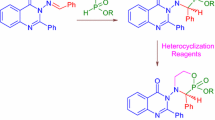
Abstract
A preparatively convenient and efficient method is proposed for the synthesis of novel 1-oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxylic acids, based on the reaction of (3-oxopiperazine-2-ylidene)ethanoates with 2-bromo-1,1-diethoxyethane and accomplished through the stage of intermediate methyl 1-oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxylates, which were also isolated as individual compounds. A method of directly transforming 1-oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxylic acids into 1-oxo-N-(alkyl)aryl-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxamides via the former’s interaction with aliphatic and aromatic amines in the presence of DIPEA and HATU was developed, with yields of 31–78%. A reliable structural determination of all the synthesized compounds has been performed by elemental analysis and a number of spectroscopic methods (1H and 13C NMR, HPLC/MS) as well as by X-ray diffraction analysis. Biological screening of all types of synthesized compounds revealed their moderate antibacterial and antifungal activity. The antioxidant effect level of the most active carboxamides was in the range of 59.3–74.5%, as compared to ascorbic acid (97.3%).
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tetrahydropyrrolo[1,2-a]pyrazinones are heterocyclic systems, the nucleus of which is part of a wide array of natural bioactive products isolated from fungi, plants, or marine sponges [1]. Striking representatives of such natural compounds are bromopyrrole alkaloids: mukanadin C [2] and longamide A [3] were moderate inhibitors of GSK-3, DYRK1A, CK-1 and may have future development potential for treating various diseases [4, 5], longamide B [6] being a powerful trypanocidal and antileishmanial agent [7]. In turn, hanishin demonstrates cytotoxicity against human non-small-cell-lung carcinoma NSCLC-N6 [8], stylisine D is an effective inhibitor of the Gram-negative bacteria biofilm formation [9] (Fig. 1).
The most important synthetic derivatives of tetrahydropyrrolo[1,2-a]pyrazine are represented by ranirestat, a potent aldose reductase inhibitor used to treat diabetic neuropathy [10]. In addition, compounds with tetrahydropyrrolo[1,2-a]pyrazine skeleton act as antagonists of melanin-concentrating hormone (MCH-R1) and can be used in anti-obesity therapy [11], are highly selective ERK1/2 inhibitors [12], inhibitors of PIM-kinases with low nanomolar activity and selectivity against a large panel of kinases [13], noncompetitive antagonists of mGluR1 [14], and inhibitors of HIV-1 replication [15] (Fig. 2).
Thus, the pronounced biological and pharmacological effects of both natural and synthetic compounds with the tetrahydropyrrolo[1,2-a]pyrazinone fragment are an incentive to obtain their new derivatives, which may prove to be very promising for further biomedical research. That is why the search for preparatively convenient approaches to tetrahydropyrrolo[1,2-a]pyrazines with synthetically potent functional groups, which can be advantageously used for targeted structural modification, remains relevant. Since the carboxyl function fully meets these requirements, the subject of our study was previously unknown derivatives of pyrrolo[1,2-a]pyrazine-8-carboxylic acids.
Results and discussion
The analysis of the available literature showed that 1-oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxylic acids remain obscure compounds, although a small number of their esters with substituents at positions 6 and 7 of the bicyclic system have been described. These were synthesized in a three-component reaction of ethane-1,2-diamine with esters of acetylenedicarboxylic acid and a limited number of 1,2-bielectrophilic reagents: ethyl bromopyruvate [16, 17], nitrostyrenes [18], and glyoxals [19].
It seemed reasonable for us to investigate the behavior of other available reagents in such cyclocondensation: chloroacetaldehyde and bromoacetaldehyde diethyl acetal, which have been used to form a pyrrole cycle based on enaminones and their synthetic analogues [20,21,22,23], thus making it possible to obtain pyrrolo[1,2-a]pyrazine-8-carboxylate derivatives, unsubstituted at positions 6 and 7.
The model three-component reactions of ethane-1,2-diamine (1a), dimethyl acetylenedicarboxylate (2), and chloroacetaldehyde (3a) or bromoacetaldehyde diethyl acetal (3b) showed that their heating in acetonitrile [16] or acetic acid did not lead to the expected result, which is most likely due to competitive condensation of the aldehyde or acetal groups with ethylenediamine. That is why the realization of the set goal required to implement a sequential transformation with isolation of intermediate products–(3-oxopiperazine-2-ylidene)ethanoates. For this purpose, in the first step, various 1,2-diamines—ethane-1,2-diamine (1a), propane-1,2-diamine (1b), 2-methylpropane-1,2-diamine (1c), and trans-cyclohexane-1,2-diamine (1d)–were reacted with dimethyl acetylenedicarboxylate (DMAD, 2) in methanol at 0 °C. In the case of symmetrical diamines 1a, 1d, (3-oxopiperazine-2-ylidene)ethanoates 4a, 4d were isolated with high yields (see Scheme 1), their physicochemical constants matching those described in the literature [24,25,26].

At the same time, the literature data concerning the interaction of nonsymmetric propane-1,2-diamine (1b) with acetylenedicarboxylates are ambiguous. For example, the authors of [27, 28] proposed the structure of methyl (6-methyl-3-oxopiperazine-2-ylidene)ethanoate (A) as the reaction product of diamine 1b with dimethyl acetylenedicarboxylate (DMAD). However, the work [29] asserts that the product of a similar reaction with diethyl acetylenedicarboxylate (DEAD) is ethyl (5-methyl-3-oxopiperazine-2-ylidene)ethanoate (B) (Fig. 3).
Thus, we carried out a more detailed study of nonsymmetric diamines 1b, 1c reaction with DMAD under the conditions used for diamines 1a, 1d. It was found that in the case of propane-1,2-diamine, the reaction yielded a mixture of two regioisomers 4b and A in a ratio of 7:3, from which the major product 4b was isolated with yield of 64%. Its 1H–1H COSY NMR analysis confirmed the formation of methyl (5-methyl-3-oxopiperazine-2-ylidene)ethanoate (4b). A previously undescribed similar interaction involving 2-methylpropane-1,2-diamine (1c) proceeds with a rather high regioselectivity to form the 5,5-dimethyl derivative 4c, the structure of which was also confirmed by 1H–1H COSY NMR spectroscopy (see supplementary).
To evaluate the efficiency of the compounds 3a and 3b as bielectrophilic cyclizing reagents, their interaction with the model piperazinone 4a was studied. It was found that in the acetic acid solution in the presence of a catalytic amount of HCl for 2 h at room temperature, the product of both reactions is methyl 1-oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxylate (5a), although its yield in the case of chloroacetaldehyde reaches 31% and in the case of bromoacetaldehyde diethyl acetal–43%. Therefore, it was the latter that was used under the above conditions to synthesize a series of pyrrolo[1,2-a]pyrazinecarboxylates 5b–5d, which were isolated with yields of 49–66%. It is characteristic that further heating of the reaction mixture containing esters 5a–5d for 2–8 h in acetic acid (method a) or cyclization of piperazinones 4a–4d under the same conditions (method b) leads to the formation of 1-oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxylic acids 6a–6d with yields of 69–87% (see Scheme 2, Table 1).

The achieved result clearly proves that the hydrolysis of esters 5b–5d to acids 6a–6d is catalyzed by HBr, which is a by-product of the cyclocondensation of pyrazinones 4a–4d with bromoacetaldehyde diethyl acetal. The structures of the synthesized acids 6a–6d were confirmed by elemental analysis, HPLC/MS, 1H and 13C NMR spectroscopy. The dihydropyrrolo[1,2-a]pyrazinone ring formation is most clearly evidenced by the 1H NMR doublet signals of the H6 and H7 protons for 5a–5c in the range of 6.71–6.98 (3JHH = 2.7–2.3 Hz, C6H), 6.44–6.66 (3JHH = 2.5–2.6 Hz, C7H) ppm and for 6a–6c in the range of 7.17–7.21 (3JHH = 2.4–2.7 Hz, C6H), 6.67–6.68 (3JHH = 2.5–2.7 Hz, C7H) ppm and the doublet signals of the H1 and H2 protons for 5d at 6.80 (3JHH = 3.0 Hz, C1H), 6.65 (3JHH = 3.0 Hz, C2H) ppm, for 6d at 6.96 (3JHH = 2.7 Hz, C1H), 6.94 (3JHH = 2.7 Hz, C2H) ppm, which agree with the spectral characteristics of structural analogues [16, 19]. The structure of trans-4-oxo-4,5,5a,6,7,8,9,9a-octahydropyrrolo[1,2-a]quinoxaline-3-carboxylic acid (6d) was established by its X-ray structural study.
It is known that amide bond is a key structural unit of peptides, many synthetic and natural products, and functional materials [30,31,32,33]. It is also an important element of a large number of drug candidates and pharmaceuticals [34, 35], which makes amide synthesis a fundamental process in modern medicinal chemistry. Given the potent pharmacological potential of the pyrrolo[1,2-a]pyrazinone nucleus, it seemed reasonable to carry out its amide functionalization using the acids 6a–6d as basic substrates for the design of potentially bioactive amides.
