Abstract
New thiosemicarbazones were synthesized in excellent yield reaction of indole derivatives with thiosemicarbazides. These thiosemicarbazones were reacted with ethyl bromoacetate to produce original heterocyclic-substituted indole derivatives possessing a 4-oxo-thiazolidine group. Analytical IR and NMR spectra and elemental analysis were performed to reveal their structures. The antimicrobial activity of all synthesized compounds was evaluated for antibacterial activity in vitro against Gram-positive and Gram-negative bacteria. Antibacterial screening data showed that two compounds demonstrated activity against Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa. These preliminary results indicate that some of these newly synthesized compounds show a promising antibacterial potency.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Indoles and several N-substituted indoles are an important class of heterocycles. These compounds have been attracting attention for their wide range of biological activities and their potential in synthetic chemistry. Indole derivatives possess a broad spectrum of pharmacological activities such as antimicrobial [1], analgesic [2], anticonvulsant [3], antiviral [4], antihypertensive [5], anti-asthmatic [6], antidepressant [7], anti-Alzheimer [8], antimalaria [9], antidiabetic [10], and anti-HIV activities [11]. They offer particular promise in the search for new antitumor agents [12, 13]. Thiosemicarbazones represent a broad family of molecules with various pharmacological properties, including antiviral [14], antibacterial [15], and antitumor activities [16]. Moreover, thiazolidinone derivatives possess potential biological and medicinal activities [17], among them antimycobacterial [18], antioxidant [19], anticancer [20, 21], anticonvulsant [22], anti-inflammatory [23], analgesic [24], and antimicrobial [25,26,27,28]. In antibacterial activities, thiazolidinones are more active on Gram-negative bacteria than on Gram-positive bacteria. In an attempt to design and synthesize new antimicrobial molecules [29,30,31,32], we report here a synthesis of new thiosemicarbazone and thiazolidinone derivatives containing the indole moiety, with the aim of obtaining new biologically active compounds. The drug design process is based on the synthesis combining two potential pharmacophore systems in one molecule.
Results and discussion
The starting materials, N4-substituted thiosemicarbazide derivatives 3a, 3b, were prepared through the nucleophilic addition reaction of 85% hydrazine hydrate with aryl isothiocyanate 1a, 1b in ethanol at room temperature [33] (Scheme 1). On the other hand, substituted N-benzylindole-3-carboxaldehyde derivatives 6a–6e were obtained in 90–95% yield using procedures described in the literature [34]. The appropriate commercially available indole-3-carboxaldehyde (4) was treated with various substituted benzyl halides 5a–5e in the presence of K2CO3 in N,N-dimethylformamide (DMF) (Scheme 1).

Thiosemicarbazone derivatives 7a–7j were obtained in good yields (86–95%), via condensation of substituted N-benzylindole-3-carboxaldehyde derivatives 6a–6e with N4-substituted thiosemicarbazides 3a, 3b in the presence of a catalytic amount of AcOH in ethanol at 90 °C as solvent, as shown in Scheme 1 and Table 1.
After recrystallization of all thiosemicarbazones 7a–7j, they were analyzed by 1H and 13C NMR, IR, as well as by elemental analysis. The IR spectra of compounds 7a–7j showed characteristic absorptions in the range of 3343–3124 cm−1 (N–H bond), 1278–1189 cm−1 (C=S), and 1537–1550 cm−1 (CH=N bond). Thus, the presence and predominance of the thione form of synthesized thiosemicarbazones 7a–7j were confirmed by the absence of absorption in the 2500–2600 cm−1 region [35, 36].
The most characteristic signals in the 1H NMR spectrum of this family of thiosemicarbazones were those corresponding to the –CH=N and N–H protons. 1H NMR analysis showed the CH=N protons to be in the 8.02–8.15 ppm range, whereas the thiourea N–H protons were found to be in the 9.50–11.63 ppm interval for N–H adjacent to the mono-substituted phenyl ring and for the N–H adjacent to the CH=N moiety, respectively. In the 13C NMR spectra, the chemical shift of the azomethine group (H–C=N) appeared at region 140.77–141.14 ppm, while for the C=S group, it occurred in the range 175–175.65 ppm, both comparable to the literature [37, 38]. All the thiosemicarbazone compounds synthesized were in configuration E, as confirmed by 1H NMR spectroscopy, where a single peak was detected, indicating a chemical shift of 8.02–8.15 ppm for hydrogen azomethine (H–C=N–) simple [39]. In addition, a single thin-layer spot was observed under chromatography (TLC), indicating the presence of a single isomeric form. It has been noted by a number of authors that compounds possessing a Z isomer generally exhibit an NH-3 signal in the 14–15 ppm range, whereas those compounds possessing an E form display a signal in the 9–12 ppm range [40].
The reaction between ethyl bromoacetate 8 and thiosemicarbazone derivatives 7a–7j in absolute ethanol containing anhydrous sodium acetate afforded the corresponding 4-thiazolidinone compounds 9a–9j in good yields (82–95%), as shown in Scheme 2 and Table 2.

The chemical structures of thiazolidinones 9a–9j were confirmed by their IR, 1H NMR, and 13C NMR spectra. The IR spectra of the thiazolidin-4-ones showed absorption bands due to the (NCS), (C=O) groups in the regions 1364–1328 and 1724–1711 cm−1, respectively.
Further support was obtained from the 1H NMR spectra, which showed no signs of the 4-phenyl-3-thiosemicarbazone (NH) protons. On the other hand, the 1H NMR spectra exhibited resonances assigned to the SCH2 group of the thiazolidine ring appearing as a singlet at 4.08–4.11 ppm due to the methylene protons. The CH=N protons in these structures were observed in the 8.45–8.84 ppm region. Similarly, in the 13C NMR spectra, the chemical shift of the carbon of the carbonyl group of 4-thiazolidinone ring occurred between 172.30 and 172.52 ppm next to signals in the 48.32–50.01 ppm region attributed to methylene groups. The formation of thiazolidinones 9a–9j occurred in two steps. The first step of this reaction is thought to be S-alkylation of thiosemicarbazide in its thiol form. The second step involved loss of ethanol to give the thiazolidin-4-ones as shown in Scheme 3.

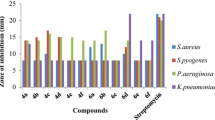
Previous studies have shown that thiazolidinones with C-2- and N-3-substituted positions possess varying degrees of inhibition against many pathogens [17]. We are therefore considering a preliminary study of the antibacterial activity for thiosemicarbazone intermediates and thiazolidinone targets. The antibacterial activity in vitro of the synthesized thiosemicarbazone 7a–7j and thiazolidinone 9a–9j derivatives was evaluated against a Gram-positive bacterial strain, Staphylococcus aureus (ATCC-25923), and two Gram-negative bacterial strains, Escherichia coli (ATCC-25922) and Pseudomonas aeruginosa (ATCC-27853), using the conventional agar dilution method [41]. Minimum inhibitory concentrations (MIC) are defined as the concentration of a compound that inhibits the growth of the tested microorganisms.
