Abstract
The lysosome is a membrane-bound organelle involved in the turnover of various intracellular and extracellular macromolecules. These are degraded by acidic hydrolases in the lumen of lysosome. The lysosomal membrane is important not only in retaining the acidic hydrolases to protect cells against cytosolic proteolysis, but it also facilitates protein trafficking though organelle fusion. In this study, we report on a novel lysosomal membrane protein transmembrane 6 superfamily 1 (Tm6sf1). Expression of Tm6sf1-DsRed fusion proteins in HEK293A cells displayed punctate or ringlike vesicles, which colocalized with conventional lysosome markers including LAMP1/2, RAB7, and Rnf167. Using fluorescence time-lapse live cell imaging, we demonstrated the fusion of Tm6sf1 vesicles with lysosomes and the integration of Tm6sf1 into the lysosomal membrane. We also examined the expression of Tm6sf1 in mouse tissues and found immunopositive signals in major organs such as the cerebellum, kidney, and intestine. These data suggest that Tm6sf1 is a widely expressed lysosomal transmembrane protein and can be used as a novel marker of lysosome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The lysosome is a highly acidic membrane-bound organelle involved in the turnover of both intracellular and extracellular macromolecules. More than 50 acidic hydrolases are contained in the lysosome for the purpose of cellular component digestion. To prevent the leakage of these hydrolases and protons that may damage the surrounding cytosolic environment (cytosolic acidification and proteolysis) and thus inducing cell death (Boya 2012; Schwake et al. 2013), a lysosomal membrane that bears various highly glycosylated integral proteins is required to withstand the luminal acidic microenvironment. Furthermore, the lysosomal membrane is an important bridge for interacting with target organelles, such as endosomes and autophagosomes, thereby facilitating effective intracellular protein trafficking (Luzio et al. 2007; Saftig and Klumperman 2009; Boya 2012; Schwake et al. 2013).
A number of lysosomal membrane components, including membrane receptors, molecular transporters, and structural proteins, have been identified in recent proteomic studies (Callahan et al. 2009; Lubke et al. 2009; Schroder et al. 2010; Schwake et al. 2013). However, some low abundant lysosomal membrane constituents may be under the level of detection when using standard proteomic methods. Besides, the hydrophobic properties of lysosomal membrane proteins may hinder their purification and subsequent functional analysis. Impurities in lysosomal fractions may also desensitize the identification of membrane proteins by mass spectrometry (Schroder et al. 2010). For these reasons, many lysosomal membrane proteins have yet to be identified.
When we investigated the function of LIM-homeodomain transcription factor Lhx1 during embryonic kidney development, we found that Transmembrane 6 superfamily 1 (Tm6sf1) is downregulated 7.4-fold in the kidney of Lhx1 knockout mutant as determined by microarray analysis (Potter et al. 2007). TM6SF1 locates at human chromosome 15q24-q26 and mouse chromosome 7, with a transcript size of 1.4 kb mRNA and 1.1 kb open reading frame, which encodes a 370 amino acid protein and is predicted to have six transmembrane domains with unknown function (Carim-Todd et al. 2000). In this study, we characterized Tm6sf1, which was found to be a novel lysosomal membrane protein. We demonstrated that expression of Tm6sf1-DsRed or Tm6sf1-myc fusion proteins largely overlapped with conventional lysosome markers LAMP1/2, RAB7, and Rnf167 in the human embryonic kidney HEK293A cell line. Furthermore, by time-lapse live cell imaging, we observed the fusion of Tm6sf1+ve vesicles with lysosomes and the integration of Tm6sf1 into the lysosomal membrane. By immunohistochemical staining, we also found extensive expression of Tm6sf1 in various mouse tissues including the cerebellum, liver, intestine, and kidney. These data suggest that Tm6sf1 is a widely expressed lysosomal transmembrane protein and Tm6sf1 can be used as a novel lysosome marker.
Materials and methods
Antibodies
The following antibodies were used in this study: TM6SF1 (Sigma, HPA016051); EEA1 (BD, #610456); GM130 (BD, #610822); COXIV (Cell Signaling, #4850); LC3B (Cell Signaling, #3868); LAMP1 (Cell Signaling, #9091); RAB7 (Cell Signaling, #9367); CD44 (Cell Signaling, #3570); LAMP2 (DSHB, H4B4), CD63 (DSHB, H5C6); α-tubulin (Millipore, #05-829); DsRed (Clontech, 632496); Myc (Cell Signaling, #2276); Alexa Fluor-conjugated secondary antibodies (Invitrogen).