The preparatively simple method of converting the acids 6a–6d into the corresponding chloroanhydrides by subjecting them to various chlorinating reagents did not lead to the expected success, which is probably due to the presence of a chlorination-prone C(O)NH group in their structure. Other methods of activating the carboxyl group using reagents such as CDI and TBTU also proved ineffective. A positive result was obtained by using such a coupling reagent as HATU (N-[(dimethylamino)(3H-[1,2,3]triazolo[4,5-b]pyridin-3-yloxy)methylidene]-N-methylmethanaminium hexafluorophosphate) [36, 37]. It is demonstrated that tetrahydropyrrolo[1,2-a]pyrazine-8-carboxylic acids 6a–6d react with aliphatic (7a–7d) and aromatic (7e–7i) amines in the presence of DIPEA and HATU in DMF at 50 °C for 8–25 h to form a series of carboxamides 8a–8t with yields of 31–78% (see Scheme 3, Table 2).

The structures of the synthesized compounds 4a–4d, 5a–5d, 6a–6d, 8a–8t have been confirmed by elemental analysis, HPLC/MS, 1H and 13C NMR spectroscopy. In order to accurately establish the structure of trans-4-oxo-4,5,5a,6,7,8,9,9a-octahydropyrrolo[1,2-a]quinoxaline-3-carboxylic acid (6d) in the crystalline state, its X-ray structural study was performed (Fig. 4).
The X-ray diffraction study has shown that the tricyclic fragment of compound 6d has an angular structure (Fig. 4). The six-membered partially saturated cycles are disordered over two positions (A and B) with equal populations. The heterocycle adopts a half-chair conformation in both conformers (the puckering parameters [38] are: S = 0.72, Θ = 31.84°, Ψ = 27.55° in conformer A or S = 0.75, Θ = 34.57°, Ψ = 27.63° in conformer B). Atoms C1 and C6 deviate from the mean plane of the remaining atoms of this cycle on 0.36 Å and −0.33 Å in conformer A or −0.38 Å and 0.36 Å in conformer B. The cyclohexane ring adopts a chair conformation in conformers A and B (the puckering parameters are: S = 1.14, Θ = 3.33°, Ψ = 5.92° in conformer A or S = 1.12, Θ = 1.87°, Ψ = 17.71° in conformer B). Atoms C2 and C5 deviate from the mean plane of the remaining atoms of this cycle on -0.71 Å and 0.63 Å in conformer A or 0.69 Å and −0.64 Å in conformer B.
An intramolecular hydrogen bond O3–H…O1 (the H…O distance is 1.72 Å, O–H…O bond angle is 162°) has been found between the carboxyl and the carbonyl groups, resulting in elongation of the C11–O1 bond up to 1.237(7) Å as compared to its mean values [39] 1.210 Å, while the C12–O3 (1.318(9) Å) is typical for the carboxyl group (mean value is 1.305 Å).
In the crystal phase, the 6d molecules are bound by the intermolecular hydrogen bond N1–H…O2 (symmetry operation is 0.5 + x, 1.5−y, −0.5 + z; the H…O distance is 2.01 Å, the N–H…O bond angle is 170°).
Study of antimicrobial and antioxidant activity
The developed convenient synthetic approaches to pyrrolo[1,2-a]pyrazine-8-carboxylic acids, their carboxamides, and esters have created good conditions for conducting their biological screening. Taking into account the previously discovered antimicrobial [40,41,42,43] and antioxidant [44,45,46,47] properties of condensed pyrroles and pyrazines, it seemed reasonable to test the compounds 5a–5d, 6a–6d, and 8a–8t, which are combinations of structurally modified pyrrole and pyrazine cycles, for these activity types.
Antibacterial and antifungal activity in vitro of the synthesized compounds 5a–5d, 6a–6d, and 8a–8t was determined using the two-fold serial dilutions technique with the reference strains of various Gram-negative (Escherichia coli ATCC 25922, Proteus vulgaris ATCC 4636, Pseudomonas aeruginosa ATCC 27853), Gram-positive (Staphylococcus aureus ATCC 25923, Enterococcus faecalis ATCC 6783, Bacillus cereus ATCC 10702) bacteria and fungi (Candida albicans ATCC 885/653, Aspergillus niger K9, Aspergillus amstelodami K12). DMSO was used as a diluent and negative control, the antibacterial agent decasan [48]—as a positive control for antibacterial activity, and clotrimazole [49], a fungicidal agent–as a positive control for antifungal activity. The antibacterial and antifungal activity was assessed by the values of minimum inhibitory (MIC) and minimal bactericidal (MBC) or fungicidal (MFC) concentrations. Analysis of the antimicrobial screening data (see Tables 3, 4) showed that the minimum inhibitory concentration of the tested compounds against the bacteria and the fungi varied from 31.25 to 125 μg/cm3, the minimum bactericidal concentration varied from 31.25 to 250 μg/cm3, and the minimum fungicidal concentration from 31.25 to 125 μg/cm3 which attests the presence of antimicrobial activity in this class of compounds. From analyzing the antibacterial activity results, it can be concluded that some amides of the 1-oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxylic acids (MIC = 31.25–62.5 μg/cm3) display twice the activity of the corresponding acids and esters (MIC = 62.5–125 μg/cm3). In particular, the minimum inhibitory concentration is 31.25 μg/cm3 for the amides 8a, 8j, 8n, 8p with Staphylococcus aureus ATCC 2592, 8e, 8h, 8j, 8n, 8p, 8q, 8s with Bacillus cereus ATCC 10702, 8d, 8j, 8m-8p, 8t with Escherichia coli ATCC 25922, 8e, 8f, 8h, 8k, 8n-8p, 8r, 8t with Pseudomonas aeruginosa ATCC 27853. For the antifungal activity, the best results were obtained with the pyrroloquinoxaline derivatives 5d, 6d, and 8q with the fungal strain Aspergillus niger K9 (MIC = 31.25 μg/cm3).
Antioxidant action of the synthesized compounds
The antioxidant activity assessment of the synthesized compounds was carried out using the DPPH radical scavenging assay according to the method described in [50]. 1 cm3 of DPPH solution (0.08 mg/cm3) was added to each of the methanol solutions of test compounds, as well as to a standard solution of ascorbic acid used as reference, and left at room temperature in a dark place for 1 h. The absorption value was determined with a UV-1800 spectrophotometer (Shimadzu, Japan) at 517 nm wavelength relative to the control. Each sample was analyzed in three repeats. The inhibition percentage was calculated relative to the blank sample:
wherein Ablank is the absorption of the control reaction (including all reagents except the tested compounds); Asample+DPPH is the absorption of the tested compounds after 60 min of incubation with the DPPH solution; Asample is the absorption of the tested compounds without the DPPH solution.
The synthesized carboxylates 5a–5d, carboxylic acids 6a–6d, and carboxamides 8a–8t were evaluated for their ability to inhibit the DPPH (1,1-diphenyl-2-picrylhydrazyl) radicals [50]. The assessment of DPPH radical scavenging activity was done at 5 μM concentration (a methanol solution, measurement after 60 min). This approach allows for quick identification of potential hit compounds while saving time and the compounds’ quantities. Ascorbic acid was used as a standard compound. The results of the radical scavenging activity screening for the 5a–5d, 6a–6d, and 8a–8t compounds are shown in Fig. 5.
So, the carboxylates 5a–5d are characterized by a moderate antioxidant effect with a DPPH radical scavenging level of 45.2–57.7%. While the acids 6a–6d demonstrate a higher level of radical scavenging in comparison with the corresponding esters, in the range of 52.6–59.2%. Most likely, the stronger antioxidant activity of the 6a–6d compounds occurs because of the presence of an active hydrogen atom of the carboxyl group.
In turn, the carboxamides 8a–8t demonstrated a broad distribution of radical scavenging values, from 26.8 to 74.5%, which is probably due to the varying nature of the substituents in the amide fragment. For instance, the N-alkyl-substituted derivatives 8a, 8b, 8f, 8h, 8i, 8n, 8p, and 8r are characterized by a lower radical scavenging level in comparison with the N-aryl derivatives 8c–8e, 8g, 8j–8m, 8o, 8q, and 8s–8t. This resulted in the identification of the hit compounds 6d, 8e, 8g, and 8o, which can be used for in-depth pharmacological research and potential synthetic antioxidant design.
Conclusion
To summarize, we have shown that the interaction of (3-oxopiperazine-2-ylidene)ethanoates and 2-bromo-1,1-diethoxyethane is a convenient method for the synthesis of the novel 1-oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxylate derivatives, which, under the conditions of HBr catalysis, have a tendency to hydrolyze into the corresponding acids. For the latter, a method of direct amidation with alkyl- and arylamines in the presence of DIPEA and HATU in DMF was developed, which allowed to obtain a focused library of 1-oxo-N-(alkyl)aryl-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxamides. The results of testing the synthesized compounds against Gram-positive and Gram-negative bacteria and fungi showed that they are characterized by moderate activity, although the majority of carboxamides display a twice stronger antibacterial effect as compared to acids and esters. Amongst the carboxamides, the antioxidant properties screening revealed compounds with maximum DPPH scavenging levels in the range of 59.3–74.5% in comparison with ascorbic acid (97.3%).
