staining. Hydrogen peroxide production is correlated with the stiffening of cell walls as growth ceases in many cell types (Gapper and Dolan 2006). To verify if H2O2 accumulation in SNP- or Spm-treated roots was implicated in the inhibition of root elongation, a pharmacological approach using CAT to eliminate H2O2 was used. Catalase had a clear behavior in the reversion of the SNP inhibitory effect on root and shoot growth, confirming that H2O2 was undoubtedly involved in the reduction of root length. However, the reduction in root elongation observed in Spm-treated roots was not reversed using CAT, probably due to even higher H2O2 levels formed in these tissues, and due to the presence of other signaling molecules, as NO, that were also acting as inhibitors of root elongation. In this sense, the addition of cPTIO strongly reduced DAB staining in Spm-treated roots (Fig. 4b) and, thus, recovered root length (Table 1). Besides, NBT staining was partially recovered in Spm + CAT treated roots compared to Spm-treated roots, which suggests that certain amount of H2O2, that was inhibiting O2·− formation, was eliminated, but the balance O2·-/H2O2 still remained inadequate to restore growth.
It is widely recognized that many stresses are associated with accumulation of ROS and PAs. In addition, export of PAs to the apoplast, after being taken up by the root tissues and catabolized by amineoxidases (CuAOs and PAOs), produced H2O2 and other ROS including hydroxyl radicals (HO·) (Angelini et al. 2010; Pottosin et al. 2014). Moschou et al. (2008) have shown that exogenous application of Spd to tobacco plants leads to an important raise in H2O2 content generated by the action of PAO, but no comments about growth was reported. In tobacco leaf discs, 1 and 5 mM of Put, Spd or Spm greatly increased H2O2 as well as PAO activity (Iannone et al. 2013), while Diao et al. (2017) reported that during chilling stress, the application of Spd and Spm (but not Put), elevated NO and H2O2 levels in tomato plants. The use of AG and 1,8-DO, recognized inhibitors of CuAO or PAO activity respectively, induced a remarkable recovery in the inhibition of root and shoot length in SNP treated plants, as well as a visible reduction in DAB staining and a recovery of O2·− formation. These results suggest that an increased activity of both catabolic H2O2-producing enzymes induced by SNP-derived NO led to an increased H2O2 formation which, concomitantly to NO released from SNP, reduced root and shoot elongation due to a simultaneous oxidative and nitrosative effect. In addition, as AG has been proposed as an inhibitor of the animal inducible nitric oxide synthase (Griffiths et al. 1993), a reduction in NO formation that contributed to inhibition of root elongation could not be discarded. The PAO inhibitor 1,8-DO used together with Spm neither reverse the inhibition of root or shoot length nor NBT staining. It could be possible that H2O2 remained high in Spm-treated roots due to CAT and GPOX decreased activities or because both DAO and PAO enzymes were present in wheat plants, H2O2 was being produced mainly by DAO so DAO inhibition by AG had a greater effect in the reduction of H2O2 formation, as it is shown in Fig. 4b. However, NO was at least partially responsible for Spm-induced inhibition of root length, as a significant recovery in root elongation was observed when cPTIO was added together with Spm.
It is well-known that NADPH oxidation activity localized in the tips of growing root hairs can be modulated by cytosolic Ca2+ (Gupta et al. 2016; Takeda et al. 2008). An interplay exists between PAs and ROS that causes a variation in intracellular Ca2+ concentration by modifying both Ca2+ influx and efflux transport systems at the root cell plasma membrane (Pottosin et al. 2012, 2014; Zepeda-Jazo et al. 2011). Roots and shoots of Spm-treated plants recovered 50% of length when Ca2+ was exogenously added, NBT staining was restored and H2O2 levels decreased compared to treatments without Ca2+. Put and Spd-treated plants also partially recovered shoot growth when CaCl2 was added. This result suggested that PAs, particularly Spm, could inhibit Ca2+ channels inducing a decrease in Ca2+ influx, thereby inhibiting a NOX-dependent O2·- formation. However, SNP-treated plants not only did not show any growth restoration upon CaCl2 treatment but also O2·- staining was completely abolished and DAB-dependent H2O2 staining remained elevated, denoting that a different mechanism, probably not involving NADPH oxidation activity but DAO or PAO activities for H2O2 formation was operating in root growth reduction.