Plasmids
The mouse Tm6sf1 full-length cDNAs was cloned into pDsRed monomer-N1, -C1, and pCMV-myc vectors (Clontech). The human LAMP1, GalTase, and mouse Rnf167 cDNAs were cloned into pEGFP-N1 vector (Clontech).
Cell culture and transfection
HEK293A cells were grown in DMEM supplemented with 10 % FBS, 1× glutamine, and 1× penicillin/streptomycin (Invitrogen) in a 5 % CO2 incubator at 37 °C. Stable cell lines were maintained in full medium supplemented with 500 μg/ml of G418. Cells were transfected with JetPRIME (Polyplus Transfection) and incubated for 24 h at 37 °C. Cells were rinsed with PBS and fixed with 4 % paraformaldehyde (PFA) for 15 min at room temperature for immunofluorescence staining. Nuclei were stained with Hoechst 33342 (Invitrogen). Cells were viewed under an epifluorescent microscope, and photos were captured using a SPOT camera (Diagnostic Instruments).
Live cell imaging
Cells were plated on a 35-mm-thin bottom culture dish and maintained at 5 % CO2, 37 °C incubation chamber of a microscope. Live cell imaging was performed using Live Cell Imaging System (Olympus IX81-ZDC). Images were collected at 4-min interval for 24 h.
Immunohistochemical staining
Dissected tissues were fixed in 4 % PFA at 4 °C overnight. Fixed tissues were dehydrated, paraffin-embedded, and sectioned at 5-μm thickness. For immunostaining, antigen retrieval was performed by microwave treatment in Tris-EDTA buffer (pH 9.0). Anti-TM6SF1 was incubated at 4 °C overnight while respective secondary antibody was incubated at room temperature for 1 h. Negative control was performed with no primary antibody added. Brown-colored immunopositive signals were envisaged by incubating the sections with diaminobenzidine (Dako), and the sections were then counterstained with hematoxylin (Sigma).
Western blotting
Proteins were extracted from the stable cell lines and separated by SDS-PAGE. Western blotting was performed by incubating with anti-TM6SF1 or anti-DsRed antibodies.
Results
Tm6sf1 is present in vesicular structures in HEK293A cells
It has been suggested that TM6SF1 is a transmembrane protein with unknown function (Carim-Todd et al. 2000). To characterize its cellular function, we first generated HEK293A cell lines stably expressing N- or C-terminally DsRed monomer-tagged mouse Tm6sf1, which shares 94 % homology of human TM6SF1. Both the N- and C-terminally tagged forms of Tm6sf1 were observed as punctate signals or as ring-shaped structures in the cytoplasm (Fig. 1a, b). However, N-terminally tagged Tm6sf1 was observed mainly as punctae, while C-terminally tagged Tm6sf1 predominantly existed as ringlike structures (Fig. 1c). We stained the stable cell lines with an antibody directed specifically against Tm6sf1, and the signals of DsRed-fusion proteins and anti-Tm6sf1 were colocalized (Fig. 1d), showing that these punctae or ringlike structures truly reflected the Tm6sf1-fusion protein. We further tested the specificity of the anti-TM6SF1 antibody by Western blotting. Two prominent bands (at approximately 60 and 100 kDa) were detected using anti-Tm6sf1 antibody. However, when comparing with a parallel blot incubated with anti-DsRed antibody, one common band at 100 kDa was observed in both blots (Fig. 1e), suggesting that this 100 kDa protein was the Tm6sf1 fusion protein. Overall, these results confirm that when expressed in HEK293A cells, Tm6sf1 is incorporated as punctate or ringlike structures.
Punctate or ringlike vesicular structures of Tm6sf1-DsRed fusion proteins expressed in HEK293A cells. N-terminally (a) and C-terminally (b) DsRed monomer-tagged Tm6sf1 fusion protein expressed in HEK293A cells. Cell counting (n > 200 for each cell line) indicated that most of the N-terminally tagged Tm6sf1 showed punctate signals, while the C-terminally tagged Tm6sf1 appeared as ringlike vesicular structures (c). That these punctae or ringlike vesicles are truly Tm6sf1 fusion proteins was shown by colocalized signals of Tm6sf1-DsRed fusion proteins (red) and anti-Tm6sf1 (green) (d). Western blottings showed a common 100-kDa band from both stable cell lysates using either anti-TM6SF1 or anti-DsRed antibody (e). Scale bars a, b 50 μm; d 20 μm
Tm6sf1 localizes to multivesicular bodies, lysosomes, and autophagosomes
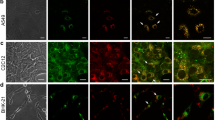
To examine which cellular compartments that these Tm6sf1+ve punctae or ringlike structures corresponded to, we stained the Tm6sf1 stable cell lines with different organelle markers. We found that both Tm6sf1 punctae and the ringlike structures colocalized with multivesicular body/lysosome (CD-63), lysosome (LAMP1/2), and autophagosome markers (LC3B) (Fig. 2), but not with other organelle markers including early endosome (EEA1), cis-Golgi (GM130), trans-Golgi (GalTase-EGFP), mitochondria (COXIV), plasma membrane (CD44), and the cytoskeleton (α-tubulin) (Supplementary Fig. 1).