Experimental
All commercially available chemicals were purchased from Sigma-Aldrich Chemicals (Steinheim, Germany), Merck Chemicals (Darmstadt, Germany), Enamine Ltd (Kyiv, Ukraine). Melting points were determined on a Kofler bench. 1H NMR spectra were acquired on a Varian VXR-400 spectrometer (400 MHz) in CDCl3 solution (for compounds 5a, 5d, 6d, 8a, 8b, 8f, 8i) and in DMSO-d6 solution (for compounds 4b, 4c, 5b, 5c, 6a–6c, 8c–8e, 8g, 8h, 8j–8t) with TMS as an internal standard. 13C NMR spectra were acquired on a Varian Mercury 300 spectrometer (76 MHz), Bruker Avance DRX-500 spectrometer (125 MHz), and a Agilent 600 MHz spectrometer (150 MHz) in CDCl3 solution (for compounds 5a, 8a, 8b, 8f) and in DMSO-d6 solution (for compounds 4b, 4c, 5b–5d, 6a–6d, 8c–8e, 8g–8s, 8t) with TMS as an internal standard. 19F NMR spectra were acquired on a Varian Mercury-400 spectrometer (376 MHz) in CDCl3 solution (for compounds 8c, 8k) and in DMSO-d6 solution (for compounds 8e, 8j, 8o, 8t). Mass spectra were recorded on an Agilent LC/MSD SL instrument; column Zorbax SB-C18, 4.6 × 15 mm, 1.8 μm (PN 82(c) 75–932); solvent DMSO, at atmospheric pressure, electrospray ionization. Merck 60 (40–63 μ) silica gel was used for column chromatography.
Synthesis of methyl (3-oxopiperazin-2-ylidene)ethanoates 4a-4c and methyl trans-(3-oxooctahydroquinoxalin-2(1H)-ylidene)ethanoate (4d) (general method)
To a cooled (0 °C) solution of DMAD (29.9 mmol) in 40 cm3 MeOH, a solution of diamines 1a–1d (29.9 mmol) in 20 cm3 MeOH was added. The resulting mixture was stirred at 0 ℃ for 2 h. After the reaction was completed, the insoluble materials were filtered off, washed with cold MeOH (2 × 2 cm3), hexane (2 × 4 cm3), and dried under reduced pressure. For the compounds 4a [24] and 4d [25, 26], the 1H NMR and 13C NMR spectra were found to be identical with the ones described in Refs. [24,25,26].
Methyl (5-methyl-3-oxopiperazin-2-ylidene)ethanoate (4b, C8H12N2O3)
Yield: 3.54 g (64%); m.p.: 189–190 °C; 1H NMR (400 MHz, DMSO-d6): δ = 8.49 (s, NH), 8.32 (s, NH), 5.21 (s, =CH), 3.65–3.59 (m, 1H), 3.57 (s, OCH3), 3.40 (dt, 2JHH = 13.0, 3JHH = 4.0 Hz, 1H), 3.02–2.95 (m, 1H), 1.10 (d, 3JHH = 6.5 Hz, C5CH3) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 18.14 (C5CH3), 44.99 (C6), 45.56 (C5CH3), 50.15 (OCH3), 82.93 (=CH), 149.36 (C2), 159.52 (C3), 169.47 (O=C−OCH3) ppm; MS (70 eV): m/z = 185 ([M + H]+).
Methyl (5,5-dimethyl-3-oxopiperazin-2-ylidene)ethanoate (4c, C9H14N2O3)
Yield: 5.40 g (91%); m.p.: 165–166 °C; 1H NMR (400 MHz, DMSO-d6): δ = 8.50 (s, NH), 8.33 (s, NH), 5.22 (s, = CH), 3.57 (s, OCH3), 3.18 (d, 3JHH = 2.7 Hz, C6H2), 1.18 (s, C5(CH3)2) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 26.04 (C5(CH3)2), 49.53 (C5), 50.13 (OCH3), 50.47 (C6), 82.94 (=CH), 148.82 (C2), 159.02 (C3), 169.43 (O=C−OCH3) ppm; MS (70 eV): m/z = 199 ([M + H]+).
Synthesis of methyl 1-oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxylates 5a-5c and methyl trans-4-oxo-4,5,5a,6,7,8,9,9a-octahydropyrrolo[1,2-a]quinoxaline-3-carboxylate (5d) (general method)
To a solution of 2.7 mmol of methyl (3-oxopiperazine-2-ylidene)ethanoates 4a–4c or methyl trans-[3-oxooctahydroquinoxalin-2(1H)-ylidene]ethanoate (4d) in 5 cm3 AcOH, 0.53 g bromoacetaldehyde diethyl acetal (2.7 mmol) and a few drops of an HCl solution in 1,4-dioxane were added. The resulting mixture was stirred at room temperature for 2–4 h. After the reaction was completed, sodium acetate was added to the obtained mixture until pH 7, and it was dried under reduced pressure. The formed precipitate was dissolved in 50 cm3 ethyl acetate, washed with saturated aqueous NaHCO3 (5 cm3), with H2O (2 × 5 cm3), and brine (2 × 5 cm3), the organic phase was dried over Na2SO4 and evaporated under reduced pressure. The residue was purified by column chromatography on silica gel, eluent CHCl3–MeOH, 10:1 (for compounds 5a, 5c), and EtOAc (for compounds 5b, 5d).
Methyl 1-oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxylate (5a, C9H10N2O3)
Yield: 0.21 g (43%); m.p.: 122–123 °C; 1H NMR (400 MHz, CDCl3): δ = 6.79 (s, NH), 6.71 (d, 3JHH = 2.6 Hz, C6H), 6.66 (d, 3JHH = 2.6 Hz, C7H), 4.13 (dd, 3JHH = 6.9, 4.7 Hz, C4H2), 3.87 (s, CH3), 3.68 (td, 3JHH = 6.5, 5.9, 3.7 Hz, C3H2) ppm; 13C NMR (126 MHz, CDCl3): δ = 39.93 (C3), 44.91 (C4), 51.79 (O−CH3), 112.71 (C7), 119.65 (C8), 122.30 (C6), 124.35 (C8a), 159.87, 164.35 (C1=O + O=C−OCH3) ppm; MS (70 eV): m/z = 195 ([M + H]+).
Methyl 3-methyl-1-oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxylate (5b, C10H12N2O3)
Yield: 0.27 g (49%); m.p.: 109–110 °C; 1H NMR (400 MHz, DMSO-d6): δ = 7.92 (s, NH), 6.98 (d, 3JHH = 2.3 Hz, C6H), 6.44 (d, 3JHH = 2.5 Hz, C7H), 4.19 (dd, 2JHH = 11.6, 3JHH = 2.3 Hz, C4H), 3.87–3.72 (m, C3H + C4H), 3.68 (s, OCH3), 1.17 (d, 3JHH = 6.1 Hz, C3CH3) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 17.75 (C3CH3), 45.93 (C3), 50.08 (C4), 51.23 (OCH3), 110.97 (C7), 117.92 (C8), 122.60 (C6), 123.77 (C8a), 157.63 (C1=O), 164.34 (O=C−OCH3) ppm; MS (70 eV): m/z = 209 ([M + H]+).
Methyl 3,3-dimethyl-1-oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxylate (5c, C11H14N2O3)
Yield: 0.34 g (58%); m.p.: 135–136 °C; 1H NMR (400 MHz, DMSO-d6): δ = 7.92 (s, NH), 6.97 (d, 3JHH = 2.6 Hz, C6H), 6.46 (d, 3JHH = 2.7 Hz, C7H), 3.97 (s, C4H2), 3.69 (s, OCH3), 1.19 (s, C3(CH3)2) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 25.91 (C3(CH3)2), 51.23 (OCH3), 51.27 (C3), 54.43 (C4), 111.06 (C7), 117.84 (C8), 122.78 (C6), 123.16 (C8a), 157.06 (C1 = O), 164.29 (O=C−OCH3) ppm; MS (70 eV): m/z = 223 ([M + H]+).
Methyl trans-4-oxo-4,5,5a,6,7,8,9,9a-octahydropyrrolo[1,2-a]quinoxaline-3-carboxylate (5d, C13H16N2O3)
Yield: 0.44 g (66%); m.p.: 173–174 °C; 1H NMR (400 MHz, CDCl3): δ = 6.80 (d, 3JHH = 2.6 Hz, C1H), 6.65 (d, 3JHH = 2.6 Hz, C2H), 5.83 (s, NH), 3.87 (s, CH3), 3.71 (td, 3JHH = 10.9, 3.7 Hz, C9aH), 3.44 (td, 3JHH = 11.2, 3.8 Hz, C5aH), 2.52 (d, 3JHH = 12.6 Hz, 1H), 2.01 (d, 3JHH = 12.5 Hz, 2H), 1.89 (d, 3JHH = 12.1 Hz, 1H), 1.59–1.37 (m, 4H) ppm; 13C NMR (151 MHz, DMSO-d6): δ = 23.05, 23.35 (C7 + C8), 27.15, 29.03 (C6 + C9), 51.29 (OCH3), 55.01 (C5a), 58.30 (C9a), 110.83 (C2), 118.64 (C3), 119.02 (C1), 124.69 (C3a), 157.76 (C4), 164.59 (O=C–OCH3) ppm; MS (70 eV): m/z = 249 ([M + H]+).
