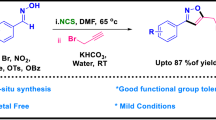
Abstract
A highly efficient, simple, and environmental friendly protocol for the synthesis of 1-hydroxy-2-arylimidazole-3-oxide derivatives was devised using inexpensive and unconventional copper borate (CuB4O7) catalyst under solvent-free condition. The documented method features a wide range of chemical substrate scope. All the products formed were characterized by different analytical and spectroscopic techniques.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the past, drugs containing heterocyclic scaffold have occupied a unique position because of their high therapeutic values. A number of drugs containing the heterocyclic core are in clinical use to treat many infectious diseases [1]. Among a large number and diverse heterocyclic compounds, imidazole, a five-membered nitrogen-containing heterocyclic compound, has gained a lot of interest in the field of drug discovery and has occupied a special position in the field of heterocyclic chemistry [2]. The polar nature of imidazole scaffold could be largely exploited to improve the pharmacokinetic property of drug molecule and help in improving the aqueous solubility of many drugs which are poorly soluble in water [3]. A large variety of compounds containing the imidazole scaffold show promising therapeutic activities such as antiviral [4], antitumor [5], antiinflamatory [6], antidiabetic [7], anticonvulsant [8], antiasthamatic [9] and antiamoebic [10] properties. Etonitazene (analgesic), enviroxime (antiviral), pantoprazole (antiulcer) [11], metronidazole (antibacterial) [12], and carbimazole (antithyroid) [13] are commercially available drugs which contain the imidazole scaffold (Fig. 1).
Interestingly, 1-hydroxyimidazole-3-oxide is regarded as a versatile heterocyclic compound, as these compounds are mostly involved in the synthesis of room temperature ionic liquids [14]. Particularly, the incorporation of the N–O moiety in imidazolium-based ionic liquid may give rise to a greener solvent, which is degradable by design and may fulfill the primary aspect of green chemistry principles [15]. Moreover, the imidazolium salt, namely, 1-alkoxy-3-alkyl-imidazolium salts, were found to be liquid at room temperature, the synthesis of which requires a two-step alkylation of the precursor 1-hydroxyimidazole [16].
The precursor 1-hydroxyimidazole-3-oxide could readily be obtained by cyclization of glyoxime and formaldehyde [17]. The employment of alkyl or aryl aldehydes in the synthesis of 1-hydroxyimidazole results in the formation of the corresponding 1-hydroxy-2-alkylimidazole-3-oxide and 1-hydroxy-2-arylimidazole-3-oxide derivatives, respectively. In general, heterocyclic compounds commonly containing a dipolar R3–N–O linkage, in which the nitrogen is either sp3 or sp2 hybridized, are known as heterocyclic N-oxides [18, 19]. Interestingly, incorporation of a negative charge on the oxygen of heterocyclic N-oxides changes most of the physical properties including the reactivity of the compounds, and the most common heterocyclic N-oxide is pyridine-N-oxide which has excellent donor properties as compared to normal pyridine [20, 21]. Imidazole-N-oxides fall under the category of N-substituted imidazole which has a wide variety of applications. These compounds have gained popularity due to their anti-protozoal [22], fungicidal, herbicidal, pesticidal [23], hypotensive [24], antitumor [25], and antiviral [26] properties (Fig. 2).
Apart from their pharmaceutical applications, imidazole-N-oxides are also used for the generation of N-containing heterocyclic carbenes (NHCs) which act as intermediates in various organic reactions [27]. A current survey revealed that only few works on the synthesis of 1-hydroxy-2-arylimidazole-3-oxide have been documented in the literature [28]. Moreover, the present-day organic synthesis demands a designing of an operationally simple, useful, and practical strategy, which is cost-effective and has less detrimental impact on the environment. A rational design in multicomponent reactions (MCRs) plays a chief role in the current scenario because of the convergent nature, higher atom economy, and easy experimental procedures in the production of target compounds by the introduction of diverse elements in a single operation, with minimization of waste, labor, time, and cost [29,30,31,32,33]. A lot of effort has been incorporated to extend the scope of the well-known classical multicomponent reactions to newer systems. Thus, it was thought worthwhile to undertake the research work in the synthesis of 1-hydroxy-2-arylimidazole-3-oxide derivatives under green chemical condition by employing inexpensive and unconventional copper borate as a catalyst.