Tm6sf1 localized in multivesicular bodies, lysosomes, and autophagosomes. Both N-terminally tagged Tm6sf1 punctae (a) and C-terminally tagged Tm6sf1 ringlike vesicle (b) signals (red) colocalized with multivesicular body (MVB, marked by CD-63, green), lysosome (marked by CD-63 and LAMP1/2, green), and autophagosome markers (marked by LC-3B, green). Scale bars a, b 20 μm
To further confirm the lysosomal residency of Tm6sf1, we stained the Tm6sf1 stable cell lines with another lysosome marker RAB7 (a lysosomal GTPase). Tm6sf1 punctae exclusively colocalized with RAB7, while some of the RAB7 signals colocalized with Tm6sf1 ringlike structures (Fig. 3a). Furthermore, we cotransfected Tm6sf1-myc vector with either LAMP1-DsRed or Rnf167-EGFP (another lysosome marker) vector and found that the Tm6sf1+ve vesicles colocalized with LAMP1+ve and Rnf167+ve vesicles (Fig. 3b). These data indicate that Tm6sf1 is a lysosomal protein.
Tm6sf1 colocalized with lysosome markers RAB7, LAMP1, and Rnf167. Both N-terminally and C-terminally DsRed monomer-tagged fusion Tm6sf1 (red) colocalized with RAB7 (green) in HEK293A cells (a). Tm6sf1-myc fusion protein (stained by anti-myc antibody) colocalized with lysosome markers LAMP1 and Rnf167 (b). Scale bars 20 μm
Tm6sf1 vesicle budding and fusion with lysosome
To study the involvement of Tm6sf1 in lysosomal pathway, we adopt fluorescence time-lapse live cell imaging to further study the dynamics of the Tm6sf1 vesicular structures. We transfected a LAMP1-EGFP plasmid into the C-terminally DsRed monomer-tagged Tm6sf1 stable cell line to label the lysosomes. We found that many of the Tm6sf1 vesicles were also LAMP1+ve (Fig. 4a). Interestingly, small Tm6sf1+ve vesicles were seen to bud off from large Tm6sf1+ve vesicles and then fuse with LAMP1+ve lysosomes (Fig. 4a, Supplementary Video 1). We also observed the integration of Tm6sf1 into part of the lysosomal membrane (Fig. 4b, Supplementary Video 2), supporting our claim that Tm6sf1 is a lysosomal membrane protein.
Tm6sf1 vesicle budding and fusion with lysosome. Images captured from a fluorescence time-lapse live imaging video showing budding of Tm6sf1 vesicle (red, arrowhead) and fusion with LAMP1+ve lysosome (green) (a). Tm6sf1 and LAMP1+ve fused vesicles were in yellow color (a). Tm6sf1 (red) fused with LAMP1+ve lysosome (green) and integrated into part of the lysosomal membrane (arrowhead) (b). Scale bars a, b 10 μm
Tm6sf1 expression in various mouse tissues
Finally, we examined the expression of Tm6sf1 in various mouse tissues by immunohistochemistry. Tm6sf1 was expressed in the molecular layer of the cerebellum, hepatocytes of the liver, epithelial cells of gut lining, and outer cortex and inner medulla of the kidney (Fig. 5). These data illustrated a broad expression of Tm6sf1 in major organs.
Tm6sf1 expressed in various mouse tissues at postnatal day 10. Tm6sf1 expressed in the molecular layer of the cerebellum (a), hepatocytes of the liver (c), epithelial cells of the intestine (e), and outer cortex and inner medulla of the kidney (g). Left panels are sections stained with anti-Tm6sf1 (a, c, e, g). Right panels are negative controls (b, d, f, h). Scale bars a, b, g, h 500 μm; c–f 50 μm
Discussion
In this study, we demonstrated that Tm6sf1 is a lysosomal membrane protein on the basis of various experimental evidence. First, both Tm6sf1 punctae and ringlike vesicular structures colocalized with multiple lysosome markers including CD-63, LAMP1/2, RAB7, and Rnf167 (Figs. 2 and 3). Second, fluorescence time-lapse live imaging showed the fusion of Tm6sf1 vesicles with lysosomes to become integrated into the lysosomal membrane (Fig. 4, Supplementary Video 1 and 2). Hence, we suggest that Tm6sf1 is a lysosomal transmembrane protein and can be used as a novel lysosome marker.
