Abstract
Ca isotopes have gained increasing interest as a diagnostic tool for bone diseases due to the variations in abundances as a consequence of changes in bone-mineral balance. Optimized Ca/matrix separation prior to analysis is a prerequisite for reliable isotope ratio measurements in complex biological matrices such as blood, serum, or urine. The online analysis of analyte/matrix separation by ICP-MS enables direct assessment of elution profiles supporting the optimization process. The integration of transient signals and signal suppression challenge the quantification and interpretation of the elution profiles. Mn and Co remain unretained by the DGA Resin (TrisKem International) from nitric acid. Hence, in the present study, these elements were investigated for their application as standards to monitor signal suppression. Successful analyte quantification was accomplished using a dynamic correction strategy applying a linear gradient of a suppression factor based on Mn and Co intensities. An optimized Ca/matrix separation procedure using DGA Resin is proposed based on the results during online ICP-MS analysis.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
DGA Resin (N,N,N′,N′-tetra-n-octyldiglycolamide, TrisKem International, Bruz, France) was developed as an extraction resin in the early 2000s by Horwitz et al. [1]. The primary advantage of this resin is the high affinity for trivalent REEs and actinides [2, 3], which originated from the need to treat high-level radioactive waste [1, 3]. Therefore, DGA Resin has been applied, first and foremost, for analyte/matrix separation in the nuclear isotopic analysis. Applications include the separation of radioactive isotopes of Sr [4, 5], Y [6], Po [7], Ac [8], Pu [9,10,11], Am [12], and Cm [13]. The beneficial features and characteristics of DGA Resin are also applicable for analyte/matrix separation of stable isotopes in the fields of geochemical, archaeological, environmental, and biomedical sciences. Table 1 summarizes analyte/matrix separation procedures for isotopic systems using DGA Resin. Future development of analyte/matrix separation procedures might target further stable isotope systems, which are retained by DGA Resin [1, 14, 15].
Calcium isotope abundance analysis is a growing field of research in biomedicine and can be used as a diagnostic tool for bone diseases like osteoporosis (e.g. [16,17,18,19]. Herein, the natural variation of Ca isotopic composition is determined with multicollector thermal ionization mass spectrometry (MC TIMS) or multicollector inductively coupled plasma mass spectrometry (MC ICP–MS). Both analytical techniques require a quantitative Ca/matrix separation for complex matrices such as blood, serum, or urine, to reduce the effects of spectral interferences (e.g. K, Mg, Al, Ti, Sr) and mass fractionation effects (e.g. by Na. Fe, P, Ba) [20]. Since MC TIMS was the preferred method for the reliable determination of Ca isotopic composition during the last decades, the offline Ca/matrix separation protocol from Feng et al. [21] was selected in the present study for further investigation. Feng et al. [21] employed the DGA Resin for Ca purification. Unwanted matrix elements, such as Na, Mg, Al, K, Ti, and Ba, are unretained using nitric acid as eluent [15].
The development and optimization of analyte/matrix separation procedures is a tedious and time-consuming task including the optimization of parameters such as resin volume (RV), eluent composition and acidity, eluent volume, and elution speed. In conventional offline optimization, small defined volumes (e.g. 0.5 cm3 or 1 cm3) are collected during incremental elution and analyzed for their elemental composition, leading to a elution profile (see e.g. [22, 23]). Online analysis of analyte/matrix separation provides a powerful alternative to batch separation. The advantages of online analysis of analyte/matrix separation lie in the continuous observation of a transient signal during the whole separation procedure. The elution profile provides information about the height, sharpness and broadening of the peaks, which enables the determination of the distinct elution volume required. Further, the mixing behavior and equilibration of the eluents can be monitored, as shown in this work. Pooled elution fractions of batch separation do not provide this information, which is crucial for loading and elution of the target element.
Inductively coupled plasma mass spectrometry (ICP-MS) is a preferable method for the measurement of eluted elements in online separation provided by high-performance liquid chromatography (HPLC) or ion chromatography (IC) [24], using micro-columns based on online flow injection [25, 26] or automated systems [22, 23]. The transient signals measured by the ICP–MS must be integrated for the quantification of analytes in the different elution steps to evaluate the separation efficiency and analyte recovery. Variations in the composition of the eluent can cause significant signal suppression, which is a challenge for accurate quantification.