Results and discussion
Considering the diverse applications of imidazole-3-oxides and 1-hydroxyimidazole-3-oxide, various methodologies have been developed in the past for the synthesis of these compounds. The most common and the conventional method for the synthesis of imidazole-3-oxides 4 is from mono-oximes (α-hydroxyiminoketones) 1 and aldehydes 2 in the presence of amines 3 [34, 35] (Scheme 1). Substitution of the amine precursor with NH4OAc for the above reaction results in the formation of the 1-hydroxyimidazole derivatives 4 [36, 37] (Scheme 2). However, when hydroxylamine hydrochloride (NH2OH‧HCl) is used instead of amine and NH4OAc, the corresponding 1-hydroxy-2-alkylimidazole-3-oxide or 1-hydroxy-2-arylimidazole-3-oxide is formed depending on the aldehyde precursor used [38] (Scheme 3).



Although the above-mentioned procedures are widely used for the synthesis of imidazole-N-oxides and 1-hydroxyimidazole-3-oxides, there are very few reports on the solvent-free and green procedures for the synthesis of the above-mentioned compounds [34]. These factors prompted us to devise a synthetic method for the synthesis of 1-hydroxy-2-arylimidazole-3-oxide derivatives under solvent-free conditions using copper borate as a catalyst. Thus, in this article, we represent the catalytic efficacy of inexpensive copper borate (CuB4O7) as a catalyst for the solvent-free synthesis of 1-hydroxy-2-aryl-4,5-dimethylimidazole-3-oxide derivatives. Initially, for the synthesis of 1-hydroxy-2-aryl-4,5-dimethylimidazole-3-oxide 4, we selected diacetyl monoxime 1, benzaldehyde (2), and hydroxylamine hydrochloride (NH2OH‧HCl, 3) as model compounds under different reaction conditions at 100 °C for 2 h using varied amounts of catalyst (0.5–3.5 mol%) (Scheme 4).

Again for the optimization of the reaction condition, different solvent systems were chosen taking five polar protic and polar aprotic solvents to screen the efficacy of the employed catalyst for the desired reaction (Table 1). Interestingly it was observed that the above reaction proceeds well in more polar protic and aprotic solvent such as alcohol and DMF and DMSO, respectively (Table 1, entries 1, 2, 3, and 4), but under this condition the reaction needs more time for completion. Therefore, the focus was made toward the green protocol by utilizing solvent-free condition for the synthesis of 1-hydroxy-2-arylimidazole-3-oxide. Moreover, it was observed that under solvent-free condition, the reaction proceeds well and requires less time for completion without forming the by-product and therefore making the workup procedure more facile and efficient (Table 1, entry 6).
The above reaction was carried out with model components using different mol% of the catalyst to optimize the catalyst loading for the desired product and it was observed that the reaction proceeds well when the amount of catalyst loading is 2.5 mol% at the optimized reaction temperature of 100 °C (Table 2). Then the reaction time was optimized for the model reaction and it was found that the reaction is completed within 10 min under solvent-free condition. Therefore, it may be concluded that the reaction is completed when the amount of catalyst loading is 2.5 mol% at 100 °C and at 10 min of reaction time (Table 2, entry 5). It was also observed that only a negligible amount of product was formed in the absence of catalyst (Table 2, entry 1). It was observed that when the amount of catalyst loading is more or less than 2.5 mol%, the isolated yield of the product was found to be decreased by a negligible amount.
After recognizing the optimum reaction conditions, this protocol was utilized to extend the scope by using other aromatic/heteroaromatic aldehydes to synthesize 1-hydroxy-2-aryl/heteroaryl-4,5-dimethylimidazole-3-oxide derivatives 4, and in almost all the cases the reaction proceeded in a short time with excellent yield of the products. With this optimized condition, the reaction protocol was extended for a number of aromatic/heteroaromatic aldehydes having electron-withdrawing and electron-releasing groups and it was observed that this catalytic protocol works well with both types of aldehyde precursors. However, this study shows that aldehydes having electron-withdrawing group successfully afforded the desired product more efficiently in excellent yield than aldehydes having the electron-releasing group (Fig. 3).
Furthermore, the recyclability of the catalyst for the given reaction protocol was adjudged and it was observed that the catalyst eas easily recoverable with simple filtration from the reaction mixture. Thus, the catalyst recovered after the first run of the reaction was purified by simply washing with methanol and drying at 100 °C in an oven. The recovered catalyst was again used for further reaction, and interestingly it was observed that the catalyst did not lose its efficiency up to the fifth run of the reaction (Table 3). However, after the fifth run of the reaction, the morphology of the catalytic surface has changed significantly which resulted in the decrease in yield of the product. Thus, we can infer that the catalyst could be used up to the fifth run for the studied reaction protocol (Figs. 4, 5).