The MIC varied from 0.25 to 128 μg/cm3 according to the tested compound, compared with the standard ceftazidime (MIC = 0.5–4 μg/cm3), imipenem (MIC = 0.5–1 μg/cm3), and gentamicin (MIC = 0.06–2 μg/cm3). It has been shown that each compound exhibited different action. According to Table 3, the product 7g (R1 = 3-Cl, R2 = 4-OCH3) and 9a (R1 = H, R2 = H) presented the best antimicrobial activity for the three strains followed by 7h (R1 = 4-F, R2 = 4-OCH3) which exhibited a positive action against the Gram-negative bacteria (E. coli and P. aeruginosa). Among the other synthesized derivatives, for 7c (R1 = 4-F, R2 = H), 7j (R1 = 2-CN, R2 = 4-OCH3), and 9i (R1 = 3-CF3, R2 = 4-OCH3), the activity toward P. aeruginosa is to be underlined with an activity higher (at least four times) than that of the reference products.
In terms of structure–activity relationships, it can been seen that for the thiosemicarbazones active against P. aeruginosa, the most active derivatives (7g, 7h, 7j) are substituted in R1 by an electron-withdrawing group (3-Cl, 4-F, 2-CN) and R2 by an electron-donating group (4-OCH3). Regarding the possible mechanism of thiosemicarbazones, we suggest membrane perturbing as well as intracellular mode of action of this class of compounds, as proposed recently [42]. For thiazolidinones, the substitution seems unfavorable, since the unsubstituted derivative 9a (R1 = R2 = H) is the most active for the three strains.
Conclusion
In this study, a series of hybrid molecules indole-thiazolidinones easily was synthesized in very good yields (82–95%). The synthesized indole-thiazolidinones and their precursor thiosemicarbazones showed good activity in antibacterial assays. The products 7g (R1 = 3-Cl, R2 = 4-OCH3) and 9a (R1 = H, R2 = H) showed the best antimicrobial activity for the three strains tested with an MIC value 0.25 μg/cm3, followed by 7 h (R1 = 4-F, R2 = 4-OCH3) which exhibited a positive action against Gram-negative bacteria (E. coli and P. aeruginosa) with an MIC value 0.25 μg/cm3. Among the other synthesized derivatives, for 7c (R1 = 4-F, R2 = H), 7j (R1 = 2-CN, R2 = 4-OCH3), and 9i (R1 = 3-CF3, R2 = 4-OCH3), the activity toward P. aeruginosa is to be underlined with an activity higher (at least four times) than that of the reference products. These results are comparable or more potent regarding their activity than the reference drugs. The first structure–activity relationship conclusions show that for thiosemicarbazones active against P. aeruginosa, the most active derivatives (7g, 7h, 7j) are substituted in R1 by an electron-withdrawing group (3-Cl, 4-F, 2-CN) and R2 by an electron-donating group (4-OCH3). For thiazolidinones, the substitution seems unfavorable, since the unsubstituted derivative 9a (R1 = R2 = H) is the most active. The pharmacomodulation of these series to confirm and complete their structure–activity relationship is currently under investigation. In addition, the most active products 9a and 7g will be tested on resistant strains in the near future. The potential interactions of lead compounds will be studied by means of docking for example.
Experimental
Melting points were determined on a Büchi B-540 apparatus and are uncorrected. IR spectra were taken on Perkin-Elmer Spectrum two FT-IR spectrometer and the wave numbers reported in cm−1. Elemental analyses were carried out at the Spectropole; Faculté des Sciences site Saint-Jérome. 1H NMR spectra were recorded on a BRUKER AC 300P (300 MHz) spectrometer (Bruker, Bremen, Germany), 13C NMR spectra on a BRUKER AC 300 P (75 MHz, Bruker) spectrometer in DMSO-d6. The 1H NMR chemical shifts were reported as parts per million downfield from tetramethylsilane (Me4Si), and the 13C NMR chemical shifts were referenced to the solvent peaks: DMSO-d6 (39.6 ppm). Silica gel 60 (Merck, 230–400 mesh) was used for column chromatography: thin-layer chromatography was performed with silica gel Merck 60F-254 (0.25 mm layer thickness).
General procedure for the preparation of compounds 6a–6e
1H-Indole-3-carbaldehyde (4, 1.45 g, 0.01 mol), the appropriate benzyl chloride 6a–6e (0.011 mol), and 2.76 g anhydrous K2CO3 (0.02 mol) in 30 cm3 N,N-dimethylformamide. The reaction mixture was refluxed for 2 h. Progress of the reaction was monitored by thin-layer chromatography. Upon cooling to room temperature, the mixture was poured into ice-cold water, and then, the precipitate was collected by filtration and dried. The crude product was purified by recrystallization from ethanol to give solids 6a-6e in good yields.
1-Benzyl-1H-indole-3-carbaldehyde (6a, C16H13NO)
Yield: 90%; white solid; m.p.: 107.9–108.2 °C; 1H NMR (DMSO-d6): δ = 9.98 (s, 1H, CHO), 8.51 (s, 1H, indole H-2), 8.17–8.14 (m, 1H, Ar–H), 7.64–7.60 (m, 1H, Ar–H), 7.40–7.20 (m, 7H, Ar–H), 5.58 (s, 2H, CH2) ppm; 13C NMR (DMSO-d6): δ = 184.70 (C=O), 141.00 (C), 137 (CH), 136.74 (C), 128.71 (2CH), 127.78 (CH), 127.23 (2CH), 124.78 (CH), 123.61 (CH), 122.5 (CH), 121.07 (C), 117.38 (C), 111.40 (CH), 49.78 (CH2) ppm.
1-(3-Chlorobenzyl)-1H-indole-3-carbaldehyde (6b, C16H12ClNO)
Yield: 93%; white solid; m.p.: 80.4–80.8 °C; 1H NMR (DMSO-d6): δ = 10 (s, 1H, CHO), 8.54 (s, 1H, indole H-2), 8.19–8.12 (m, 1H, Ar–H), 7.68–7.61 (m, 1H, Ar–H), 7.45 (s, 1H, Ar–H), 7.44–7.35 (m, 2H, Ar–H), 7.33–7.25 (m, 3H, Ar–H), 5.58 (s, 2H, CH2) ppm; 13C NMR (DMSO-d6): δ = 184.79 (C=O), 141.00 (C), 139.28 (CH), 136.44 (C), 133.30 (C), 130.66 (CH), 127.80 (CH), 127.23 (CH), 126.02 (CH), 124.75 (CH), 123.74 (CH), 122.66 (CH), 121.12 (C), 117.51 (C), 111.31 (CH), 49.06 (CH2) ppm.
1-(4-Fluorobenzyl)-1H-indole-3-carbaldehyde (6c, C16H12FNO)
Yield: 94%; white solid; m.p.: 117.1–117.7 °C; 1H NMR (DMSO-d6): δ = 10 (s, 1H, CHO), 8.51 (s, 1H, indole H-2), 8.16–8.13 (m, 1H, Ar–H), 7.66–7.62 (m, 1H, Ar–H), 7.45–7.39 (m, 2H, Ar–H), 7.35–7.27 (m, 2H, Ar–H), 7.21 (t, 2H, J = 8.85 Hz, Ar–H), 5.56 (s, 2H, CH2) ppm; 13C NMR (DMSO-d6): δ = 184.70 (C=O), 163.58 (d, 1JCF = 244.05 Hz, C), 140.87 (CH), 136.83 (C), 133.0 (d, 4JCF = 3.22 Hz, C), 129.65 (d, 3JCF = 8.27 Hz, 2CH), 124.80 (CH), 123.64 (CH), 122.60 (CH), 121.08 (C), 117.43 (C), 115.71 (d, 2JCF = 21.60 Hz, 2CH), 111.37 (CH), 49.0 (CH2) ppm.