Our previous study demonstrates that the subcellular localization of EMP proteins in Arabidopsis is altered by the position of GFP tag (Gao et al. 2012). EMP12 properly resides in Golgi apparatus when the GFP is tagged at the N-terminal. In contrast, C-terminal GFP tag leads to mislocalization of EMP12 to post-Golgi compartments likely due to the masking of the Golgi retention signal located at the C-terminal of EMP12 (Gao et al. 2012). Because of this, we also investigated if the position of DsRed fusion tag affects the subcellular localization of Tm6sf1. Interestingly, although the location of the DsRed fluorescence tag had an effect on the Tm6sf1 punctae or vesicle formation (Fig. 1), the colocalization of Tm6sf1 punctae or vesicle with these lysosome markers was not altered (Fig. 2). We further confirmed the lysosomal localization of Tm6sf1 by tagging with myc, which is a commonly used short fusion tag assumingly with less effect on protein topology. Overall, these data supports Tm6sf1 as a novel lysosomal protein.
TM6SF1 has been shown to be expressed in the human brain, lung, liver, kindney, spleen, testis, and peripheral blood leukocytes (Carim-Todd et al. 2000; Tao et al. 2011; Expression Atlas, http://www.ebi.ac.uk). Our immunohistochemical staining also showed the Tm6sf1 expression in the mouse cerebellum, liver, intestine, and kidney (Fig. 5). It is therefore conceivable that Tm6sf1 may play a role in lysosomal function, which is, in turn, critical to the normal physiological function of these organs. Further studies are required to determine if Tm6sf1 is a structural protein, a molecular channel, or has some other molecular function. Of note is that Tm6sf1 was also colocalized with the autophagosome marker LC3B (Fig. 2). It would therefore be interesting to investigate any role played by Tm6sf1 in the formation of autophagosomes as well as autolysosomes, which are formed through the fusion of lysosomes and autophagosomes (Luzio et al. 2007; Schroder et al. 2010; Yu et al. 2010).
The regulation of TM6SF1 expression is largely unknown, but this gene is one of the targets of miR-155 and miR-K12-11 (Skalsky et al. 2007). In addition, several studies have demonstrated that TM6SF1 is hypermethylated in liver and kidney cancer (Tao et al. 2011; Ricketts et al. 2014). These may imply that expression of Tm6sf1 is highly regulated in an epigenetic fashion.
Recently, TM6SF2 was found to be a regulator of fat metabolism in the liver and thus associated with the development of nonalcoholic fatty liver disease (Holmen et al. 2014; Kozlitina et al. 2014; Mahdessian et al. 2014; Wong et al. 2014). TM6SF2 colocalizes with endoplasmic reticulum (ER) and ER-Golgi intermediate compartment markers, but not Golgi markers, suggesting that this protein is involved in the early ER-Golgi transportation pathway (Mahdessian et al. 2014). Knockdown of TM6SF2 results in increased cellular triglyceride and lipid droplet formation in liver cell lines (Mahdessian et al. 2014). In vivo knockdown of Tm6sf2 in mouse also increases the hepatic triglyceride content (Kozlitina et al. 2014). Further investigations are required to examine, if any functional relationship between Tm6sf1 and Tm6sf2 exists, although the two are expressed in different cellular compartments.