A plausible mechanism for the synthesis of 1-hydroxy-2-aryl-4,5-dimethylimidazole-3-oxide 4 by the condensation of diacetyl monoxime, substituted benzaldehyde, and hydroxylamine hydrochloride is depicted in Fig. 6. As per the literature, it was presumed that the aromatic aldehyde in the presence of hydroxylamine hydrochloride forms the corresponding aldoxime 5 in the presence of the catalyst, which helps in polarizing the oxygen of carbonyl carbon for the nucleophilic attack by hydroxyl amine [39]. A subsequent nucleophilic addition of nitrogen atom of aldoxime 5 to the carbon atom of carbonyl group of diacetyl monoxime (1) followed by cyclization gives the intermediate 6. The intermediate 6 undergoes dehydration, followed by deprotonation to produce 1-hydroxy-2-aryl-4,5-dimethylimidazole-3-oxide 4.
Conclusion
In this article, we have reported an efficient, simple, and green protocol for the synthesis of 1-hydroxy-2-aryl-4,5-dimethylimidazole-3-oxides 4a–4p using an unconventional copper borate catalyst under solvent-free condition. The reaction provides a method for the synthesis of a variety of 1-hydroxy-2-aryl/heteroaryl-4,5-dimethylimidazole-3-oxides with good to excellent yields, and it has been observed that the catalyst is highly recyclable up to the fifth run of the reaction.
Experimental
All starting materials of high purity for the desired synthesis were purchased commercially and used as received. The FT-IR spectra of the prepared compounds were recorded in Bruker Alpha III spectrophotometer operating in the wave number region 4000–400 cm−1 in dry KBr. The melting points of the synthesized compounds were determined by the open capillary method. NMR spectra of the synthesized 1-hydroxy-2aryl/heteroarylimidazole-3-oxide derivatives were recorded at room temperature on an FT-NMR (Bruker Advance-II 400 MHz) spectrometer using DMSO-d6 as solvents and the chemical shifts are quoted in ppm downfield of internal standard tetramethyl silane (TMS). The surface morphology of the catalyst was studied using field emission scanning electron microscopy (FESEM, INSPECT F50, FEI, The Netherlands).
General procedure for the synthesis of 1-hydroxy-2-aryl/heteroaryl-4,5-dimethylimidazole-3-oxides 4a–4p
In a typical green procedure, a mixture of diacetyl monoxime (1 mmol), substituted benzaldehyde/heteroaryl aldehyde (1 mmol), hydroxylamine hydrochloride (2.5 mmol), and copper borate (2.5 mmol) were thoroughly ground and mixed in a mortar and pestle to make a homogeneous mixture. The mixture was then transferred to a test tube. The mixture was heated at 100 °C for 10 min. The progress of the reaction was monitored by TLC using hexane/ethyl acetate (80:20) solvent. After completion of the reaction, the reaction mixture was dissolved in methanol and filtered. The filtrate was evaporated under vacuum and subsequently dried to afford the desired product. All the synthesized products, 4a–4p, were recrystallized from ethanol, characterized by their analytical and spectroscopic data (FT-IR and NMR) and compared with the literature values.
Data availability
All the analytical and spectroscopic data for the synthesized compounds have been embeded in the supplimentary information file.