1-[3-(Trifluoromethyl)benzyl]-1H-indole-3-carbaldehyde (6d, C17H12F3NO)
Yield: 93%; white solid; m.p.: 134.8–135.6 °C; 1H NMR (DMSO-d6): δ = 10 (s, 1H, CHO), 8.57 (s, 1H, indole H-2), 8.17–8.14 (m, 1H, Ar–H), 7.81 (s, 1H, Ar–H), 7.71–7.58 (m, 4H, Ar–H), 7.33–7.25 (m, 2H, Ar–H), 5.70 (s, 2H, CH2) ppm; 13C NMR (DMSO-d6): δ = 184.70 (C=O), 138.41 (CH), 137.29 (C), 136.52 (C), 131.33 (q, 2JCF = 33 Hz, C-CF3), 130.3 (CH), 129.82 (CH), 125.51(C), 125.37 (q, 4JCF = 3.9 Hz, CH), 124.50 (CH), 123.85 (q, 3JCF = 3.8 Hz, CH), 123.34 (CH), 122.34 (CH), 122.0 (q, 1JCF = 272.35 Hz, C-F), 118.82 (C), 111.20 (CH), 50.47 (CH2) ppm.
1-(2-Cyanobenzyl)-1H-indole-3-carboxaldehyde (6e, C17H12N2O)
Yield: 95%; white solid; m.p.: 147.8–148.2 °C; 1H NMR (DMSO-d6): δ = 10 (s, 1H, CHO), 8.45 (s, 1H, indole H-2), 8.20–8.17 (m, 1H, Ar–H), 7.97 (1H, dd, J = 1.26 Hz, 7.58 Hz, Ar–H), 7.67 (1H, td, J = 1.42 Hz, 7.74 Hz, Ar–H), 7.60–7.52 (m, 2H, Ar–H), 7.35–7.25 (m, 2H, Ar–H), 7.07 (1H, d, J = 7.58 Hz, Ar–H), 5.83 (s, 2H, CH2) ppm; 13C NMR (DMSO-d6): δ = 185.00 (C=O), 141.25 (C), 140.0 (C), 137.07 (CH), 133.84 (CH), 133.50 (CH), 128.71 (CH), 127.81 (CH), 124.67 (CH), 123.91 (CH), 122.79 (CH), 121.20 (C), 117.72 (C), 117.19 (C), 111.13 (C), 110.39 (CH), 48.19 (CH2) ppm.
General procedure for the preparation of compounds 7a–7j
To a solution of N4-substituted thiosemicarbazide 3a, 3b (6 mmol, 1 eq) in 33 cm3 ethanol, 3-(4-substitutedphenyl)-1H-indole-3-carbaldehyde 6a–6e (6.3 mmol, 1.05 eq) and 0.50 cm3 acetic acid were added with stirring and the resulting reaction mixture was stirred at 90 °C for 3 h. The reaction was monitored by thin-layer chromatography (TLC) for completion. The solid separated was then filtered and recrystallized from ethanol-DMF (3:1) to give compounds 7a–7j.
(E)-1-[(1-Benzyl-1H-indol-3-yl)methylene]-4-phenylthiosemicarbazide (7a, C23H20N4S)
Yield: 94%; white solid; m.p.: 200–200.5 °C; IR (ATR): \(\vec{v}\) = 3342 and 3124 (NH), 1538 (C=N), 1202 and 1272 (C=S) cm−1; 1H NMR (DMSO-d6): δ = 11.61 (s, 1H, NH), 9.63 (s, 1H, NH), 8.43 (s, 1H, indole H-2), 8.26 (dd, 1H, J = 6.60 Hz, 1.4 Hz, Ar–H), 8.10 (s, 1H, CH=N), 7.65 (d, 2H, J = 8.62 Hz, Ar–H), 7.53 (dd, 1H, J = 7.15 Hz, 1.1 Hz, Ar–H), 7.18–7.41 (m, 10H, Ar–H), 5.48 (s, 2H, PhCH2) ppm; 13C NMR (DMSO-d6): δ = 175 (C=S), 141.14 (CH), 139.75 (C), 137.90 (C), 137.38 (C), 134.71 (CH), 129.12 (2CH), 128.61 (2CH), 128.07 (CH), 127.64 (2CH), 125.60 (2CH), 125.44 (CH), 125.23(C), 123.38 (CH), 122.63 (CH), 121.56 (CH), 111.20 (CH), 111.12 (C), 49.90 (CH2) ppm.
(E)-1-[[1-(3-Chlorobenzyl)-1H-indol-3-yl]methylene]-4-phenylthiosemicarbazide (7b, C23H19ClN4S)
Yield: 87%; white solid; m.p.: 178.4–178.9 °C; IR (ATR): \(\vec{v}\) = 3343 and 3143 (NH), 1541 (C=N), 1268 and 1204 (C=S) cm−1; 1H NMR (DMSO-d6): δ = 11.63 (s, 1H, NH), 9.64 (s, 1H, NH), 8.43 (s, 1H, indole H-2), 8.28 (dd, 1H, J = 6.7 Hz, 1.3 Hz, Ar–H), 8.12 (s, 1H, CH=N), 7.64 (dd, 2H, J = 8.25 Hz, 0.83 Hz, Ar–H), 7.55 (dd, 1H, J = 8.25 Hz, 1.28 Hz, Ar–H), 7.41–7.32 (m, 5H, Ar–H), 7.27–7.17 (m, 4H, Ar–H), 5.50 (s, 2H, PhCH2) ppm; 13C NMR (DMSO-d6): δ = 175 (C=S), 141.09 (CH), 139.75 (C), 137.28 (C), 137.69 (C), 133.72 (CH), 131.1 (2CH), 128.61 (2CH), 128.07 (CH), 127.45 (CH), 126.32 (CH), 125.67 (2CH), 125.19 (C), 123.55 (CH), 122.76 (CH), 121.70 (CH), 111.36 (C), 111.11 (CH), 49.16 (CH2) ppm.
(E)-1-[[1-(4-Fluororobenzyl)-1H-indol-3-yl]methylene]-4-phenylthiosemicarbazide (7c, C23H19FN4S)
Yield: 95%; white solid; m.p.: 193.5–193.9 °C; IR (ATR): \(\vec{v}\) = 3292 and 3160 (NH), 1537 (C=N), 1268 and 1196 (C=S) cm−1; 1H NMR (DMSO-d6): δ = 11.61 (s, 1H, NH), 9.62 (s, 1H, NH), 8.42 (s, 1H, indole H-2), 8.28 (dd, 1H, J = 6.7 Hz, 1.3 Hz, Ar–H), 8.10 (s, 1H, CH=N), 7.64 (dd, 2H, J = 8.25 Hz, 0.83 Hz, Ar–H), 7.55 (dd, 1H, J = 8.25 Hz, 1.28 Hz, Ar–H), 7.41–7.32 (m, 5H, Ar–H), 7.27–7.17 (m, 4H, Ar–H), 5.46 (s, 2H, PhCH2) ppm; 13C NMR (DMSO-d6): δ = 175.03 (C=S), 163.64 (d, 1JCF = 243.2 Hz, C), 141.12 (CH), 139.74 (C), 137.26 (C), 134.60 (CH), 134.13 (d, 4JCF = 3.30 Hz, C), 129.88 (d, 3JCF = 8.48 Hz, 2CH), 128.61 (3CH), 125.62 (2CH), 125.46 (CH), 125.23 (C), 123.43 (CH), 122.67 (CH), 121.62 (CH), 116.07 (d, 2JCF = 21.46 Hz, 2CH), 111.20 (CH), 49.12 (CH2) ppm.