Synthesis of 1-oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxylic acids 6a-6c and trans-4-oxo-4,5,5a,6,7,8,9,9a-octahydropyrrolo[1,2-a]quinoxaline-3-carboxylic acid (6d) (general method)
To a solution of 26.6 mmol of methyl (3-oxopiperazine-2-ylidene)ethanoates 4a–4c or methyl [3-oxooctahydroquinoxalin-2(1H)-ylidene]ethanoate (4d) in 10 cm3 AcOH, 5.24 g bromoacetaldehyde diethyl acetal (26.6 mmol) was added. The resulting mixture was stirred at 80 ℃ for 6–12 h. After the reaction was completed, the mixture was cooled and the insoluble materials were filtered off, washed with AcOH (2 × 2 cm3), MTBE (2 × 2 cm3), hexane (2 × 4 cm3) and dried under reduced pressure.
1-Oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxylic acid (6a, C8H8N2O3)
Yield: 3.30 g (69%); m.p.: 274–275 °C; 1H NMR (400 MHz, DMSO-d6): δ = 15.36 (s, OH), 9.08 (s, NH), 7.20 (d, 3JHH = 2.7 Hz, C6H), 6.67 (d, 3JHH = 2.7 Hz, C7H), 4.23 (t, 3JHH = 6.2 Hz, C4H2), 3.66–3.61 (m, C3H2) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 39.30 (C3), 43.28 (C4), 113.20 (C7), 121.27, 121.37 (C8 + C8a), 125.05 (C6), 161.94, 162.73 (C1=O + HO−C=O) ppm; MS (70 eV): m/z = 181 ([M + H]+).
3-Methyl-1-oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxylic acid (6b, C9H10N2O3)
Yield: 3.72 g (72%); m.p.: 222–223 °C; 1H NMR (400 MHz, DMSO-d6): δ = 15.37 (s, OH), 9.14 (s, NH), 7.18 (d, 3JHH = 2.4 Hz, C6H), 6.67 (d, 3JHH = 2.5 Hz, C7H), 4.32 (dd, 2JHH = 12.5, 3JHH = 3.9 Hz, C4H), 4.06–3.97 (m, C3H), 3.92 (dd, 2JHH = 12.4, 3JHH = 9.5 Hz, C4H), 1.23 (d, 3JHH = 6.4 Hz, CH3) ppm; 13C NMR (151 MHz, DMSO-d6): δ = 17.56 (CH3), 46.41 (C3), 48.88 (C4), 113.22 (C7), 120.83, 121.16 (C8 + C8a), 125.00 (C6), 161.54 (C1=O), 162.59 (OH−C=O) ppm; MS (70 eV): m/z = 195 ([M + H]+).
3,3-Dimethyl-1-oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxylic acid (6c, C10H12N2O3)
Yield: 4.37 g (79%); m.p.: 243–244 °C; 1H NMR (400 MHz, DMSO-d6): δ = 15.36 (s, OH), 9.20 (s, NH), 7.17 (d, 3JHH = 2.5 Hz, C6H), 6.68 (d, 3JHH = 2.6 Hz, C7H), 4.11 (s, C4H2), 1.27 (s, 2CH3) ppm; 13C NMR (151 MHz, DMSO-d6): δ = 25.63 (2CH3), 52.50 (C3), 53.36 (C4), 113.31 (C7), 120.26, 121.28 (C8 + C8a), 125.22 (C6), 160.89 (C1=O), 162.57 (OH−C=O) ppm; MS (70 eV): m/z = 209 ([M + H]+).
trans-4-Oxo-4,5,5a,6,7,8,9,9a-octahydropyrrolo[1,2-a]quinoxaline-3-carboxylic acid (6d, C12H14N2O3)
Yield: 5.44 g (87%); m.p.: 245–246 °C; 1H NMR (400 MHz, CDCl3): δ = 14.75 (s, OH), 7.07 (s, NH), 6.96 (d, 3JHH = 2.7 Hz, C1H), 6.94 (d, 3JHH = 2.7 Hz, C2H), 3.81 (td, 3JHH = 11.3, 4.0 Hz, C9aH), 3.57 (td, 3JHH = 11.5, 3.9 Hz, C5aH), 2.58 (dd, 3JHH = 12.0, 2.9 Hz, 1H), 2.14–2.11 (m, 1H), 2.07–2.00 (m, 1H), 1.96–1.93 (m, 1H), 1.69–1.57 (m, 2H), 1.55–1.41 (m, 2H) ppm; 13C NMR (151 MHz, DMSO-d6): δ = 22.97, 23.21 (C7 + C8), 26.74, 28.75 (C6 + C9), 55.34 (C5a), 57.50 (C9a), 113.40 (C2), 121.58 (C1), 121.75, 121.94 (C3 + C3a), 161.89 (C4=O), 162.76 (HO−C=O) ppm; MS (70 eV): m/z = 235 ([M + H]+).
X-ray diffraction study of compound 6d
The colourless crystals of compound 6d (C12H14N2O3) are monoclinic. At 293 K a = 8.033(2) Å, b = 11.939(4) Å, c = 12.172(3) Å, β = 98.645(15)°, V = 1154.0(6) Ǻ3, Mr = 234.25, Z = 4, space group P21/n, dcalc = 1.348 g/cm3, m(MoKa) = 0.098 mm−1, F(000) = 496. Intensities of 13,891 reflections (2033 independent, Rint = 0.025) were measured on the Bruker APEX II diffractometer (graphite monochromated MoKα radiation, CCD detector, ω-scaning, 2Θmax = 50°). The structure was solved by direct method using SHELXTL package [51]. Some restrictions were applied for the bond distances within the disordered fragment (Csp3–Csp3 = 1.54 Å, Csp3–N = 1.47 Å). Positions of the hydrogen atoms were located from electron density difference maps and refined using “riding” model with Uiso = 1.2 Ueq of the carrier atom. Hydrogen bond of the carboxylic group is refined isotropycally. Full-matrix least-squares refinement against F2 in anisotropic approximation for non-hydrogen atoms using 2033 reflections was converged to wR2 = 0.246 (R1 = 0.119 for 1718 reflections with F > 4σ(F), S = 1.114). The final atomic coordinates, and crystallographic data for molecule 6d have been deposited to with the Cambridge Crystallographic Data Centre, 12 Union Road, CB2 1EZ, UK (fax: + 44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk) and are available on request quoting the deposition numbers CCDC 2225234).
Synthesis of N-substituted 1-oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxamides 8a-8o and N-substituted trans-4-oxo-4,5,5a,6,7,8,9,9a-octahydropyrrolo[1,2-a]quinoxaline-3-carboxamides 8p–8t (general method)
To a solution of 1.28 mmol of 1-oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxylic acids 6a–6c or trans-4-oxo-4,5,5a,6,7,8,9,9a-octahydropyrrolo[1,2-a]quinoxaline-3-carboxylic acid (6d) in 3 cm3 DMF, 1.34 mmol of corresponding amines 7a–7i, 0.22 g DIPEA (1.66 mmol), and 0.58 g HATU (1.54 mmol) were added. The resulting mixture was stirred at 50 ℃ for 8–25 h. After the reaction was completed, for the compounds 8c, 8e, 8g, 8j–8m, 8o, 8q, 8s, and 8t the reaction mixture was cooled and water (5 cm3) added, the insoluble materials were filtered off, washed with H2O (2 × 5 cm3), hexane (2 × 4 cm3) and dried under reduced pressure. For the compounds 8a, 8b, 8d, 8f, 8h, 8i, 8n, 8p, 8r the obtained mixture was evaporated under reduced pressure. The formed precipitate was dissolved in 50 cm3 CHCl3, washed with with H2O (2 × 2 cm3) and brine (2 × 2 cm3), the organic phase was dried over Na2SO4 and evaporated under reduced pressure. The formed precipitate was purified by column chromatography on silica gel, eluent CH2Cl2–MeOH, 25:1 (for compound 8d), CHCl3–MeOH, 20:1 (for compounds 8a, 8b, 8f, 8i, 8n, 8p), and CHCl3–MeOH, 10:1 (for compounds 8h, 8r).
N-Benzyl-1-oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxamide (8a, C15H15N3O2)
Yield: 0.13 g (38%); m.p.: 164–165 °C; 1H NMR (400 MHz, CDCl3): δ = 10.90 (s, NH), 7.38 (d, 3JHH = 7.4 Hz, 2HAr), 7.30 (t, 3JHH = 7.5 Hz, 2HAr), 7.22 (t, 3JHH = 7.3 Hz, HAr), 7.02 (d, 3JHH = 2.7 Hz, C6H), 6.76 (d, 3JHH = 2.8 Hz, C7H), 6.69 (s, C1NH), 4.62 (d, 3JHH = 5.3 Hz, CH2Ph), 4.11 (t, 3JHH = 5.9 Hz, C4H2), 3.58–3.46 (m, C3H2) ppm; 13C NMR (151 MHz, CDCl3): δ = 39.92 (C3), 43.62, 44.39 (C4 + C−Ph), 114.08 (C7), 120.17 (C8), 123.39 (C6), 126.16 (C8a), 126.92 (CAr), 127.80 (2CAr), 128.50 (2CAr), 139.31 (CAr), 162.19, 162.78 (C1=O + O = C−NHBn) ppm; MS (70 eV): m/z = 270 ([M + H]+).