Considering that NO is a molecule related to PAs by a common precursor arginine, and that previous reports indicated that PAs can be a source of NO by a still unknown pathway (Groppa et al. 2008; Tun et al. 2006), we explored if NO was involved in PAs-derived root growth inhibition in a complex network with O2·- and H2O2 using cPTIO to trap NO. When cPTIO was included simultaneously with the respective treatment, a remarkable reversion in the inhibition of root elongation was observed, particularly in SNP or Spm treated roots, demonstrating the involvement of this signaling molecule in root elongation, as was previously reported for wheat roots (Groppa et al. 2008), or in sunflower root architecture (Corti Monzón et al. 2014). Likewise, the addition of cPTIO evidently reversed the inhibition of NBT staining as well as DAB precipitation in roots of SNP or Spm-wheat treated roots and restored root elongation, sustaining the fact that a balance between O2·- and H2O2 are necessary for growth and this balance could be altered by NO, possibly through the inhibition of a NADPH oxidation activity. Although it is well established that H2O2 induces the synthesis and accumulation of NO, it has been suggested that NO also modulates H2O2 levels (He et al. 2005; Li et al. 2009). Correa-Aragunde et al. (2004, 2015) reported that NO plays an important role during lateral and adventitious root formation in tomato plants and an acute ROS burst induces NO and other RNS formation that could affect enzymes of the antioxidant system like ascorbate peroxidase, by simultaneous carbonylation, tyrosine nitration, and S-nitrosylation that would irreversibly affect its activity (de Pinto et al. 2013; Tanou et al. 2014), thus modifying the antioxidant status of the plant and consequently altering root growth. Most probably, H2O2 functions as a cofactor in endogenous NO synthesis. Thus, it can be clearly said that both of their production pathways are inter-related and can regulate production of each other. The possible multi-step interactions between ROS, PAs, and NO showed a complex scenario to be analyzed.
In view of the values recorded for lipid peroxidation and the behavior of the antioxidant enzymes, it seems that ROS levels, particularly H2O2, depended not only on PAs catabolism but was also regulated through the activity of H2O2 detoxifying enzymes CAT and GPOX. The strong decay in GPOX activity in SNP-treated roots and CAT and GPOX activities in Spm-treated roots allowed H2O2 to be accumulated. Surprisingly, no lipid peroxidation measured as TBARS was detected in roots, suggesting that the inhibition of root elongation was not associated to oxidative damage in these treatments. On the contrary Put neither modified CAT or GPOX activities nor increased H2O2 content over the controls, while Spd-induced lipid oxidation correlated well with a remarkably decrease in CAT and an increased in SOD activities compared to controls. In tobacco leaf discs, PAs showed a variable effect, decreasing or increasing the activity of H2O2 detoxifying enzymes according to the PA concentration and exposure time (Iannone et al. 2013). Liu et al. (2017) reported that exogenous application of 0.1 mM Put, Spd, or Spm resulted in enhanced activities of SOD, CAT, APX, and GR in centipedegrass, but in conditions of drought stress. Moreover, treatment with polyamines increased activities of antioxidant enzymes and reduces oxidative damages in Cicer arietinum L. (Nayyar and Chander 2004), Brassica juncea (Verma and Mishra 2005), and white clover (Peng et al. 2016) but in every case, an abiotic stress condition was additional to PAs application. In this case, other ROS or NO sources are active due to the stress situation, thus PAs might be acting by other ways. The complex scenario observed in relation to ROS formation and degradation, oxidative damage and root growth inhibition suggested that other factors beyond ROS are associated to growth inhibition. H2O2-induced toxicity and the activity of the detoxifying enzymes might depend on multiple factors related to the subcellular localization of ROS and enzymes, PAs export and catabolization, as well as formation, degradation and transport of different PAs-derived ROS (Pottosin et al. 2014).