(E)-1-[[1-[3-(Trifluoromethyl)benzyl]-1H-indol-3-yl]methylene]-4-phenylthiosemicarbazide (7d, C24H19F3N4S)
Yield: 86%; white solid; m.p.: 188.3–188.9 °C; IR (ATR): \(\vec{v}\) = 3340 and 3141 (NH), 1538 (C=N), 1278 and 1195 (C=S) cm−1; 1H NMR (DMSO-d6): δ = 11.61 (s, 1H, NH), 9.63 (s, 1H, NH), 8.43 (s, 1H, Ar–H), 8.29 (dd, 1H, J = 6.8 Hz, 1.3 Hz, Ar–H), 8.15 (s, 1H, CH=N), 7.72–7.62 (m, 4H, Ar–H), 7.60–7.50 (m, 3H, Ar–H), 7.41–7.35 (m, 2H, Ar–H), 7.27–7.17 (m, 3H, Ar–H), 5.59 (s, 2H, PhCH2) ppm; 13C NMR (DMSO-d6): δ = 175.6 (C=S), 156.30 (C), 140.85 (CH), 139.47 (C), 137.28 (C), 134.57 (CH), 132.70 (C), 131.7 (CH), 130.32 (CH), 129.54 (q, 2JCF = 32 Hz, C-CF3), 127.77 (2CH), 125.17 (C), 124.90 (q, 3JCF = 4.0 Hz, CH), 124.26 (q, 4JCF = 3.8 Hz, CH), 123.55 (CH), 122.93 (CH), 122.75 (q, 1JCF = 272.35 Hz, C-F), 121.65 (CH), 113.77 (2CH), 111.5 (C), 111.0 (CH), 55.72 (OCH3), 49.20 (CH2) ppm.
(E)-1-[[1-(2-Cyanobenzyl)-1H-indol-3-yl]methylene]-4-phenylthiosemicarbazide (7e, C24H19N5S)
Yield: 90%; white solid; m.p.: 221.7–222.1 °C; IR (ATR): \(\vec{v}\) = 3305 and 3147 (NH), 1536 (C=N), 1265 and 1198 (C=S) cm−1; 1H NMR (DMSO-d6): δ = 11.63 (s, 1H, NH), 9.65 (s, 1H, NH), 8.43 (s, 1H, Ar–H), 8.33–8.31 (m, 1H, Ar–H), 8.04 (s, 1H, CH=N), 7.92 (dd, 1H, J = 7.70 Hz, 1.10 Hz, Ar–H), 7.66–7.60 (m, 3H, Ar–H), 7.53–7.48 (m, 2H, Ar–H), 7.41–7.36 (m, 2H, Ar–H), 7.28–7.18 (m, 3H, Ar–H), 7.00 (d, 1H, J = 7.70 Hz, Ar–H), 5.72 (s, 2H, PhCH2) ppm; 13C NMR (DMSO-d6): δ = 175 (C=S), 141.16 (C), 141 (CH), 139.75 (C), 137.52 (C), 134.87 (CH), 134.28 (CH), 133.89 (CH), 129.05 (CH), 128.61 (2CH), 128.14 (CH), 125.70 (2CH), 125.50 (CH), 125.14 (CH), 123.73 (CH), 122.88 (CH), 121.86 (CH), 117.75 (C), 111.68 (C), 111 (CH), 110.75 (C), 49.16 (CH2) ppm.
(E)-1-[(1-Benzyl-1H-indol-3-yl)methylene]-4-(4-methoxyphenyl)thiosemicarbazide (7f, C24H22N4OS)
Yield: 95%; white solid; m.p.: 252.9–253.2 °C; IR (ATR): \(\vec{v}\) = 3285 and 3160 (NH), 1538 (C=N), 1260 and 1191 (C=S) cm−1; 1H NMR (DMSO-d6): δ = 11.52 (s, 1H, NH), 9.50 (s, 1H, NH), 8.40 (s, 1H, indole H-2), 8.28 (dd, 1H, J = 7.00 Hz, 1.0 Hz, Ar–H), 8.10 (s, 1H, CH=N), 7.52 (d, 1H, J = 7.52 Hz, Ar–H), 7.46 (dt, 2H, J = 3.4 Hz, 2.1 Hz Ar–H), 7.36–7.31 (m, 2H, Ar–H), 7.29–7.24 (m, 3H, Ar–H), 7.23–7.19 (m, 1H, Ar–H), 7.15 (dd, 1H, J = 7.24 Hz, 1.1 Hz, Ar–H), 6.93 (dt, 2H, J = 3.48 Hz, 2.2 Hz Ar–H), 5.47 (s, 2H, PhCH2), 3.77 (s, 3H, OCH3) ppm; 13C NMR (DMSO-d6): δ = 175.52 (C=S), 157.3 (C), 141 (CH), 137.92 (C), 137.33 (C), 134.58 (CH), 132.67 (C), 129.12 (3CH), 128.07 (CH), 127.75 (CH), 127.64 (3CH), 125.19 (C), 123.36 (CH) 122.74 (CH), 121.50 (CH), 113.77 (2CH), 111.15 (C), 55.72 (OCH3), 49.86 (CH2) ppm.
(E)-1-[[1-(3-Chlorobenzyl)-1H-indol-3-yl]methylene]-4-(4-methoxyphenyl)thiosemicarbazide (7g, C24H21ClN4OS)
Yield: 95%; white solid; m.p.: 237.7–238.5 °C; IR (ATR): \(\vec{v}\) = 3310 and 3140 (NH), 1548 (C=N), 1273 and 1195 (C=S) cm−1; 1H NMR (DMSO-d6): δ = 11.53 (s, 1H, NH), 9.51 (s, 1H, NH), 8.41 (s, 1H, indole H-2), 8.29 (dd, 1H, J = 7.15 Hz, 0.73 Hz, Ar–H), 8.10 (s, 1H, CH=N), 7.53 (d, 1H, J = 7.70 Hz, Ar–H), 7.46 (dt, 2H, J = 3.4 Hz, 2.2 Hz, Ar–H), 7.27–7.18 (m, 2H, Ar–H), 7.15 (dd, 1H, J = 7.24 Hz, 1.0 Hz, Ar–H), 6.93 (dt, 2H, J = 3.4 Hz, 2.2 Hz, Ar–H), 5.5 (s, 2H, PhCH2), 3.77 (s, 3H, OCH3) ppm; 13C NMR (DMSO-d6): δ = 175.57 (C=S), 157.3 (C), 140.86 (CH), 140.38 (C), 137.25 (C), 134.55 (CH), 133.72 (C), 133.72 (C), 132.67 (C), 131.1 (CH), 128.07 (CH), 127.78 (2CH), 127.44 (CH), 126.33 (C), 125.17 (CH), 123.53 (CH), 122.85 (CH), 121.64 (CH), 113.8 (CH), 111.4 (C), 111.06 (CH), 55.72 (OCH3), 49.16 (CH2) ppm.