References
Brahmbhatt H, Molnar M, Pavić V (2018) Karbala Int J Mod Sci 4:200
Verma A, Joshi S, Singh D (2013) J Chem 2013:329412
Manocha P, Wakode SR, Kaur A, Kumar H (2016) Int J Pharm SciRes 1:12
Reyes-Arellano A, Gómez-García O, Torres-Jaramillo (2016) J Med Chem 6:561
Romero DH, Torres Heredia VH, García-Barradas O, López MEM, Pavón ES (2014) J Chem Biochem 2:45
Anand K, Wakode S (2017) Int J Chem Stud 5:350
Verma BK, Kapoor S, Kumar U, Pandey S, Arya P (2017) Ind J Pharm BioRes 5:1
Bhade MW, Rajput PR (2016) Int J Appl Pure Sci Agric 2:80
Katikireddy R, Kakkerla R, Krishna MPSM, Durgaiah G, Reddy YN, Satyanarayana M (2019) Heterocycl Commun 25:27
Goyal A, Singh J, Pathak DP (2013) J Pharm Technol Res Manag 1:69
MacFarlane DR, Seddon KR (2007) Aust J Chem 60:3
Ceruelos AH, Romero-Quezada LC, Ledezma JCR, Contreras LL (2019) Eur Rev Med Pharmacol Sci 23:397
Laus G, Sladlwieser J, Klötzer W (1989) Synthesis 1989:773
Hayes K (1975) J Heterocycl Chem 11:615
Albini A, Pietra S (1991) Heterocyclic N-oxides. CRC Press, Boca Raton
Albini A (1993) Synthesis 1993:263
Wang Y, Zhang L (2015) Synthesis 47:289
Quagliano JV, Fujita J, Franz G, Phillips DJ, Walmsley JA, Tyree SY (1961) J Am Chem Soc 83:3770
da Silva RB, Loback VB, Salomão K, de Castro SL, Wardell JL, Wardell SMSV, Costa TEMM, Penido C, de Oliveira HMDGM, Carvalho SA, da Silva EF, Fraga CAM (2013) Molecules 18:3445
Witschel M (2009) Bioorg Med Chem 17:4221
Stensbøl TB, Uhlmann P, Morel S, Eriksen BL, Felding J, Kromann H, Hermit MB, Greenwood JR, Braüner-Osborne H, Madsen U, Junager F, Krogsgaard-Larsen P, Begtrup M, Vedsø P (2002) J Med Chem 45:19
Richardson ML, Croughton KA, Matthews CS, Stevens MFG (2004) J Med Chem 47:4105
Kim OK, Garrity-Ryan LK, Bartlett VJ, Grier MC, Verma AK, Medjanis G, Donatelli JE, Macone AB, Tanaka SK, Levy SB, Alekshun MN (2009) J Med Chem 52:5626
Mloston G, Celeda M, Jasinski M, Urbaniak K, Boratynski PJ, Schreiner PR, Heimgartner H (2019) Molecules 24:4398
Amitina SA, Tikhonov AY, Grigor’Ev IA, Gatilov YV, Selivanov BA (2009) Chem Heterocycl Comp 45:691
Edward TG, Fisher C (2018) Antiviral Res 152:68
Nikitina PA, Kuzmina LG, Perevalov VP, Tkach II (2013) Tetrahedron 69:3249
Nikitina PA, Peregudov AS, Koldaeva TY, Kuzmina LG, Adiulin EI, Tkach II, Perevalov VP (2015) Tetrahedron 71:5217
Chen ME, Mo LP, Cui ZS, Zhang ZH (2018) Curr Opin Green Sustain Chem 15:27
Zhang M, Liu YH, Shang ZR, Hu HC, Zhang ZH (2017) Catal Commun 88:39
Zhang WH, Chen MN, Hao Y, Jiang X, Zhou L, Zhang ZH (2019) J Mol Liq 278:124
Zeng M, Xue Y, Qin Y, Peng F, Li Q, Zeng MH (2022) Chin Chem Lett 33:4891
Zhang M, Chen MN, Zhang ZH (2019) Synth Catal 361:1
Pradhan K, Tiwary BK, Hossain M, Chakraborty R, Nanda AK (2016) RSC Adv 6:10743
Bartz S, Blumenröder B, Kern A, Fleckenstein J, Frohnapfel S, Schatz J, Wagner A (2009) Z Naturforsch 64b:629
Chua SO, Cook MJ, Katritzky AR (1971) J Chem Soc B 1971:2350
Schilf W, Stefaniak L, Witanowski M, Webb GA (1986) J Mol Struct 140:311
Boiani M, Cerecetto H, Gonzalez M, Piro OE, Castellano EE (2004) J Phys Chem A 108:11241
Wright JB (1964) J Org Chem 29:1620
Acknowledgements
The authors are grateful to the research facilities available at the incubation center of St. Joseph’s College, Darjeeling, and University of North Bengal, Darjeeling.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chettri, S., Tamang, S., Sinha, B. et al. An efficient and green protocol for the synthesis of 1-hydroxy-2-arylimidazole-3-oxide derivatives under solvent-free condition using inexpensive copper borate (CuB4O7) catalyst. Monatsh Chem 154, 635–643 (2023). https://doi.org/10.1007/s00706-023-03068-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-023-03068-1