N-Butyl-1-oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxamide (8b, C12H17N3O2)
Yield: 0.10 g (33%); m.p.: 138–139 °C; 1H NMR (400 MHz, CDCl3): δ = 10.38 (s, NH), 7.01 (d, 3JHH = 2.8 Hz, C6H), 6.77 (d, 3JHH = 2.8 Hz, C7H), 5.99 (s, C1NH), 4.21–4.16 (m, C4H2), 3.74–3.68(m, C3H2), 3.44–3.38 (m, NH–CH2–CH2), 1.64–1.56 (m, CH2–CH2–CH3), 1.48–1.38 (m, CH2–CH3), 0.94 (t, 3JHH = 7.3 Hz, CH3) ppm; 13C NMR (151 MHz, CDCl3): δ = 13.92 (CH3), 20.34 (CH2–CH3), 31.60 (CH2–CH2–CH3), 39.23, 40.13 (NH–CH2 + C3), 44.49 (C4), 113.92 (C7), 120.04 (C8), 123.30 (C6), 126.58 (C8a), 162.19, 162.67 (C1=O + O=C−NHBu) ppm; MS (70 eV): m/z = 236 ([M + H]+).
N-(4-Fluorophenyl)-1-oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxamide (8c, C14H12FN3O2)
Yield: 0.21 g (60%); m.p.: 205–206 °C; 1H NMR (400 MHz, DMSO-d6): δ = 13.24 (s, NH), 8.58 (s, C1−NH), 7.67 (dd, 3JHH = 9.0, 4JHF = 5.0 Hz, 2HAr), 7.20–7.13 (m, 2HAr + C6H), 6.78 (d, 3JHH = 2.7 Hz, C7H), 4.23 (t, 3JHH = 6.1 Hz, C4H2), 3.62–3.56 (m, C3H2) ppm; 13C NMR (151 MHz, DMSO-d6): δ = 39.19 (C3), 43.84 (C4), 112.52 (C7), 115.49 (d, 2JCF = 22.3 Hz, CHAr), 120.23 (C8), 120.60 (d, 3JCF = 7.7 Hz, CHAr), 124.13 (C6), 124.58 (C8a), 135.98 (d, 4JCF = 1.8 Hz, CAr), 157.84 (d, 1JCF = 239.4 Hz, F−CAr), 160.14, 161.58 (C1=O + O=C−NHAr) ppm; 19F NMR (376 MHz, CDCl3): δ = −120.1 (CF) ppm; MS (70 eV): m/z = 274 ([M + H]+).
N-(2-Methoxyphenyl)-1-oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxamide (8d, C15H15N3O3)
Yield: 0.16 g (44%); m.p.: 173–174 °C; 1H NMR (400 MHz, DMSO-d6): δ = 13.25 (s, NH−Ar), 8.65 (s, NH), 7.44 (s, 1HAr), 7.23 (t, 3JHH = 8.1 Hz, 1HAr), 7.16 (d, 3JHH = 2.8 Hz, C6H), 7.11 (d, 3JHH = 8.0 Hz, 1HAr), 6.78 (d, 3JHH = 2.2 Hz, C7H), 6.63 (d, 3JHH = 8.3 Hz, 1HAr), 4.22 (t, 3JHH = 6.0 Hz, C4H2), 3.75 (s, OCH3), 3.61–3.56 (m, C3H2), ppm; 13C NMR (126 MHz, DMSO-d6): δ = 39.18 (C3) 43.84 (C4), 54.95 (OCH3), 104.76, 108.46, 111.30, 112.55, 120.19 (C8), 124.11, 124.73 (C8a), 129.66, 140.74 (CAr−NH), 159.69 (CAr−OCH3), 160.24, 161.58 (C1=O + O=C−NHAr) ppm; MS (70 eV): m/z = 286 ([M + H]+).
1-Oxo-N-[4-(trifluoromethyl)phenyl]-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxamide (8e, C15H12F3N3O2)
Yield: 0.29 g (71%); m.p.: 259–260 °C; 1H NMR (400 MHz, DMSO-d6): δ = 13.63 (s, NH−Ar), 8.71 (s, C1−NH), 7.86 (d, 3JHH = 7.5 Hz, 2HAr), 7.70 (d, 3JHH = 7.6 Hz, 2HAr), 7.19 (s, C6H), 6.80 (s, C7H), 4.27–4.21 (m, C4H2), 3.63–3.58 (m, C3H2) ppm; 13C NMR (151 MHz, DMSO-d6): δ = 39.18 (C3), 43.85 (C4), 112.64 (C7), 118.86 (2CHAr), 120.48 (C8), 122.93 (q, 2JCF = 31.9 Hz, CAr−CF3), 124.18 (C8a), 124.31 (C6), 124.45 (q, 1JCF = 271.3 Hz, CF3), 126.24 (q, 3JCF = 3.4 Hz, 2CHAr), 143.13 (CAr), 160.70, 161.56 (C1=O + O=C−NHAr) ppm; 19F NMR (376 MHz, DMSO-d6): δ = −60.7 (CF3) ppm; MS (70 eV): m/z = 324 ([M + H]+).
N-Butyl-3-methyl-1-oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxamide (8f, C13H19N3O2)
Yield: 0.10 g (31%); m.p.: 194–195 °C; 1H NMR (400 MHz, CDCl3): δ = 10.40 (s, NH), 6.99 (d, 3JHH = 2.8 Hz, C6H), 6.74 (d, 3JHH = 2.7 Hz, C7H), 6.40 (s, C1−NH), 4.14 (dd, 2JHH = 12.2, 3JHH = 4.0 Hz, C4HH), 4.07–3.98 (m, C3H), 3.85 (dd, 2JHH = 12.3, 3JHH = 9.8 Hz, C4HH), 3.44–3.38 (m, NH−CH2), 1.63–1.55 (m, CH2−CH2−CH3), 1.47–1.35 (m, CH2−CH3 + C3−CH3), 0.93 (t, 3JHH = 7.3 Hz, CH2−CH3) ppm; 13C NMR (151 MHz, CDCl3): δ = 13.93 (CH3−CH2), 18.29 (C3−CH3), 20.39 (CH2−CH3), 31.74 (CH2−CH2−CH3), 39.26 (CH2−CH2−CH2−CH3), 46.95 (C3), 50.70 (C4), 114.08 (C7), 119.49 (C8), 123.35 (C6), 126.57 (C8a), 162.03, 162.62 (C1=O + O=C−NHBu) ppm; MS (70 eV): m/z = 250 ([M + H]+).
N-(4-Chlorophenyl)-3-methyl-1-oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxamide (8g, C15H14ClN3O2)
Yield: 0.29 g (74%); m.p.: 199–200 °C; 1H NMR (400 MHz, DMSO-d6): δ = 13.34 (s, NH−Ar), 8.63 (s, C1−NH), 7.69 (d, 3JHH = 8.4 Hz, 2HAr), 7.38 (d, 3JHH = 8.4 Hz, 2HAr), 7.13 (d, 3JHH = 2.8 Hz, C6H), 6.79 (d, 3JHH = 2.8 Hz, C7H), 4.31 (d, 3JHH = 10.1 Hz, C4H), 3.94–3.89 (m, C3H + C4H), 1.23 (d, 3JHH = 5.6 Hz, CH3) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 17.75 (CH3), 46.10 (C3), 49.51 (C4), 112.65 (C7), 119.81 (C8), 120.47 (2CHAr), 124.26 (C6), 124.35 (C8a), 126.46 (CAr), 128.85 (2CHAr), 138.49 (CAr), 160.30, 161.25 (C1=O + O=C−NHAr) ppm; MS (70 eV): m/z = 304, 306 ([M + H]+).
N,3-Dimethyl-1-oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxamide (8h, C10H13N3O2)
Yield: 0.08 g (30%); m.p.: 206–207 °C; 1H NMR (400 MHz, DMSO-d6): δ = 10.56 (s, NH), 8.28 (s, C1−NH), 7.01 (d, 3JHH = 2.7 Hz, C6H), 6.66 (d, 3JHH = 2.9 Hz, C7H), 4.28–4.21 (m, C4H), 3.91–3.80 (m, C3H + C4H), 2.77 (d, 3JHH = 4.4 Hz, NH–CH3), 1.20 (d, 3JHH = 5.8 Hz, C3–CH3) ppm; 13C NMR (151 MHz, DMSO-d6): δ = 17.77 (CH3−C3), 25.49 (CH3−NH), 46.04 (C3), 49.55 (C4), 112.14 (C7), 119.70 (C8), 123.58 (C6), 124.29 (C8a), 160.89, 162.51 (C1=O + O=C−NHCH3) ppm; MS (70 eV): m/z = 208 ([M + H]+).
3-Methyl-1-oxo-N-(2-propyl)-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxamide (8i, C12H17N3O2)
Yield: 0.18 g (59%); m.p.: 154–155 °C; 1H NMR (400 MHz, CDCl3): δ = 10.33 (s, NH−iPr), 7.00 (d, 3JHH = 2.6 Hz, C6H), 6.74 (d, 3JHH = 2.7 Hz, C7H), 5.99 (s, C1−NH), 4.26–4.18 (m, CH−(CH3)2), 4.15 (dd, 2JHH = 12.2, 3JHH = 3.7 Hz, C4HH), 4.06–3.98 (m, C3H), 3.85 (dd, 2JHH = 12.2, 3JHH = 9.6 Hz, C4HH), 1.37 (d, 3JHH = 6.4 Hz, C3−CH3), 1.25 (dd, 3JHH = 6.5, 4.5 Hz, 2CH3) ppm; 13C NMR (151 MHz, DMSO-d6): δ = 17.83 (C3−CH3), 22.55, 22.60 (N−CH(CH3)2), 40.29 (N−CH(CH3)2), 46.01 (C3), 49.55 (C4), 112.15 (C7), 119.63 (C8), 123.51 (C6), 124.75 (C8a), 160.90, 160.93 (C1=O + O=C−NHiPr) ppm; MS (70 eV): m/z = 236 ([M + H]+).