The maintenance of ROS homeostasis is crucial for plant development and the root apex is a zone of active ROS production (Zinta et al. 2016). If ROS are involved in basic cell growth, then they might be expected to influence or be influenced by phytohormones or other growth-related molecules like PAs or NO. The dual role of PAs in the regulation of cell growth and cell death as well as the involvement of PAs, H2O2 and NO in plant differentiation events and PCD signaling keeps on the still unresolved question whether amine oxidases play their roles mainly through modulation of PAs homeostasis or H2O2/ NO production via PAs catabolism (Tisi et al. 2011). This work demonstrates that a complex interaction among PAs-derived ROS and NO is taking place after the exogenous addition of PAs to wheat plants, triggering a plethora of events at different cellular levels that led to growth inhibition. Further studies addressing the cellular and molecular mechanisms underlying the intriguing crosstalk between PAs-derived ROS or NO will be essential to identify their roles in plant growth.
Abbreviations
- AG:
-
Aminoguanidine
- 1,8-DO:
-
1,8-diaminoctane
- SNP:
-
Sodium nitroprusside
References
Alcázar R, Tiburcio AF (2014) Plant polyamines in stress and development: an emerging area of research in plant sciences. Front Plant Sci 5:319
Alcázar R, García-Martínez JL, Cuevas JC, Tiburcio AF, Altabella T (2005) Overexpression of ADC2 in Arabidopsis induces dwarfismand late-flowering through GA deficiency. Plant J 43:425–436
Alcázar R, Cuevas JC, Planas J, Zarza X, Bortolotti C, Carrasco P, Salinas J, Tiburcio AF, Altabella T (2011) Integration of polyamines in the cold acclimation response. Plant Sci 180:31–38
Andronis EA, Moschou PN, Toumi I, Roubelakis-Angelakis KA (2014) Peroxisomal polyamine oxidase and NADPH-oxidase cross-talk for ROS homeostasis which affects respiration rate in Arabidopsis thaliana. Front Plant Sci 5:1–10
Angelini R, Cona A, Federico R, Fincato P, Tavladoraki P, Tisi A (2010) Plant amine oxidases “on the move”: an update. Plant Physiol Biochem 48:560–564
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Becana M, Aparicio-Tejo P, Irigoyen JJ, Sánchez-Díaz M (1986) Some enzymes of hydrogen peroxide metabolism in leaves and root nodules of Medicago sativa. Plant Physiol 82:1169–1171
Beligni MV, Lamattina L (2001) Nitric oxide in plants: the history is just beginning. Plant Cell Environ 24:267–278
Carol RJ, Dolan L (2006) The role of reactive oxygen species in cell growth: lessons from root hairs. J Exp Bot 57:1829–1834
Chance B, Sies H, Boveris A (1979) Hydroperoxide metabolism in mammalian organs. Physiol Rev 59:527–605
Correa-Aragunde N, Graziano M, Lamattina L (2004) Nitric oxide plays a central role in determining lateral root development in tomato. Planta 218:900–905
Correa-Aragunde N, Foresi N, L L (2015) Nitric oxide is a ubiquitous signal for maintaining redox balance in plant cells: regulation of ascorbate peroxidase as a case study. J Exp Bot 66:2913–2921
Corti Monzón G, Pinedo M, Di Rienzo J, Novo-Uzal E, Pomar F, Lamattina L, de la Canal L (2014) Nitric oxide is required for determining root architecture and lignin composition in sunflower. Supporting evidence from microarray analyses. Nitric Oxide 39:20–28
de Agazio M, Grego S, Ciofi-Luzzatto A, Rea E, Zaccaria ML, Federico R (1995) Inhibition of maize primary root elongation by spermidine: effect on cell shape and mitotic index. Plant Growth Regul 14:85–89
de Pinto MC, Locato V, Sgobba A, Romero-Puertas M, Gadaleta C, Delledonne M, De Gara L (2013) S-nitrosylation of ascorbate peroxidase is part of programmed cell death signaling in tobacco Bright Yellow-2 cells. Plant Physiol 163:1766–1775
Diao Q, Song Y, Shi D, Qi H (2017) Interaction of polyamines, abscisic acid, nitric oxide, and hydrogen peroxide under chilling stress in tomato (Lycopersicon esculentum Mill.) seedlings. Front Plant Sci 8:203