Besides interfering matrix elements, Co and Mn are also unretained by DGA Resin using nitric acid as eluent [15]. These two elements do not influence Ca isotope amount ratio measurements. Therefore, the working hypothesis of the present work was to introduce Co and Mn continuously during the separation procedure to monitor for possible suppression and the mixing behavior of eluents and consequently enable a reliable quantification of the analyte and matrix elements in the transient signal. The aim of our study was to prove this concept and to demonstrate that online analysis (applying monitoring elements) is helpful to optimize offline separation protocols.
Results and discussion
Quantification of the Ca/matrix separation
The first quantification approach investigated for the transient signal (of the elements of interest) employed an external nine-point-calibration [normalized to 59Co, prepared in dilute nitric acid (w = 3%)] from offline-analysis. The signals were integrated over a time period corresponding to the incremental time intervals of the transient signal. The transient elemental signal intensities were corrected for the blank by subtracting the average signal intensity of each corresponding fraction for nitric acid (c = 3 mol dm−3) and subboiled water at baseline. Each eluent was doped with Mn and Co (1 ng g−1) to provide a continuous normalization signal. The transient signal was normalized to the signal intensity of 59Co (internal standard). 55Mn was monitored in parallel. The elution curve was divided into sample load, matrix wash, and Ca elution sections. The calculated total amounts of each of these three sections were summed up and compared to the total amount loaded (see section “Data reduction-internal normalization”). The resulting overall recoveries indicated significant overestimates in the final fraction for Ca as well as for the coeluting elements Sr, In, and Pb (recoveries of 333%, 206%, 177%, and 249%, respectively, see Supplementary Table 2). In contrast, Fe which is also partly co-eluting in the final fraction showed low recoveries (around 21%). It has to be noted, that Fe signals were close to LOD. The gradient from subboiled water to nitric acid (c = 3 mol dm−3) and accompanying suppression effects in the ICP as well as a potential delay of Co (also Mn) elution due to slight retaining by DGA Resin may have hampered this quantification strategy.
Consequently, a second quantification approach for the transient signal (of the elements of interest) was investigated. In this approach, suppression correction was applied prior to quantification using the external nine-point-calibration (mass vs. intensity, prepared in diluted nitric acid (w = 3%)) from offline-analysis. Therefore, the suppression factors of Mn and Co were determined in the different eluent matrices first, since both elements were continuously present in each elution step. Table 2 lists the suppression factors of 55Mn as well as 59Co in nitric acid (c = 3 mol dm−3), in nitric acid (c = 3 mol dm−3) including a multi-elemental test solution (β(Ca) = 975 ng g−1, see Supplementary Table 2), and in subboiled water relative to diluted nitric acid (w = 2%) during offline ICP-MS measurements. The suppression factors of nitric acid (c = 3 mol dm−3) with and without multi-element matrix overlapped within standard deviation (see Table 2). Suppression effects by multi-element matrix are considered negligible. To cope with a variation of suppression factors between elements, a mean suppression factor for each eluent (nitric acid (c = 3 mol dm−3), subboiled water) was calculated from 55Mn and 59Co intensities. Mean suppression factors of 1.57 ± 0.15 (SD, n = 14) for nitric acid (c = 3 mol dm−3; conditioning, sample load and matrix wash) and 0.15 ± 0.05 (SD, n = 18) for subboiled water (column cleaning and Ca elution), respectively, were determined.
After blank subtraction, the transient signal of each elution step was corrected using the mean suppression factors for the corresponding eluent (sample load with nitric acid (c = 3 mol dm−3), matrix wash with nitric acid (c = 3 mol dm−3), Ca elution with subboiled water). Each complete elution step was corrected with the suppression factor (static model). Following the blank subtraction, a quantification of the transient signal (for each element) was performed using an external nine-point-calibration (mass vs. intensity, see section “Data reduction-dynamic correction of suppression factors”). Then, the calculated total amount of analyte and matrix elements in the sample load step, matrix wash step and Ca elution step were summed up and compared to the amount loaded (see section “Data reduction-internal normalization”). This procedure led to a significant overestimation of all elements which elute—at least partially—in subboiled water: Ca, Fe, Sr, In, and Pb (recoveries of 901%, 449%, 695%, 591%, and 757%, respectively).