References
Boya P (2012) Lysosomal function and dysfunction: mechanism and disease. Antioxid Redox Signal 17:766–774
Callahan JW, Bagshaw RD, Mahuran DJ (2009) The integral membrane of lysosomes: its proteins and their roles in disease. J Proteomics 72:23–33
Carim-Todd L, Escarceller M, Estivill X, Sumoy L (2000) Cloning of the novel gene TM6SF1 reveals conservation of clusters of paralogous genes between human chromosomes 15q24--q26 and 19p13.3--p12. Cytogenet Cell Genet 90:255-260
Gao C, Yu CK, Qu S, San MW, Li KY, Lo SW, Jiang L (2012) The Golgi-localized Arabidopsis endomembrane protein12 contains both endoplasmic reticulum export and Golgi retention signals at its C terminus. Plant Cell 24:2086–2104
Holmen OL, Zhang H, Fan Y, Hovelson DH, Schmidt EM, Zhou W, Guo Y, Zhang J, Langhammer A, Lochen ML, Ganesh SK, Vatten L, Skorpen F, Dalen H, Pennathur S, Chen J, Platou C, Mathiesen EB, Wilsgaard T, Njolstad I, Boehnke M, Chen YE, Abecasis GR, Hveem K, Willer CJ (2014) Systematic evaluation of coding variation identifies a candidate causal variant in TM6SF2 influencing total cholesterol and myocardial infarction risk. Nat Genet 46:345–351
Kozlitina J, Smagris E, Stender S, Nordestgaard BG, Zhou HH, Tybjaerg-Hansen A, Vogt TF, Hobbs HH, Cohen JC (2014) Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 46:352–356
Lubke T, Lobel P, Sleat DE (2009) Proteomics of the lysosome. Biochim Biophys Acta 1793:625–635
Luzio JP, Pryor PR, Bright NA (2007) Lysosomes: fusion and function. Nat Rev Mol Cell Biol 8:622–632
Mahdessian H, Taxiarchis A, Popov S, Silveira A, Franco-Cereceda A, Hamsten A, Eriksson P, Van’t Hooft F (2014) TM6SF2 is a regulator of liver fat metabolism influencing triglyceride secretion and hepatic lipid droplet content. Proc Natl Acad Sci U S A 111:8913–8918
Potter SS, Hartman HA, Kwan KM, Behringer RR, Patterson LT (2007) Laser capture-microarray analysis of Lim1 mutant kidney development. Genesis 45:432–439
Ricketts CJ, Hill VK, Linehan WM (2014) Tumor-specific hypermethylation of epigenetic biomarkers, including SFRP1, predicts for poorer survival in patients from the TCGA Kidney Renal Clear Cell Carcinoma (KIRC) project. PLoS One 9:e85621
Saftig P, Klumperman J (2009) Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol 10:623–635
Schroder BA, Wrocklage C, Hasilik A, Saftig P (2010) The proteome of lysosomes. Proteomics 10:4053–4076
Schwake M, Schroder B, Saftig P (2013) Lysosomal membrane proteins and their central role in physiology. Traffic 14:739–748
Skalsky RL, Samols MA, Plaisance KB, Boss IW, Riva A, Lopez MC, Baker HV, Renne R (2007) Kaposi’s sarcoma-associated herpesvirus encodes an ortholog of miR-155. J Virol 81:12836–12845
Tao R, Li J, Xin J, Wu J, Guo J, Zhang L, Jiang L, Zhang W, Yang Z, Li L (2011) Methylation profile of single hepatocytes derived from hepatitis B virus-related hepatocellular carcinoma. PLoS One 6:e19862
Wong VW, Wong GL, Tse CH, Chan HL (2014) Prevalence of the TM6SF2 variant and non-alcoholic fatty liver disease in Chinese. J Hepatol
Yu L, McPhee CK, Zheng L, Mardones GA, Rong Y, Peng J, Mi N, Zhao Y, Liu Z, Wan F, Hailey DW, Oorschot V, Klumperman J, Baehrecke EH, Lenardo MJ (2010) Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature 465:942–946
Acknowledgments
The authors would like to thank Mr. Freddie Kwok for the technical support on live cell imaging. The work described in this paper was supported by grants from the Research Grants Council of the Hong Kong Special Administrative Region, China (Project No. CUHK2/CRF/11G and AoE/M-05/12).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Reimer Stick
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Video 1
Fluorescence time-lapse live imaging video showing budding of Tm6sf1 vesicle (red) and fusion with LAMP1+ve lysosome (green) (AVI 177 kb)
Supplementary Video 2
Fluorescence time-lapse live imaging video showing the integration of Tm6sf1 (red) into LAMP1+ve lysosomal membrane (green) (AVI 492 kb)
Supplementary Fig. 1
Tm6sf1 (red) did not colocalize with early endosome (EEA1, green), Cis-Golgi (GM130, green), Trans-Golgi (GalTase, green), mitochondria (COXIV, green), plasma membrane (CD44, green) and cytoskeleton markers (α-tubulin, green). Scale bars: 20 μm (GIF 1.7 mb)
High resolution image
(TIFF 4.81 mb)
Rights and permissions
About this article
Cite this article
Tam, W.Y., Jiang, L. & Kwan, K.M. Transmembrane 6 superfamily 1 (Tm6sf1) is a novel lysosomal transmembrane protein. Protoplasma 252, 977–983 (2015). https://doi.org/10.1007/s00709-014-0733-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-014-0733-x