Dunand C, Crèvecoeur M, Penel C (2007) Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: possible interaction with peroxidases. New Phytol 174:332–341
Farnese FS, Menezes-Silva PE, Gusman GS, Oliveira JA (2016) When bad guys become good ones: the key role of reactive oxygen species and nitric oxide in the plant responses to abiotic stress. Front Plant Sci 7:471
Fincato P, Moschou PM, Ahou A, Angelini R, Roubelakis-Angelakis KA, Federico R, Tavladoraki P (2012) The members of Arabidopsis thaliana PAO gene family exhibit distinct tissue- and organ-specific expression pattern during seedling growth and flower development. Amino Acids 42:831–841
Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, Linstead P, Costa BC, Jones JD, Davies JM, Dolan L (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422:442–446
Foyer CH, Noctor G (2009) Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxid Redox Signal 11(4):861–905
Francoz E, Ranocha P, Nguyen-Kim H, Jamet E, Burlat V, Dunand C (2015) Roles of cell wall peroxidases in plant development. Phytochemistry 112:15–21
Gapper C, Dolan L (2006) Control of plant development by reactive oxygen species. Plant Physiol 141:341–345
Griffiths MJ, Messent M, MacAllister RJ, Evans TW (1993) Aminoguanidine selectively inhibits inducible nitric oxide synthase. Br J Pharmacol 110:963–968
Groppa MD, Rosales EP, Iannone MF, Benavides MP (2008) Nitric oxide, polyamines and Cd-induced phytotoxicity in wheat roots. Phytochemistry 69:2609–2615
Gupta K, Dey A, Gupta B (2013) Plant polyamines in abiotic stress responses. Acta Physiol Plant 35:2015–2036
Gupta K, Sengupta A, Chakraborty M, Gupta B (2016) Hydrogen peroxide and polyamines act as double edged swords in plant abiotic stress responses. Front Plant Sci 7:1343
He JM, Xu H, She XP, Song XG, Zhao WM (2005) The role and the interrelationship of hydrogen peroxide and nitric oxide in the UV-B-induced stomatal closure in broad bean. Funct Plant Biol 32:237–247
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. Arch Biochem Biophys 25:189–198
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Calif Agric Exp Stn Circ 347:1–32
Iannone MF, Rosales EP, Groppa MD, Benavides MP (2013) H2O2 involvement in polyamines-induced cell death in tobacco leaf discs. J Plant Growth Regul 32:745–757
Jones RD, Hancock JT, Morice AH (2000) NADPH oxidase: a universal oxygen sensor? Free Rad Biol Med 29:416–424
Jones MA, Raymond MJ, Yang Z, Smirnoff N (2007) NADPH oxidase-dependent reactive oxygen species formation required for root hair growth depends on ROP GTPase. J Exp Bot 58:1261–1270
Joo JH, Bae YS, Lee JS (2001) Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol 126(3):1055–1060
Lamattina L, Garcia-Mata C, Graziano M, Pagnussat G (2003) Nitric oxide: the versatility of an extensive signal molecule. Ann Rev Plant Biol 54:109–136
Li JH, Liu YQ, Lü P, Lin HF, Bai Y, Wang XC, Chen YL (2009) A signaling pathway linking nitric oxide production to heterotrimeric G protein and hydrogen peroxide regulates extracellular calmodulin induction of stomatal closure in Arabidopsis. Plant Physiol 150:114–124
Liszkay A, van der Zalm E, Schopfer P (2004) Production of reactive oxygen intermediates (O2•-, H2O2, and •OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiol 136:3114–3123
Liu M, Chen J, Guo Z, Lu S (2017) Differential responses of polyamines and antioxidants to drought in a centipede grass mutant in comparison to its wild type plants. Front Plant Sci 8:792
Maehly AC, Chance B (1954) The assay of catalase and peroxidase. Meth Biochem Anal 1:357–424
Minocha R, Majumdar R, Minocha SC (2014) Polyamines and abiotic stress in plants: a complex relationship. Front Plant Sci 5:175
Montiel J, Arthikala MK, Quinto C (2012) Phaseolus vulgaris RbohB functions in lateral root development. Plant Signal Behav 8:144–146
Moreau M, Lee GI, Wang Y, Crane BR, Klessig DF (2008) AtNOS/AtNOA1 is a functional Arabidopsis thaliana cGTPase and not a nitric-oxide synthase. J Biol Chem 283:32957–32967