Hence, a third quantification approach for the transient signal (of the elements of interest) was investigated. Herein, a concentration gradient from subboiled water to nitric acid (c = 3 mol dm−3) was assumed when starting the conditioning. Further, a concentration gradient from nitric acid (c = 3 mol dm−3) to subboiled water was assumed when starting the Ca elution due to on-column mixing. Hence, the transient signal was dynamically corrected using a linear gradient of the mean suppression factors from one eluent to the other (e.g. for Ca elution from nitric acid (c = 3 mol dm−3; 1.57 ± 0.15 (SD, n = 14)) to subboiled water (0.15 ± 0.05 (SD, n = 18)). Consideration of the time required for the gradient of an entire resin volume (200 mm3, 24 s) only accounted for 30% of the overestimate observed for the second quantification approach. Therefore, a time of 141.4 s (280 data points, 1180 mm3 = 5.9 times RV) required for the gradient was estimated based on the elution profiles of Mn and Co reaching baseline intensities in nitric acid (c = 3 mol dm−3; covering conditioning, sample load and matrix wash) and in subboiled water (covering Ca elution), respectively (see Figs. 1 and 2). Under the assumption of a linear gradient, dynamic suppression factors ranging from 0.15 ± 0.05 (subboiled water) to 1.57 ± 0.15 (nitric acid, c = 3 mol dm−3) were applied for the 115.1 s of conditioning and the first 26.3 s sample load. Dynamic suppression factors from 1.57 (nitric acid, c = 3 mol dm−3) to 0.15 (subboiled water) were applied for the first 141.4 s of Ca elution. Finally, the quantifications of the transient signals for each element were performed using external nine-point-calibration (mass vs. intensity, see section “Data reduction-dynamic correction of suppression factors”). Recoveries were calculated accordingly (see section “Data reduction-internal normalization”). In agreement with Feng et al. [21], Ca recovery was 102% ± 15% in the Ca elution step using subboiled water (see Fig. 3 and Supplementary Table 3). Recoveries of Sr, In, and Pb were significantly reduced (80% ± 18%, 72% ± 30%, and 110% ± 30%, respectively) and the recovery of Fe have increased (73% ± 30%) (see Supplementary Table 3). The successful quantification (approach 2) of the online analysis of Ca/matrix separation using DGA resin, with a dynamic suppression correction proved the concept of using Mn and Co signal intensities as indicators for suppression effects.
Elution profiles of 55Mn and 59Co intensities (I) during a conditioning (V = 1 cm3 nitric acid (c = 3 mol dm−3); 2 min), sample load (V = 0.5 cm3 nitric acid (c = 3 mol dm−3); 1 min) and matrix wash (V = 1 cm3 nitric acid (c = 3 mol dm−3); 2 min) and b Ca elution (V = 5 cm3 subboiled water; 10 min) of the blank sample (nitric acid, c = 3 mol dm−3)
Elution profiles of 55Mn and 59Co blank-corrected intensities (I) during a conditioning (V = 1 cm3 nitric acid (c = 3 mol dm−3); 2 min), sample load (V = 0.5 cm3 nitric acid (c = 3 mol dm−3); 1 min) and matrix wash (V = 1 cm3 nitric acid (c = 3 mol dm−3); 2 min) and b Ca elution (V = 5 cm3 subboiled water; 10 min) of the multi-elemental test solution
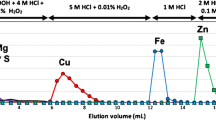
Elution profiles of several elements of interest during Ca/matrix separation of the multi-elemental test solution, shown as cumulative recoveries (R) per 50 mm3 (6 s) after quantification using approach 2, applying dynamic suppression correction and external calibration (mass vs. intensity). Additionally, raw intensities of Co (I) are shown as average per 50 mm3 (6 s)
Optimized Ca/matrix separation
Figure 3 shows the elution profile of Ca, as well as coeluting matrix elements causing spectral (K, Mg, Al, Ti, Sr) and non-spectral interferences (Na. Fe, Ba). Due to the properties of the DGA Resin, most of the matrix-based interferences (Na, Mg, Al, K, and Ti) are removed from Ca within the matrix wash. It should be noted, that a co-elution of Sr is not a concern for Ca isotopic analysis using MC TIMS [21] but a serious issue for Ca isotopic analysis using MC ICP-MS [20] because these elements cannot be separated during analysis in the mass spectrometer when ionized in the plasma source.