3-Methyl-1-oxo-N-[4-(trifluoromethyl)phenyl]-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxamide (8j, C16H14F3N3O2)
Yield: 0.34 g (78%); m.p.: 237–238 °C; 1H NMR (400 MHz, DMSO-d6): δ = 13.63 (s, NH−Ar), 8.75 (s, C1−NH), 7.86 (d, 3JHH = 8.3 Hz, 2HAr), 7.70 (d, 3JHH = 8.6 Hz, 2HAr), 7.16 (d, 3JHH = 2.2 Hz, C6H), 6.81 (d, 3JHH = 2.4 Hz, C7H), 4.32 (d, 3JHH = 10.6 Hz, C4H), 3.98–3.90 (m, C3H + C4H), 1.23 (d, 3JHH = 4.1 Hz, CH3) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 17.71 (CH3), 46.10 (C3), 49.51 (C4), 112.73 (C7), 118.84 (2CHAr), 119.99 (C8), 122.92 (q, 2JCF = 31.8 Hz), 124.10 (C8a), 124.33 (C6), 124.43 (q, 1JCF = 271.4 Hz), 126.23 (q, 3JCF = 4.2 Hz), 143.11 (CAr), 160.67, 161.24 (C1=O + O=C−NAr) ppm; 19F NMR (376 MHz, DMSO-d6): δ = −60.9 (CF3) ppm; MS (70 eV): m/z = 338 ([M + H]+).
N-(4-Fluorophenyl)-3,3-dimethyl-1-oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxamide (8k, C16H16FN3O2)
Yield: 0.26 g (68%); m.p.: 213–214 °C; 1H NMR (400 MHz, DMSO-d6): δ = 13.23 (s, NH − Ar), 8.62 (s, C1NH), 7.67 (dd, 3JHH = 8.8, 4JHF = 5.0 Hz, 2HAr), 7.16 (dd, 3JHH = 8.7, 3JHF = 8.7 Hz, 2HAr), 7.11 (d, 3JHH = 2.7 Hz, C6H), 6.80 (d, 3JHH = 2.8 Hz, C7H), 4.09 (s, C4H2), 1.27 (s, C3(CH3)2) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 25.66 (2CH3), 51.81, 53.98 (C3 + C4), 112.65 (C7), 115.48 (d, 2JCF = 22.1 Hz), 119.18 (C8), 120.60 (d, 3JCF = 7.7 Hz), 124.33 (C6), 124.52 (C8a), 135.93 (d, 4JCF = 2.2 Hz), 157.83 (d, 1JCF = 239.4 Hz), 160.10, 160.66 (C1=O + (O=C−NHAr) ppm; 19F NMR (376 MHz, CDCl3): δ = -120.1 (CF) ppm; MS (70 eV): m/z = 302 ([M + H]+).
3,3-Dimethyl-N-(4-methylphenyl)-1-oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxamide (8l, C17H19N3O2)
Yield: 0.27 g (70%); m.p.: 258–259 °C; 1H NMR (400 MHz, DMSO-d6): δ = 13.06 (s, NH−Ar), 8.55 (s, C1−NH), 7.55 (d, 3JHH = 8.1 Hz, 2HAr), 7.13 (d, 3JHH = 8.2 Hz, 2HAr), 7.09 (d, 3JHH = 2.5 Hz, C6H), 6.79 (d, 3JHH = 2.7 Hz, C7H), 4.08 (s, C4H2), 2.27 (s, CH3−Ar), 1.27 (s, C3(CH3)2) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 20.45 (CH3−Ar), 25.68 (C3(CH3)2), 51.80, 53.99 (C3 + C4), 112.64 (C7), 118.95 (2CHAr), 119.06 (C8), 124.26 (C6), 124.81 (C8a), 129.30 (2CHAr), 131.84 (CAr), 137.04 (CAr), 159.97, 160.66 (C1=O + (O=C−NHAr) ppm; MS (70 eV): m/z = 298 ([M + H]+).
N-(2-Methoxyphenyl)-3,3-dimethyl-1-oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxamide (8m, C17H19N3O3)
Yield: 0.22 g (54%); m.p.: 177–178 °C; 1H NMR (400 MHz, DMSO-d6): δ = 13.26 (s, NH−Ar), 8.74 (s, C1NH), 7.43 (s, 1HAr), 7.23 (t, 3JHH = 7.8 Hz, 1HAr), 7.13–7.10 (m, C6H + 1HAr), 6.79 (d, 3JHH = 2.3 Hz, C7H), 6.63 (d, 3JHH = 8.2 Hz, 1HAr), 4.09 (s, C4H2), 3.74 (s, OCH3), 1.26 (s, C3(CH3)2) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 25.67 (C3(CH3)2, 51.80, 53.98 (C3 + C4), 54.92 (OCH3), 104.77, 108.47, 111.28, 112.70, 119.15 (C8), 124.34, 124.69 (C8a), 129.68, 140.72 (CAr−NH), 159.68(CAr−OCH3), 160.21, 160.68 (C1=O + O=C−NHAr) ppm; MS (70 eV): m/z = 314 ([M + H]+).
3,3-Dimethyl-1-oxo-N-(2-propyl)-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxamide (8n, C13H19N3O2)
Yield: 0.20 g (64%); m.p.: 201–202 °C; 1H NMR (400 MHz, DMSO-d6): δ = 10.73 (d, 3JHH = 6.9 Hz, NH−CH), 8.34 (s, C1 − NH), 7.02 (d, 3JHH = 2.7 Hz, C6H), 6.65 (d, 3JHH = 2.5 Hz, C7H), 4.01 (s, C4H2), 3.98–3.90 (m, CH−(CH3)2), 1.22 (s, C3−(CH3)2), 1.13 (d, 3JHH = 6.6 Hz, CH−(CH3)2) ppm; 13C NMR (151 MHz, DMSO-d6): δ = 22.57 (CH−(CH3)2), 25.72 (C3−(CH3)2), 40.32 (CH−(CH3)2), 51.59 (C3), 54.03 (C4), 112.19 (C7), 119.06 (C8), 123.68 (C6), 124.77 (C8a), 160.33, 160.90 (C1=O + O=C−NiPr) ppm; MS (70 eV): m/z = 250 ([M + H]+).
3,3-Dimethyl-1-oxo-N-[4-(trifluoromethyl)phenyl]-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxamide (8o, C17H16F3N3O2)
Yield: 0.34 g (75%); m.p.: 228–229 °C; 1H NMR (400 MHz, DMSO-d6): δ = 13.64 (s, NH−Ar), 8.79 (s, C1−NH), 7.86 (d, 3JHH = 8.1 Hz, 2HAr), 7.70 (d, 3JHH = 8.3 Hz, 2HAr), 7.15 (d, 3JHH = 2.7 Hz, C6H), 6.81 (d, 3JHH = 2.7 Hz, C7H), 4.10 (s, C4H2), 1.26 (s, C3(CH3)2) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 25.66 (2CH3), 51.87, 53.97 (C3 + C4), 112.79 (C7), 118.85 (2CHAr), 119.42 (C8), 122.93 (q, 2JCF = 31.2 Hz), 124.14 (C8a), 124.42 (q, 1JCF = 270.8 Hz), 124.52 (C6), 126.24 (q, 3JCF = 4.5 Hz), 143.09 (CAr), 160.63, 160.65 (C1=O + O=C−NAr) ppm; 19F NMR (376 MHz, DMSO-d6): δ = −60.7 (CF3) ppm; MS (70 eV): m/z = 352 ([M + H]+).
trans-N-Benzyl-4-oxo-4,5,5a,6,7,8,9,9a-octahydropyrrolo[1,2-a]quinoxaline-3-carboxamide (8p, C19H21N3O2)
Yield: 0.17 g (40%); m.p.: 194–195 °C; 1H NMR (400 MHz, DMSO-d6): δ = 11.30 (t, 3JHH = 5.0 Hz, NH–Bn), 8.49 (s, C4−NH), 7.34–7.29 (m, 4HAr), 7.26–7.21 (m, 1HAr), 7.18 (d, 3JHH = 2.5 Hz, C1H), 6.72 (d, 3JHH = 2.5 Hz, C2H), 4.53–4.43 (m, CH2–Ph), 3.80–3.73 (m, C9aH), 3.43–3.34 (m, C5aH), 2.58 (d, 3JHH = 9.4, 1H), 1.99 (d, 3JHH = 10.7 Hz, 1H), 1.84 (d, 3JHH = 7.7 Hz, 1H), 1.74 (d, 3JHH = 10.2 Hz, 1H), 1.50–1.29 (m, 4H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 22.95, 23.30 (C7 + C8), 26.97, 28.93 (C6 + C9), 42.35 (CH2−Bn), 54.99 (C5a), 57.69 (C9a), 112.50 (C2), 119.97 (C1), 120.86 (C3), 124.76 (C3a), 126.71 (CAr), 127.14 (2CAr), 128.34 (2CAr), 139.56 (CAr), 161.15, 162.06 (C4=O + O=C−NHBn) ppm; MS (70 eV): m/z = 324 ([M + H]+).