Moschou P, Paschalidis KA, Delis ID, Andriopoulou AH, Lagiotis GD, Yakoumakis DI, Roubelakis-Angelakis KA (2008) Spermidine exodus and oxidation in the apoplast induced by abiotic stress is responsible for H2O2 signatures that direct tolerance responses in tobacco. Plant Cell 20:1708–1724
Nayyar H, Chander S (2004) Protective effects of polyamines against oxidative stress induced by water and cold stress in chickpea. J Agron Crop Sci 190:355–365
Ogata K, Nishimoto N, Uhlinger DJ, Igarashi K, Takeshita M, Tamura M (1996) Spermine suppresses the activation of human neutrophil NADPH oxidase in cell-free and semi-recombinant systems. Biochem J 313:549–554
Pagnussat GC, Lanteri ML, Lamattina L (2003) Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiol 132:1241–1248
Papadakis K, Roubelakis-Angelakis KA (2005) Polyamines inhibit NADPH oxidase-mediated superoxide generation and putrescine prevents programmed cell death induced by polyamine oxidase-generated hydrogen peroxide. Planta 220:826–837
Peng D, Wang X, Li Z, Zhang Y, Peng Y, Li Y (2016) NO is involved in spermidine-induced drought tolerance in white clover via activation of antioxidant enzymes and genes. Protoplasma 253:1243–1254
Planas-Portell J, Gallart M, Tiburcio AF, Altabella T (2013) Copper-containing amine oxidases contribute to terminal polyamine oxidation in peroxisomes and apoplast of Arabidopsis thaliana. BMC Plant Biol 13:109
Pottosin I, Shabala S (2014) Polyamines control of cation transport across plant membranes: implications for ion homeostasis and abiotic stress signaling. Front Plant Sci 5:154
Pottosin I, Velarde-Buendía AM, Zepeda-Jazo I, Dobrovinskaya O, Shabala S (2012) Synergism between polyamines and ROS in the induction of Ca2+ and K+ fluxes in roots. Plant Signal Behav 7:1084–1087
Pottosin I, Velarde-Buendía AM, Bose J, Zepeda-Jazo I, Shabala S, Dobrovinskaya O (2014) Cross-talk between reactive oxygen species and polyamines in regulation of ion transport across the plasma membrane: implications for plant adaptive responses. J Exp Bot 65:1271–1283
Rodríguez-Serrano M, Romero-Puertas MC, Pazmino DM, Testillano TM, Risueño MC, del Río LA, Sandalio LM (2009) Cellular response of pea plants to cadmium toxicity: cross talk between reactive oxygen species, nitric oxide and calcium. Plant Physiol 150:229–243
Rosales EP, Iannone MF, Groppa MD, Benavides MP (2012) Polyamines modulate nitrate reductase activity in wheat leaves: involvement of nitric oxide. Amino Acids 42:857–865
Sagi M, Fluhr R (2001) Superoxide production by plant homologues of the gp91phox NADPH oxidase: modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiol 126:1281–1290
Sergiev I, Alexieva V, Karanov E (1997) Effect of spermine, atrazine and combination between them on some endogenous protective systems and stress markers in plants. Compt Rend Acad Bulg Sci 51:121
Shen W, Nada K, Tachibana S (2000) Involvement of polyamines in the chilling tolerance of cucumber cultivars. Plant Physiol 124:431–439
Suzuki N, Miller G, Morales J, Shulaev V, Torres MA, Mittler R (2011) Respiratory burst oxidases: the engines of ROS signaling. Curr Opin Plant Biol 14:691–699
Takahashi T, Kakehi J (2010) Polyamines: ubiquitous polycations with unique roles in growth and stress responses. Ann Bot 105:1–6
Takeda S, Gapper C, Kaya H, Bell E, Kuchitsu K, Dolan L (2008) Local positive feedback regulation determines cell shape in root hair cells. Science 319:1241–1244
Tanou G, Ziogas V, Belghazi M, Christou A, Filippou P, Job D, Fotopoulos V, Molassiotis A (2014) Polyamines reprogram oxidative and nitrosative status and the proteome of citrus plants exposed to salinity stress. Plant Cell Environ 37:864–885
Tavladoraki P, Rossi MN, Saccuti G, Perez-Amador MA, Polticelli F, Angelini R, Federico R (2006) Heterologous expression and biochemical characterization of a polyamine oxidase from Arabidopsis involved in polyamine back conversion. Plant Physiol 141:1519–1532