(E)-1-[[1-(4-Fluorobenzyl)-1H-indol-3-yl]methylene]-4-(4-methoxyphenyl)thiosemicarbazide (7h, C24H21FN4OS)
Yield: 91%; white solid; m.p.: 239.6–240.4 °C; IR (ATR): \(\vec{v}\) = 3313 and 3159 (NH), 1547 (C=N), 1274 and 1195 (C=S) cm−1; 1H NMR (DMSO-d6): δ = 11.53 (s, 1H, NH), 9.51 (s, 1H, NH), 8.41 (s, 1H, indole H-2), 8.29 (dd, 1H, J = 7.15 Hz, 0.83 Hz, Ar–H), 8.10 (s, 1H, CH = N), 7.53 (d, 1H, J = 7.70 Hz, Ar–H), 7.46 (dt, 2H, J = 3.4 Hz, 2.2 Hz, Ar–H), 7.39–7.32 (m, 3H, Ar–H), 7.27–7.15 (m, 3H, Ar–H), 6.93 (dt, 2H, J = 3.4 Hz, 2.2 Hz, Ar–H), 5.5 (s, 2H, PhCH2), 3.77 (s, 3H, OCH3) ppm; 13C NMR (DMSO-d6): δ = 175.6 (C=S), 163.64 (d, 1JCF = 243.75 Hz, C-F), 157.31 (C), 140.87 (C), 137.27 (C), 134.42 (CH), 134.14 (d, 4JCF = 2.75 Hz, C), 132.70 (C), 129.88 (d, 3JCF = 8.25 Hz, 2CH), 127.67 (2CH), 125.25 (CH), 123.40 (CH), 122.75 (CH), 121.54 (CH), 115.78 (d, 2JCF = 21.46 Hz, 2CH), 113.80 (2CH), 111.3 (C), 111.11 (CH), 55.74 (OCH3), 49.12 (CH2) ppm.
(E)-1-[[1-[3-(Trifluoromethyl)benzyl]-1H-indol-3-yl]methylene]-4-(4-methoxyphenyl)thiosemicarbazide (7i, C25H21F3N4OS)
Yield: 92%; white solid; m.p.: 232.3–232.8 °C; IR (ATR): \(\vec{v}\) = 3316 and 3156 (NH), 1545 (C=N), 1273 and 1193 (C=S) cm−1; 1H NMR (DMSO-d6): δ = 11.54 (s, 1H, NH), 9.51 (s, 1H, NH), 8.41 (s, 1H, Ar–H), 8.31 (d, 1H, J = 7.34 Hz, Ar–H), 8.14 (s, 1H, CH=N), 7.70 (s, 1H, Ar–H), 7.65 (d, 1H, J = 7.70 Hz, Ar–H), 7.60–7.50 (m, 3H, Ar–H), 7.44 (d, 2H, J = 9.0 Hz, Ar–H), 7.27–7.15 (m, 2H, Ar–H), 6.94 (d, 2H, J = 9.0 Hz, Ar–H), 5.59 (s, 2H, CH2), 3.77 (s, 3H, OCH3) ppm; 13C NMR (DMSO-d6): δ = 175.6 (C=S), 156.30 (C), 140.85 (CH), 139.47 (C), 137.28 (C), 134.57 (CH), 132.70 (C), 131.7 (CH), 130.32 (CH), 129.54 (q, 2JCF = 32 Hz, C-CF3), 127.77 (2CH), 125.17 (C), 124.90 (q, 3JCF = 4.0 Hz, CH), 124.26 (q, 4JCF = 3.8 Hz, CH), 123.55 (CH), 122.93 (CH), 122.75 (q, 1JCF = 272.35 Hz, C-F), 121.65 (CH), 113.77 (2CH), 111.5 (C), 111.0 (CH), 55.72 (OCH3), 49.20 (CH2) ppm.
(E)-1-[[1-(2-Cyanobenzyl)-1H-indol-3-yl]methylene]-4-(4-methoxyphenyl)thiosemicarbazide (7j, C25H21N5OS)
Yield: 95%; white solid; m.p.: 271.9–272.3 °C; IR (ATR): \(\vec{v}\) = 3312 and 3163 (NH), 1550 (C=N), 1274 and 1189 (C=S) cm−1; 1H NMR (DMSO-d6): δ = 11.54 (s, 1H, NH), 9.51 (s, 1H, NH), 8.41 (s, 1H, Ar–H), 8.31 (dd, 1H, J = 7.0 Hz, 1.4 Hz, Ar–H), 8.02 (s, 1H, CH=N), 7.93 (dd, 1H, J = 7.70 Hz, 1.10 Hz, Ar–H), 7.53–7.42 (m, 4H, Ar–H), 7.27–7.17 (m, 2H, Ar–H), 7.0–6.93 (m, 3H, Ar–H), 5.72 (s, 2H, PhCH2), 3.77 (s, 3H, OCH3) ppm; 13C NMR (DMSO-d6): δ = 175.65 (C=S), 157.32 (C), 141.21 (C), 140.77 (CH), 137.50 (C), 134.73 (CH), 134.28 (CH), 133.89 (CH), 132.68 (C), 129.04 (CH), 128.10 (CH), 127.80 (2CH), 125.11 (C), 123.7 (CH), 1230 (CH), 121.80 (CH), 117.76 (C), 113.78 (3CH), 111.73 (C), 110.72 (C), 55.72 (OCH3), 49.16 (CH2) ppm.
General procedure for the preparation of compounds 9a–9j
A mixture of compound 7a–7j (3 mmol, 1 eq), 0.36 cm3 ethyl 2-bromoacetate (1.1 eq) and 1 g anhydrous sodium acetate (6 mmol, 2 eq) in 30 cm3 ethanol was stirred until reflux; the mixture was stirred under the same conditions until complete reaction (3–4 h). The reaction mixture was left to cool, and poured into ice-cold water, and the separated solid was filtered, washed with water, and recrystallized from a mixture of ethanol.
2-[2-[(1-Benzyl-1H-indol-3-yl)methylene]hydrazono]-3-phenylthiazolidin-4-one (9a, C25H20N4OS)
Yield: 94%; white solid; m.p.: 250.9–251.4 °C; IR (ATR): \(\vec{v}\) = 1715 (C=O), 1573 and 1530 (C=N), 1337 (NCS) cm−1; 1H NMR (DMSO-d6): δ = 8.84 (s, 1H, CH=N), 8.26 (m, 1H, Ar–H), 7.95 (s, 1H, CH=C), 7.52–7.41 (m, 6H,Ar–H), 7.28–7.24 (m, 7H, Ar–H), 5.46 (s, 2H, PhCH2), 4.10 (s, 2H, CH2) ppm; 13C NMR (DMSO-d6): δ = 172.52 (C=O), 161.43(C), 153.72 (CH), 137.85 (C), 137.49 (C), 135.68 (C), 135.40 (CH), 129.55 (2CH), 129.12 (3CH), 128.74 (2CH), 128.07 (CH), 127.60 (2CH), 125.64 (C), 123.47 (CH), 122.76 (CH), 121.71 (CH), 112.00 (C), 111.36 (CH), 49.88 (CH2), 32.68 (CH2) ppm.
2-[2-[[1-(3-Chlorobenzyl)-1H-indol-3-yl]methylene]hydrazono]-3-phenylthiazolidin-4-one (9b, C25H19ClN4OS)
Yield: 87%; yellow solid; m.p.: 221.4–221.9 °C; IR (ATR): \(\vec{v}\) = 1718 (C=O), 1573 and 1529 (C=N), 1339 (NCS) cm−1; 1H NMR (DMSO-d6): δ = 8.46 (s, 1H, CH=N), 8.30–8.27 (m, 1H, Ar–H), 7.98 (s, 1H, CH=C), 7.54 (t, 3H, J = 7.24 Hz, Ar–H), 7.48 (d, 1H, J = 7.00 Hz, Ar–H), 7.41 (d, 2H, J = 7.43 Hz, Ar–H), 7.34 (t, 3H, J = 4.68 Hz, Ar–H), 7.25 (t, 2H, J = 3.76 Hz, Ar–H), 7.21–7.1 (m, 1H, Ar–H), 5.50 (s, 2H, PhCH2), 4.11 (s, 2H, CH2) ppm; 13C NMR (DMSO-d6): δ = 172.52 (C=O), 153.61 (CH), 140.4 (C), 137.57 (C), 135.81 (C), 135.13 (C), 135.34 (CH), 133.81 (C), 131.0 (CH), 129.55 (2CH), 129.03 (CH), 128.76 (2CH), 128.05 (CH), 127.43 (CH), 126.23 (CH), 125.81 (C), 123.56 (CH), 122.83 (CH), 121.78 (C), 112.38 (CH), 111.16 (C), 49.29 (CH2), 32.66 (CH2) ppm.