trans-N-(4-Chlorophenyl)-4-oxo-4,5,5a,6,7,8,9,9a-octahydropyrrolo[1,2-a]quinoxaline-3-carboxamide (8q, C18H18ClN3O2)
Yield: 0.33 g (74%); m.p.: > 300 °C; 1H NMR (400 MHz, DMSO-d6): δ = 13.45 (s, NH−Ar), 8.78 (s, C4−NH), 7.69 (d, 3JHH = 8.8 Hz, 2HAr), 7.39 (d, 3JHH = 8.7 Hz, 2HAr), 7.25 (d, 3JHH = 2.9 Hz, C1H), 6.81 (d, 3JHH = 2.8 Hz, C2H), 3.83 (td, 3JHH = 10.4 Hz, 3JHH = 3.5 Hz, C9aH), 3.45 (td, 3JHH = 11.2 Hz, 3JHH = 3.5 Hz, C5aH), 2.60 (d, 3JHH = 9.0 Hz, 1H), 2.03 (d, 3JHH = 12.1 Hz, 1H), 1.85 (d, 3JHH = 10.0 Hz, 1H), 1.75 (d, 3JHH = 11.3 Hz, 1H), 1.52–1.30 (m, 4H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 22.95, 23.30 (C7 + C8), 26.92, 28.90 (C6 + C9), 54.99 (C5a), 57.74 (C9a), 112.78 (C2), 120.52 (2CHAr), 120.57 (C1), 120.78 (C3), 125.00 (C3a), 126.50 (CAr), 128.89 (2CHAr), 138.50(CAr), 160.41, 161.49 (C4=O + O=C−NHAr) ppm; MS (70 eV): m/z = 344, 346 ([M + H]+).
trans-N-Methyl-4-oxo-4,5,5a,6,7,8,9,9a-octahydropyrrolo[1,2-a]quinoxaline-3-carboxamide (8r, C13H17N3O2)
Yield: 0.10 g (32%); m.p.: 247–248 °C; 1H NMR (400 MHz, DMSO-d6): δ = 10.62 (s, NH), 8.33 (s, C4−NH), 7.12 (d, 3JHH = 2.8 Hz, C1H), 6.69 (d, 3JHH = 2.8 Hz, C2H), 3.75 (td, 3JHH = 10.8, 3JHH = 4.2 Hz, C9aH), 3.37 (td, 3JHH = 11.1, 3JHH = 4.0 Hz, C5aH), 2.77 (d, 3JHH = 4.5 Hz, CH3), 2.57 (d, 3JHH = 7.0 Hz, 1H), 2.02 (d, 3JHH = 8.2 Hz, 1H), 1.85 (d, 3JHH = 6.9 Hz, 1H), 1.75 (d, 3JHH = 10.9 Hz, 1H), 1.53–1.30 (m, 4H) ppm; 13C NMR (151 MHz, DMSO-d6): δ = 22.92, 23.29 (C7 + C8), 25.51 (CH3), 26.94, 28.91 (C6 + C9), 54.99 (C5a), 57.65 (C9a), 112.26 (C2), 119.79 (C1), 120.67, 124.94 (C3 + C3a), 161.08, 162.59 (C4=O + O=C−NHMe) ppm; MS (70 eV): m/z = 248 ([M + H]+).
trans-N-(4-Methylphenyl)-4-oxo-4,5,5a,6,7,8,9,9a-octahydropyrrolo[1,2-a]quinoxaline-3-carboxamide (8s, C19H21N3O2)
Yield: 0.28 g (69%); m.p.: > 300 °C; 1H NMR (400 MHz, DMSO-d6): δ = 13.20 (s, NH − Ar), 8.72 (s, C4−NH), 7.55 (d, 3JHH = 8.0 Hz, 2HAr), 7.24 (s, C1H), 7.13 (d, 3JHH = 8.0 Hz, 2HAr), 6.79 (s, C2H), 3.88–3.77 (m, 1H), 3.50–3.40 (m, 1H), 2.61–2.59 (m, 1H), 2.26 (s, CH3), 2.04–2.01 (m, 1H), 1.86–1.84 (m, 1H), 1.77–1.74 (m, 1H), 1.53–1.33 (m, 4H) ppm; 13C NMR (151 MHz, DMSO-d6): δ = 20.45 (CH3−Ar), 22.92, 23.28 (C7 + C8), 26.91, 28.90 (C6 + C9), 54.97 (C5a), 57.71 (C9a), 112.71 (C2), 118.96 (2CHAr), 120.34 (C1), 120.59 (C3), 125.44 (C3a), 129.29 (2CHAr), 131.84 (CAr), 137.07 (CAr), 160.05, 161.48 (C4=O + O=C−NHAr) ppm; MS (70 eV): m/z = 324 ([M + H]+).
trans-4-Oxo-N-[4-(trifluoromethyl)phenyl]-4,5,5a,6,7,8,9,9a-octahydropyrrolo[1,2-a]quinoxaline-3-carboxamide (8t, C19H18F3N3O2)
Yield: 0.35 g (73%); m.p.: > 300 °C; 1H NMR (400 MHz, DMSO-d6): δ = 13.70 (s, NH−Ar), 8.83 (s, NH), 7.86 (d, 3JHH = 8.4 Hz, 2HAr), 7.70 (d, 3JHH = 8.4 Hz, 2HAr), 7.28 (d, 3JHH = 2.9 Hz, C1H), 6.83 (d, 3JHH = 2.8 Hz, C2H), 3.85 (td, 3JHH = 10.9 Hz, 3JHH = 3.0 Hz, C9aH), 3.47 (td, 3JHH = 11.4 Hz, 3JHH = 2.8 Hz, C5aH), 2.61 (d, 3JHH = 8.3 Hz, 1H), 2.04 (d, 3JHH = 10.4 Hz, 1H), 1.86 (d, 3JHH = 8.8 Hz, 1H), 1.76 (d, 3JHH = 11.6 Hz, 1H), 1.54–1.31 (m, 4H) ppm; 13C NMR (76 MHz, DMSO-d6): δ = 22.84, 23.19 (C7 + C8), 26.83, 28.81 (C6 + C9), 54.94 (C5a), 57.70 (C9a), 112.76 (C2), 118.82 (2CHAr), 120.47 (C1), 120.88 (C3), 122.89 (q, 2JCF = 31.8 Hz), 124.33 (q, 1JCF = 271.1 Hz), 124.69 (C3a), 126.15 (2CHAr), 143.03 (CAr), 160.67, 161.39 (C4=O + O=C−NHAr) ppm; 19F NMR (376 MHz, DMSO-d6): δ = −60.7 (CF3) ppm; MS (70 eV): m/z = 378 ([M + H]+).
Study of antimicrobial activity
The antimicrobial activity of the synthesized compounds was investigated by the method of nutrient broth microdilution as recommended by EUCAST (European Committee on antimicrobial susceptibility testing) [52]. According to this method, the minimal inhibitory concentration (MIC) was determined as the concentration of every synthesized compound required to suppress the proliferation of the given microbial culture in the multihole microplate. The stock 1000 μg/cm3 solution was prepared by dissolving the required amount of a compound in dimethylsulfoxide (DMSO). Further, diluted solutions with the concentrations from 500 to 3.9 μg/cm3 (or from 500 to 0.48 μg/cm3 in the case of controls) were used to find the MIC values. The sensitivity of every microbial culture to every concentration of the synthesized compounds was tested three times. In addition, the control experiments were carried out to check the proliferation of microbes in the clean broth, in the same broth with an admixture of DMSO, and in the broth with DMSO and the control dugs (decasan and clotrimazole) (Tables 3, 4). The control clear broth remained sterile and transparent (no proliferation of the microbial cultures), while some proliferation of the cultures has been registered in the case of a mixture of DMSO and the broth.
Data availability
We declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. Should any raw data files be needed in another format, they are available from the corresponding author upon reasonable request.