Thordal-Christensen H, Zhang ZG, Wei YD, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11:1187–1194
Tiburcio F, Altabella T, Bitrián M, Alcázar R (2014) The roles of polyamines during the lifespan of plants: from development to stress. Planta 240:1–18
Tisi A, Federico R, Moreno S, Lucretti S, Moschou PN, Roubelakis-Angelakis KA, Angelini R, Cona A (2011) Perturbation of polyamine catabolism can strongly affect root development and xylem differentiation. Plant Physiol 157:200–215
Tun NN, Santa-Catarina C, Begum T, Silveira V, Handro W, Floh EI, Scherer GF (2006) Polyamines induce rapid biosynthesis of nitricoxide (NO) in Arabidopsis thaliana seedlings. Plant Cell Physiol 47:346–354
Van Gestelen P, Asard H, Caubergs RJ (1997) Solubilization and separation of a plant plasma membrane NADPH-O2 synthase from other NAD(P)H oxidoreductases. Plant Physiol 115:543–550
Velarde-Buendía M, Shabala S, Cvikrova M, Dobrovinskaya O, Pottosin I (2012) Salt-sensitive and salt-tolerant barley varieties differ in the extent of potentiation of the ROS-induced K+ efflux by polyamines. Plant Physiol Biochem 61:18–23
Verbelen JP, De Cnodder T, Le J, Vissenberg K, Baluška F (2006) The root apex of Arabidopsis thaliana consists of four distinct zones of growth activities: meristematic zone, transition zone, fast elongation zone and growth terminating zone. Plant Signal Behav 1:296–304
Verma S, Mishra SN (2005) Putrescine alleviation of growth in salt stressed Brassica juncea by inducing antioxidative defense system. J Plant Physiol 162:669–677
Wimalasekera R, Tebartz F, Scherer GFE (2011) Polyamines, polyamine oxidases and nitric oxide in development, abiotic and biotic stresses. Plant Sci 181:593–603
Wojtasik W, Kulma A, Namysl K, Preisner M, Szopa J (2015) Polyamine metabolism in flax in response to treatment with pathogenic and non-pathogenic fusarium strains. Front Plant Sci 6(291)
Yamaguchi-Shinozaki K, Shinozaki K (2006) Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Ann Rev Plant Biol 57:781–803
Zepeda-Jazo I, Velarde-Buendía AM, Enrıquez-Figueroa R, Bose J, Shabala S, Muñiz-Murguía J, Pottosin I (2011) Polyamines interact with hydroxyl radicals in activating Ca2+ and K+ transport across the root epidermal plasma membranes. Plant Physiol 157:2167–2180
Zinta G, Khan A, AbdElgawad H, Verma V, Srivastava AK (2016) Unveiling the redox control of plant reproductive development during abiotic stress. Front Plant Sci 7:700
Funding
This work was supported by grants from the Universidad de Buenos Aires, Argentina (UBACYT 20020130100178BA), from Consejo Nacional de Investigaciones Científicas y Técnicas (PIP 266, IQUIFIB-CONICET, Argentina) and ANPCyT (Agencia Nacional de Promoción Científica y Tecnológica, Argentina). L.R is a Universidad de Buenos Aires fellow, A.V is an CONICET fellow, and M.D.G and M.P.B are career investigators from CONICET.
Author information
Authors and Affiliations
Contributions
L.R and A.V performed experimental work; L.R, M.D.G and M.P.B performed data analysis, L.R and M.P.B oversaw project planning; L.R, M.D.G and M.P.B wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Bhumi Nath Tripathi
Electronic supplementary material
Supplemental Fig. 1
Root growth of wheat plants after 5 d in the respective treatment as it is indicated in the picture: 0.1 mM SNP; 1 mM Put, Spd or Spm, with the addition of ROS, NO or PAs modulators: 1000 U ml-1 CAT; 200 μM cPTIO; 0.5 mM AG; 0.3 mM 1,8 OD; 2 mM CaCl2. The figure is representative of five different experiments (PPTX 3674 kb)
Rights and permissions
About this article
Cite this article
Recalde, L., Vázquez, A., Groppa, M.D. et al. Reactive oxygen species and nitric oxide are involved in polyamine-induced growth inhibition in wheat plants. Protoplasma 255, 1295–1307 (2018). https://doi.org/10.1007/s00709-018-1227-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-018-1227-z