2-[2-[[1-(4-Fluorobenzyl)-1H-indol-3-yl]methylene]hydrazono]-3-phenylthiazolidin-4-one (9c, C25H19FN4OS)
Yield: 91%; white solid; m.p.: 263.8–264.2 °C; IR (ATR): \(\vec{v}\) = 1711 (C=O), 1562 and 1531 (C=N), 1337 (NCS) cm−1; 1H NMR (DMSO-d6): δ = 8.45 (s, 1H, CH=N), 8.30–8.25 (m, 1H, Ar–H), 7.95 (s, 1H, C=CH), 7.58–7.51 (m, 3H, Ar–H), 7.49–7.40 (m, 3H, Ar–H), 7.33–7.26 (m, 2H, Ar–H), 7.26–7.12 (m, 4H, Ar–H), 5.46 (s, 2H, N-CH2), 4.10 (s, 2H, CH2) ppm; 13C NMR (DMSO-d6): δ = 172.50 (C=O), 163.62 (d, 1JCF = 243.2 Hz, C), 163.62 (C),153.68 (CH), 137.41 (CH), 135.71 (C), 135.24 (CH), 134.13 (d, 4JCF = 3.30 Hz, C), 129.82 (d, 3JCF = 8.25 Hz, 2CH), 129.53 (2CH), 129.03 (CH), 128.76 (2CH), 125.68 (C), 123.49 (CH), 122.80 (CH), 121.75 (CH), 116.07 (d, 2JCF = 21.46 Hz, 2CH), 112.1 (CH), 111.3 (CH), 49.07 (CH2), 32.70 (CH2) ppm.
2-[2-[[1-[3-(Trifluoromethyl)benzyl]-1H-indol-3-yl]methylene]hydrazono]-3-phenylthiazolidin-4-one (9d, C26H19F3N4OS)
Yield: 86%; green solid; m.p.: 208.7–209.6 °C; IR (ATR): \(\vec{v}\) = 1718 (C=O), 1561 and 1531 (C=N), 1364 (NCS) cm−1; 1H NMR (DMSO-d6): δ = 8.47 (s, 1H, CH=N), 8.32–8.29 (m, 1H, Ar–H), 8.00 (s, 1H, CH=C), 7.64 (d, 2H, J = 10.82 Hz, Ar–H), 7.57–7.52 (m, 4H,Ar–H), 7.74 (d, 2H, J = 7.0 Hz, Ar–H), 7.42 (d, 2H, J = 7.24 Hz, Ar–H), 7.29–7.14 (m, 2H, Ar–H), 5.60 (s, 2H, N-CH2), 4.11 (s, 2H, CH2) ppm; 13C NMR (DMSO-d6): δ = 172.54 (C=O), 162.62 (C), 153.7 (CH), 139.40 (C), 137.41 (C), 135.66 (C), 135.34 (CH), 131.64 (CH), 130.31 (CH), 129.66 (q, 2JCF = 31.7 Hz, C-CF3), 129.55 (2CH), 129.07 (CH), 128.74 (2CH), 125.62 (C), 125.0 (q, 3JCF = 3.7 Hz, CH), 124.2 (q, 4JCF = 3.6 Hz, CH), 123.66 (CH), 122.85 (CH), 122.70 (q, 1JCF = 272.35 Hz, C-F), 121.88 (CH), 112.25 (CH), 111.23 (C), 49.17 (CH2), 32.70 (CH2) ppm.
2-[2-[[1-(2-Cyanobenzyl)-1H-indol-3-yl]methylene]hydrazono]-3-phenylthiazolidin-4-one (9e, C26H19N5OS)
Yield: 82%; white solid; m.p.: 264.9–265.6 °C; IR (ATR): \(\vec{v}\) = 1718 (C=O), 1572 and 1531 (C=N), 1346 (NCS) cm−1; 1H NMR (DMSO-d6): δ = 8.46 (s, 1H, CH=N), 8.35–8.29 (m, 1H, Ar–H), 8.00 (s, 1H, CH=C), 7.87 (d, 1H, J = 1 Hz, Ar–H),7.62 (td, 1H, J = 7.8 Hz, 1.4 Hz, Ar–H), 7.56–7.46 (m, 10H, Ar–H), 7.43 (d, 1H, J = 1.47 Hz, Ar–H), 7.41 (d, 1H, J = 1 Hz, Ar–H), 7.29–7.23 (m, 2H, Ar–H), 5.70 (s, 2H, N-CH2), 4.11 (s, 2H, CH2) ppm; 13C NMR (DMSO-d6): δ = 172.30 (C=O), 153.56 (CH), 141 (C), 137.73 (C), 135.80 (CH), 135.52 (CH), 135.27 (C), 134.13 (CH), 133.88 (CH), 129.42 (2CH), 129.05 (C), 128.91 (CH), 128.88 (2CH), 128.37 (CH), 128.08 (CH), 125.81 (C), 123.71 (CH), 122.90 (CH), 121.92 (C), 117.58 (C), 112.66 (C), 111.0 (CH), 48.40 (CH2), 32.67 (CH2) ppm.
2-[2-[(1-Benzyl-1H-indol-3-yl)methylene]hydrazono]-3-(4-methoxyphenyl)thiazolidin-4-one (9f, C26H22N4O2S)
Yield: 90%; white solid; m.p.: 271.6–272.0 °C; IR (ATR): \(\vec{v}\) = 1716 (C=O), 1561 and 1531 (C=N), 1343 (NCS) cm−1; 1H NMR (DMSO-d6): δ = 8.46 (s, 1H, CH=N), 8.30–8.26 (m, 1H, Ar–H), 7.91 (s, 1H, CH=C), 8.53–8.50 (m, 1H, Ar–H), 7.35–7.28 (m, 5H, Ar–H), 7.26–7.19 (m, 4H, Ar–H), 7.05 (dt, 2H, J = 9.8 Hz, 3.0 Hz, Ar–H), 5.46 (s, 2H, N-CH2), 4.07 (s, 2H, CH2), 3.8 (s, 3H, OCH3) ppm; 13C NMR (DMSO-d6): δ = 172.42 (C=O), 169.54 (C), 162.30 (C), 153.50 (CH), 137.83 (C), 137.67 (C), 135.11 (CH), 129.77 (2CH), 129.07 (2CH), 128.39 (C), 128.02 (CH), 127.59 (2CH), 125.83 (C), 123.40 (CH), 122.78 (CH), 121.61 (CH), 114.80 (2CH), 112.20 (C), 111.22 (CH), 55.59 (CH3), 50.01 (CH2), 32.67 (CH2) ppm.