References
Winant P, Horsten T, de Gil Melo SM, Emery F, Dehaen W (2021) Organics 2:118. https://doi.org/10.3390/org2020011
Uemoto H, Tsuda M, Kobayashi J (1999) J Nat Prod 62:1581. https://doi.org/10.1021/np9902542
Cafieri F, Fattorusso E, Mangoni A, Taglialatela-Scafati O (1995) Tetrahedron Lett 36:7893. https://doi.org/10.1016/0040-4039(95)01626-S
Li T, Wang N, Zhang T, Zhang B, Sajeevan TP, Joseph V, Armstrong L, He S, Yan X, Naman CB (2019) Mar Drugs 17:493. https://doi.org/10.3390/md17090493
Ebada SS, Linh MH, Longeon A, de Voogd NJ, Durieu E, Meijer L, Bourguet-Kondracki M-L, Singab ANB, Müller WEG, Proksch P (2015) Nat Prod Res 29:231. https://doi.org/10.1080/14786419.2014.947496
Cafieri F, Fattorusso E, Taglialatela-Scafati O (1998) J Nat Prod 61:122. https://doi.org/10.1021/np970323h
Scala F, Fattorusso E, Menna M, Taglialatela-Scafati O, Tierney M, Kaiser M, Tasdemir D (2010) Mar Drugs 8:2162. https://doi.org/10.3390/md8072162
Mancini I, Guella G, Amade P, Roussakis C, Pietra F (1997) Tetrahedron Lett 38:6271. https://doi.org/10.1016/S0040-4039(97)01405-6
Sun J, Wu J, An B, De Voogd NJ, Cheng W, Lin W (2018) Mar Drugs 16:9. https://doi.org/10.3390/md16010009
Mashiko T, Kumagai N, Shibasaki M (2008) Org Lett 10:2725. https://doi.org/10.1021/ol8008446
Meyers KM, Méndez-Andino J, Colson A-O, Hu XE, Wos JA, Mitchell MC, Hodge K, Howard J, Paris JL, Dowty ME, Obringer CM, Reizes O (2007) Bioorg Med Chem Lett 17:657. https://doi.org/10.1016/j.bmcl.2006.10.096
Ward RA, Bethel P, Cook C, Davies E, Debreczeni JE, Fairley G, Feron L, Flemington V, Graham MA, Greenwood R, Griffin N, Hanson L, Hopcroft P, Howard TD, Hudson J, James M, Jones CD, Jones CR, Lamont S, Lewis R, Lindsay N, Roberts K, Simpson I, St-Gallay S, Swallow S, Tang J, Tonge M, Wang Z, Zhai B (2017) J Med Chem 60:3438. https://doi.org/10.1021/acs.jmedchem.7b00267
Casuscelli F, Ardini E, Avanzi N, Casale E, Cervi G, D’Anello M, Donati D, Faiardi D, Ferguson RD, Fogliatto G, Galvani A, Marsiglio A, Mirizzi DG, Montemartini M, Orrenius C, Papeo G, Piutti C, Salom B, Felder ER (2013) Bioorg Med Chem 21:7364. https://doi.org/10.1016/j.bmc.2013.09.054
Micheli F, Cavanni P, Di Fabio R, Marchioro C, Donati D, Faedo S, Maffeis M, Sabbatini FM, Tranquillini ME (2006) Bioorg Med Chem Lett 16:1342. https://doi.org/10.1016/j.bmcl.2005.11.049
Fisher TE, Kim B, Staas DD, Lyle TA, Young SD, Vacca JP, Zrada MM, Hazuda DJ, Felock PJ, Schleif WA, Gabryelski LJ, Anari MR, Kochanskyd CJ, Wai JS (2007) Bioorg Med Chem Lett 17:6511. https://doi.org/10.1016/j.bmcl.2007.09.086
Piltan M, Moradi L, Abasi G, Zarei SA, Wolfe JP (2013) Beilstein J Org Chem 9:510. https://doi.org/10.3762/bjoc.9.55
Piltan M (2016) J Chem Res 40:410. https://doi.org/10.3184/174751916X14652279155994
Moradi L, Piltan M, Rostami H, Abasi G (2013) Chin Chem Lett 24:740. https://doi.org/10.1016/j.cclet.2013.04.038
Alizadeh A, Abadi MH, Ghanbaripour R (2014) Synlett 25:1705. https://doi.org/10.1055/s-0034-1378275
Weiss MM, Zheng X (2021) IRAK degraders and uses thereof. World Patent WO2021158634 A1 (2021) Chem Abstr 176: 10869-10870
Le Diguarher T, Casara P, Starck J-B, Henlin J-M, Davidson JEP, Murray JB, Graham CJ, Chen I-J, Geneste O, Hickman J, Depil S, Le Tiran A, Nyerges M, De Nanteuil G (2015) Indolizine compounds, a process for their preparation and pharmaceutical compositions containing them. United States Patent US20150051189A1 2015; (2013) Chem Abstr 159:290274
Gupta AK, Chakrasali RT, Ila H, Junjappa H (1989) Synthesis 1989:141. https://doi.org/10.1055/s-1989-27179
Barun O, Chakrabarti S, Ila H, Junjappa H (2001) J Org Chem 66:4457. https://doi.org/10.1021/jo010273s
Fan M-J, Li G-Q, Liang Y-M (2006) Tetrahedron 62:6782. https://doi.org/10.1016/j.tet.2006.04.100
Nami N, Neumuller B, Heravi MM, Haghdadi M (2008) Mendeleev Commun 18:153. https://doi.org/10.1016/j.mencom.2008.05.014
Choudhary G, Peddinti RK (2011) Green Chem 13:3290. https://doi.org/10.1039/C1GC15701A
Kawahara N, Shimamori T, Itoh T, Takayanagi H, Ogura H (1987) Chem Pharm Bull 35:457. https://doi.org/10.1248/cpb.35.457
Kawahara N, Nakajima T, Itoh T, Ogura H (1983) Heterocycles 20:121. https://doi.org/10.3987/r-1983-01-0121
Horsten T, Alegbejo Price TO, Van Meervelt L, da Silva EF, Dehaen W (2022) New J Chem 46:2028. https://doi.org/10.1039/D1NJ04965H
de Figueiredo RM, Suppo J-S, Campagne J-M (2016) Chem Rev 116:12029. https://doi.org/10.1021/acs.chemrev.6b00237
Massolo E, Pirola M, Benaglia M (2020) Eur J Org Chem 2020:4641. https://doi.org/10.1002/ejoc.202000080
Santos AS, Silva AMS, Marques MMB (2020) Eur J Org Chem 2020:2501. https://doi.org/10.1002/ejoc.202000106
Lundberg H, Tinnis F, Selander N, Adolfsson H (2014) Chem Soc Rev 43:2714. https://doi.org/10.1039/C3CS60345H
Carey JS, Laffan D, Thomson C, Williams MT (2006) Org Biomol Chem 4:2337. https://doi.org/10.1039/B602413K
Ghose AK, Viswanadhan VN, Wendoloski JJ (1999) J Comb Chem 1:55. https://doi.org/10.1021/cc9800071
Carpino LA (1993) J Am Chem Soc 115:4397. https://doi.org/10.1021/ja00063a082
Bhatt V, Samant SD, Pednekar S (2017) Lett Org Chem 14:764. https://doi.org/10.2174/1570178614666170710095437
Zefirov NS, Palyulin VA, Dashevskaya EE (1990) J Phys Org Chem 3:147. https://doi.org/10.1002/poc.610030304
Burgi H-B, Dunitz JD (1994) Structure correlation, vol 2. VCH, Weinheim, p 741
El-Hameed RHA, Sayed AI, Ali SM, Mosa MA, Khoder ZM, Fatahala SS (2021) J Enzyme Inhib Med Chem 36:2183. https://doi.org/10.1080/14756366.2021.1984904
Mohamed MS, Fathallah SS (2014) Mini-Rev Org Chem 11:477. https://doi.org/10.2174/1570193x113106660018
Choudhary D, Garg S, Kaur M, Sohal HS, Malhi DS, Kaur L, Verma M, Sharma A, Mutreja V (2023) Polycycl Aromat Compd 43:4512. https://doi.org/10.1080/10406638.2022.2092873
Miyazawa T, Takabatake T, Hasegawa M (1997) J Pharm Soc Japan 117:126. https://doi.org/10.1248/yakushi1947.117.2_126
El-Bayouki KAM, Basyouni WM, Mostafa EA (2010) Collect Czech Chem Commun 75:813. https://doi.org/10.1135/cccc2009566
Andreou D, Essien NB, Pubill-Ulldemolins C, Terzidis MA, Papadopoulos AN, Kostakis GE, Lykakis IN (2021) Org Lett 23:6685. https://doi.org/10.1021/acs.orglett.1c02251
Pontiki E, Hadjipavlou-Litina D, Patsilinakos A, Tran TM, Marson CM (2015) Future Med Chem 7:1937. https://doi.org/10.4155/fmc.15.104
Lima RN, Gonçalves JR, Silva VR, de Santos L, Bezerra DP, Soares MBP, Leitão A, Porto ALM (2020) Curr Bioact Compd 16:900. https://doi.org/10.2174/1573407215666190318144105
Nazarchuk OA (2016) Klin Khir 9:59. PMID: 30265488
Crowley PD, Gallagher HC (2014) J Appl Microbiol 117:611. https://doi.org/10.1111/jam.12554
Brand-Williams W, Cuvelier ME, Berset C (1995) LWT Food Sci Technol 28:25. https://doi.org/10.1016/S0023-6438(95)80008-5
Sheldrick GM (2008) Acta Crystallogr Sect A 64:112. https://doi.org/10.1107/S0108767307043930
Kowalska-Krochmal B, Dudek-Wicher R (2021) Pathogens 10:165. https://doi.org/10.3390/pathogens10020165
Acknowledgements
We are grateful to Enamine Ltd (Kyiv, Ukraine) for support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Litvinchuk, M.B., Bentya, A.V., Grozav, A.M. et al. Synthesis, antimicrobial and antioxidant activity of novel 1-oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-8-carboxylic acids, esters, and amides thereof. Monatsh Chem 154, 1145–1159 (2023). https://doi.org/10.1007/s00706-023-03118-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-023-03118-8