2-[2-[[1-(3-Chlorobenzyl)-1H-indol-3-yl]methylene]hydrazono]-3-(4-methoxyphenyl)thiazolidin4-one (9g, C26H21ClN4O2S)
Yield: 91%; white solid; m.p.: 192.0–192.6 °C; IR (ATR): \(\vec{v}\) = 1717 (C=O), 1576 and 1531 (C=N), 1329 (NCS) cm−1; 1H NMR (DMSO-d6): δ = 8.45 (s, 1H, CH=N), 8.31–8.28 (m, 1H, Ar–H), 7.94 (s, 1H, CH=C), 7.54–7.51 (m, 1H, Ar–H), 7.38–7.27 (m, 5H, Ar–H), 7.27–7.17 (m, 3H, Ar–H), 7.06 (dd, 2H, J = 9.8 Hz, J = 3.0 Hz, Ar–H), 5.50 (s, 2H, N-CH2), 4.08 (s, 2H, CH2), 3.84 (s, 3H, OCH3) ppm; 13C NMR (DMSO-d6): δ = 172.52 (C=O), 162.42 (C), 159.64 (C), 153.47 (CH), 140.41 (C), 137.58 (C), 135.07 (CH), 133.82 (C), 131.0 (CH), 129.77 (2CH), 128.38 (C), 128.05 (CH), 127.43 (CH), 126.23 (CH), 125.82 (C), 123.55 (CH), 122.84 (CH), 121.76 (CH), 114.80 (2CH), 112.42 (CH), 111.15 (C), 56.0 (CH3), 49.3 (CH2), 32.54 (CH2) ppm.
2-[2-[[1-(4-Fluorobenzyl)-1H-indol-3-yl]methylene]hydrazono]-3-(4-methoxyphenyl)thiazolidin4-one (9h, C26H21FN4O2S)
Yield: 95%; white solid; m.p.: 242.4–242.9 °C; IR (ATR): \(\vec{v}\) = 1716 (C=O), 1573 and 1532 (C=N), 1352 (NCS) cm−1; 1H NMR (DMSO-d6): δ = 8.44 (s, 1H, CH=N), 8.28–8.25 (m, 1H, Ar–H), 7.96 (s, 1H, CH=C), 7.56–7.54 (m, 1H, Ar–H), 7.34–7.27 (m, 4H, Ar–H), 7.25–7.21 (m, 2H, Ar–H), 7.16 (d, 2H, J = 8.9 Hz, Ar–H), 7.06 (d, 2H, J = 8.9 Hz, Ar–H), 5.45 (s, 2H, N-CH2), 4.07 (s, 2H, CH2), 3.82 (s, 3H, OCH3) ppm; 13C NMR (DMSO-d6): δ = 172.66 (C=O), 163.62 (d, 1JCF = 243.74 Hz, C-F), 162.78 (C), 159.54 (C), 153.54 (CH), 137.38 (C), 135.23 (CH), 134.11 (d, 4JCF = 2.75 Hz, C), 129.84 (2CH), 129.72 (d, 3JCF = 8.25 Hz, 2CH), 128.19 (C), 125.67 (C), 123.50 (CH), 122.80 (CH), 121.75 (CH), 115.78 (d, 2JCF = 21.46 Hz, 2CH), 114.73 (2CH), 112.1 (C), 111.32 (CH), 55.85 (CH3), 49.07 (CH2), 32.60 (CH2) ppm.
2-[2-[[1-[3-(Trifluoromethyl)benzyl]-1H-indol-3-yl]methylene]hydrazono]-3-(4-methoxyphenyl)thiazolidin-4-one (9i, C27H21F3N4O2S)
Yield: 92%; white solid; m.p.: 186.7–187.1 °C; IR (ATR): \(\vec{v}\) = 1724 (C=O), 1563 and 1530 (C=N), 1328 (NCS) cm−1; 1H NMR (DMSO-d6): δ = 8.46 (s, 1H, CH=N), 8.32–8.28 (m, 1H, Ar–H), 7.93 (s, 2H, CH=C), 7.61 (t, 1H, J = 7.70 Hz, Ar–H), 7.49 (t, 2H, J = 6.70 Hz, Ar–H), 7.31 (d, 2H, J = 8.62 Hz, Ar–H), 7.27–7.24 (m, 2H, Ar–H), 7.06 (d, 2H, J = 8.62 Hz, Ar–H), 7.31 (d, 1H, J = 7.70 Hz, Ar–H), 5.72 (s, 2H, N-CH2), 4.08 (s, 2H, CH2), 3.84 (s, 3H, OCH3) ppm; 13C NMR (DMSO-d6): δ = 172.42 (C=O), 162.47 (C), 159.64 (C), 153.47 (CH), 139.41 (C), 137.60 (C), 135.05 (CH), 131.60 (CH), 130.24 (CH), 129.96 (q, 2JCF = 31.36 Hz, C-CF3), 129.77 (2CH), 128.37 (C), 125.82 (C), 124.83 (q, 3JCF = 3.7 Hz, CH), 124.15 (q, 3JCF = 3.7 Hz, CH), 123.58 (CH), 122.86 (CH), 122.72 (q, 1JCF = 272.35 Hz, C-F), 121.80 (CH), 114.8 (2CH), 112.50 (C), 111.10 (CH), 56.0 (CH3), 49.34 (CH2), 32.54 (CH2) ppm.
2-[2-[[1-(2-Cyanobenzyl)-1H-indol-3-yl]methylene]hydrazono]-3-(4-methoxyphenyl)thiazolidin-4-one (9j, C27H21N5O2S)
Yield: 83%; white solid; m.p.: 191.4–192.1 °C; IR (ATR): \(\vec{v}\) = 1720 (C=O), 1562 and 1530 (C=N), 1351 (NCS) cm−1; 1H NMR (DMSO-d6): δ = 8.46 (s, 1H, CH=N), 8.32–8.28 (m, 1H, Ar–H), 7.93 (s, 2H, CH=C), 7.61 (t, 1H, J = 7.70 Hz, Ar–H), 7.49 (t, 2H, J = 6.70 Hz, Ar–H), 7.31 (d, 2H, J = 8.62 Hz, Ar–H), 7.27–7.24 (m, 2H, Ar–H), 7.06 (d, 2H, J = 8.62 Hz, Ar–H), 7.31 (d, 1H, J = 7.70 Hz, Ar–H), 5.72 (s, 2H, N-CH2), 4.08 (s, 2H, CH2), 3.84 (s, 3H, OCH3) ppm; 13C NMR (DMSO-d6): δ = 172.42 (C=O), 162.58 (C), 159.64 (C), 153.42 (CH), 140.00 (C), 137.73 (C), 135.21 (CH), 134.13 (CH), 133.82 (CH), 129.77 (2CH), 129.05 (CH), 128.37 (2CH), 125.81 (C), 123.71 (CH), 122.91 (CH), 121.91 (CH), 117.59 (C), 114.80 (2CH), 113.42 (C), 112.69 (C), 112.52 (C), 110.99 (CH), 55.95 (CH3), 48.39 (CH2), 32.55 (CH2) ppm.
Minimum inhibitory concentration (MIC) evaluation
All compounds were screened in vitro for their antibacterial activities against three human pathogens’ microorganisms: Staphylococcus aureus (ATCC-25923), Escherichia coli (ATCC-25922), and Pseudomonas aeruginosa (ATCC-27853). All strains were maintained on tryptic soy broth (TSB). Ceftazidime, imipenem (third-generation cephalosporin), and gentamicin (aminoglycosides) are used as standard antibacterial drugs. All the tested antibiotics were supplied from Oxoid.
The in vitro minimum inhibitory concentration (MIC) of the various compounds against bacterial strains was determined by the agar dilution method as recommended by NCCLS [41]. DMSO was used to prepare different concentrations ranging from 0.25 to 128 μg/cm3 by serial dilutions. Petri dishes were spot-inoculated with 2 mm3 of each bacterial suspension (1 × 104 CFU/spot) and incubated at 37 °C for 24 h. At the end of the incubation period, MIC was determined as the lowest concentration for the tested chemical that did not result in any visible growth on the plate. A control test was also conducted with DMSO media added to the same dilutions as used in the experiment, to ensure that the solvent had no influence on bacterial growth.
References
Abo-Ashour MF, Eldehna WM, George RF, Abdelaziz MM, Elaasser MM, Abdel Gawad NM, Gupta A, Bhakta S, Abou-Seri SM (2018) Eur J Med Chem 160:49
Radwan MAA, Ragab EA, Sabry NM, El-Shenawy SM (2007) Bioorg Med Chem 15:3832
Ahuja P, Siddiqui N (2014) Eur J Med Chem 80:509
Zhang MZ, Chen Q, Yang GF (2015) Eur J Med Chem 89:421
Kim HS, Kim Y, Doddareddy MR, Seo SH, Rhim H, Tae J, Pae AN, Choo H, Cho YS (2017) Bioorg Med Chem Lett 17:476
Velankar AD, Quintini G, Prabhu A, Weber A, Hunaeus G, Voland B, Wuest M, Orjeda C, Harel D, Varghese S, Gore V, Patil M, Gayke D, Herdemann M, Heit I, Zaliani A (2010) Bioorg Med Chem 18:4547
Zhou D, Zhou P, Evrard DA, Meagher K, Webb M, Harrison BL, Huryn DM, Golembieski J, Hornby GA, Schechter LE, Smith DL, Andree TH, Mewshaw RE (2008) Bioorg Med Chem 16:6707
Catto M, Aliano R, Carotti A, Cellamare S, Palluotto F, Purgatorio R, Stradis AD, Campagna F (2010) Eur J Med Chem 45:1359
Chiyanzu I, Clarkson C, Smith PJ, Lehman J, Gut J, Rosenthal PJ, Chibale K (2005) Bioorg Med Chem 13:3249
Lamotte Y, Martres P, Faucher N, Laroze A, Grillot D, Ancellin N, Saintillan Y, Beneton V, Gampe RT (2010) Bioorg Med Chem Lett 20:1399
Chiummiento L, Funicello M, Lupattelli P, Tramutola F, Campaner P (2009) Tetrahedron 65:5984
Alvarez R, Puebla P, Díaz JF, Bento AC, García Navas R, de la Iglesia VJ, Mollinedo F, Andreu JM, Medarde M, Pelaez R (2013) J Med Chem 56:2813
Oishi S, Watanabe T, Sawada JI, Asai A, Ohno H, Fujii N (2010) J Med Chem 53:5054
Pirrung MC, Pansare SV, das Sarma K, Keith KA, Kern ER (2005) J Med Chem 48:3045
De Aquino TM, Liesen AP, da Silva REA, Lima VT, Carvalho CS, de Faria AR, de Araujo JM, de Lima JG, Alves AJ, de Melo EJT, Alexandre JSG (2008) Bioorg Med Chem 16:446
Hu WX, Zhou W, Xia CN, Wen X (2006) Bioorg Med Chem Lett 16:2213
Jain AK, Vaidya A, Ravichandran V, Kashaw SK, Agrawal RK (2012) Bioorg Med Chem 11:3378
Srivastava T, Gaikwad AK, Haq W, Sinha S, Katti SB (2005) ARKIVOC 2:120
Aly AA, Ishak EA, El Malah T, Brown AB, Elayat WM (2015) J Heterocycl Chem 52:1758
Gududuru V, Hurh E, Dalton JT, Miller DD (2004) Bioorg Med Chem Lett 14:5289
Zhou H, Wu S, Zhai S, Liu A, Sun Y, Li R, Zhang Y, Ekins S, Swaan PW, Fang B, Zhang B, Yan B (2008) J Med Chem 51:1242
Kaur H, Kumar S, Vishwakarma P, Sharma M, Saxena KK, Kumar A (2010) Eur J Med Chem 45:2777
Kumar A, Rajput CS, Bhati SK (2007) Bioorg Med Chem 15:3089
Knutsen LJS, Hobbs CJ, Earnshaw CG, Fiumana A, Gilbert J, Mellor SL, Radford F, Smith NJ, Birch PJ, Burley R, Thomas D, Ward SDC, James IF (2007) Bioorg Med Chem Lett 17:662
Vicini P, Geronikaki A, Incerti M, Zani F, Dearden J, Hewitt M (2008) Bioorg Med Chem 16:3714
Patel NB, Patel SD (2010) Acta Pol Pharm 67:45
Omar K, Geronikaki A, Zoumpoulakis P, Camoutsis C, Sokovic M, Ciric A, Glamoclija J (2010) Bioorg Med Chem 18:426
Angapelly S, Sri R, SunithaRani R, Kumar CG, Kamal A, Arifuddin M (2017) Tetrahedron Lett 58:4632
Benmohammed A, Khoumeri O, Djafri A, Terme T, Vanelle P (2014) Molecules 19:3068
Kabri Y, Azas N, Dumetre A, Hutter S, Laget M, Verhaeghe P, Gellis A, Vanelle P (2010) Eur J Med Chem 45:616
Montana M, Correard F, Khoumeri O, Esteve MA, Terme T, Vanelle P (2014) Molecules 19:14987
Khoumeri O, Montana M, Terme T, Vanelle P (2012) Tetrahedron Lett 53:2410
Huang H, Chen Q, Ku X, Meng L, Lin L, Wang X, Zhu C, Wang Y, Chen Z, Li M, Jiang H, Chen K, Ding J, Liu H (2010) J Med Chem 53:3048
Ma J, Bao G, Wang L, Li W, Xu B, Du B, Lv J, Zhai X, Gong P (2015) Eur J Med Chem 96:173
Bharti N, Sharma SS, Naqvi F, Azam A (2003) Bioorg Med Chem 11:2923
Antonini I, Claudi F, Franchetti P, Grifantini M, Marteli S (1977) J Med Chem 20:447
Afrasiabi Z, Sinn E, Chen J, Ma Y, Rheingold AL, Zakharov LN, Rath N, Padhye S (2004) Inorg Chim Acta 357:271
Chen J, Huang YW, Liu G, Afrasiabi Z, Sinn E, Padhye S, Ma Y (2004) Toxicol Appl Pharmacol 197:40
Karabatsos GJ, Vane FM, Talle RA, His N (1964) J Am Chem Soc 86:3351
Serda M, Malecki JG, Mrozek-Wilczkiewicz A, Musiol R, Polanski J (2013) J Mol Struct 1037:63
CLSI/NCCLS Guidelines, 8th edn (2009) Clinical and Laboratory Standards Institute, Pennsylvania, USA
Singh M, Singh SD, Gangwar M, Nath G, Singh SK (2016) Med Chem Res 25:263
Acknowledgements
This work was supported by the CNRS (Centre National de la Recherche Scientifique) and Aix-Marseille University.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Benmohammed, A., Rekiba, N., Sehanine, Y. et al. Synthesis and antimicrobial activities of new thiosemicarbazones and thiazolidinones in indole series. Monatsh Chem 152, 977–986 (2021). https://doi.org/10.1007/s00706-021-02823-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-021-02823-6