Following the results of the successful quantification during online matrix separation, an optimized Ca/matrix separation procedure is proposed in Table 3 based on the following considerations: (1) when re-using the DGA Resin, a pre-cleaning step with diluted hydrochloric acid as applied in previous studies [22, 23, 27] should be considered to remove remaining REEs (e.g. Y and U, see Supplementary Table 3) from the DGA Resin. (2) Since the time required for the concentration gradient from subboiled water to nitric acid (c = 3 mol dm−3) exceeds the time required for the conditioning step and interferes with the accurate loading acidity of the nitric acid (c = 3 mol dm−3), an extension of the conditioning step to 1.5 cm3 (3 min at 500 mm3 min−1 pump speed) should be considered. (3) Based on the observed elution profile, the Ca elution volume can be reduced to 750 mm3 (1.5 min at 500 mm3 pump speed, 3.75 RV), while maintaining a recovery of 102% (see Supplementary Table 3: Ca elution (750 mm3), Fig. 3) (Note: Total mass fraction of Ca in the sample load was 721 ng ± 72 ng, the collected mass fraction of Ca in the Ca elution using 5 cm3 was 741 ng ± 111 ng, the collected mass fraction of Ca in Ca elution using 750 mm3 was 740 ng ± 111 ng).
Conclusion
The quantification of a transient signal during an online separation is challenging because of the dynamic gradient of eluents in an online analysis of analyte/matrix separation following on-column mixing. A modified approach using dynamic suppression correction, based on indicator elements (Mn, Co) that were unretained by the DGA resin, proved to be a robust approach for accurate quantification as a prerequisite for the optimization of analyte/matrix separation procedures.
The online analysis of Ca/matrix separation using DGA Resin enabled insights in the mixing behavior of the eluents (subboiled water, nitric acid (c = 3 mol dm−3)) which was revealed by the elution behavior of indicator elements Mn and Co. Based on the results during online separation, an optimized Ca/matrix separation procedure using DGA Resin is proposed for subsequent Ca isotope ratio analysis by TIMS analysis.
The approach is advantageous for separation procedures with alternating elution solutions and can be applied to any system once suitable monitoring elements (i.e. will not be retained on the resin) are found. This can dramatically increase the efficiency of the method development and allow for rapid checks of ion exchange methods for various different matrices.
Experimental
Type I reagent-grade water (18 MΩ cm) (F + L GmbH, Vienna, Austria) was further purified by sub-boiling distillation (Milestone-MLS GmbH, Leutkirch, Germany). Analytical reagent-grade nitric acid (w = 65%; Merck-Millipore, Darmstadt, Germany) was purified by double sub-boiling using a Savillex DST-1000 sub-boiling distillation system (AHF Analysentechnik, Tübingen, Germany). Polyethylene flasks, tubes and pipette tips (VWR International, Radnor, USA) were pre-cleaned in a two-stage washing procedure using diluted nitric acid (w = 10% and w = 1%, respectively).
Single-element standards were purchased from Inorganic Ventures (Christiansburg, USA: Na, Mg, Al, K, Ca, Fe, Mn, Co, Cu, Zn, Sr, Y, Cd, In, Ba, Pb, and U), Merck (Ti, Cr, Ni, and Mo) and VWR International (Radnor, USA: Tl, Rb (Aristar)).
Two grams of the unbranched DGA Resin (TrisKem International) with a particle size of 50 µm–100 μm was soaked in diluted ethanol (w ≈ 30%, Merck) and shaken for 1 h. For the online analysis of Ca/matrix separation, an empty PFA column with a resin volume of 200 mm3 (part. no. CF-0200, ESI) was utilized and self-packed with the soaked DGA Resin following the standard procedure [22].
A multi-elemental test solution containing 10 ng g−1 of Na, Mg, Al, K, Ti, Fe, Cr, Ni, Cu, Zn, Rb, Sr, Y, Mo, Cd, In, Ba, Tl, Pb, and U and 1000 ng g−1 of Ca was gravimetrically prepared in nitric acid (c = 3 mol dm−3) including 1 ng g−1 of Mn and Co spike. The nitric acid (c = 3 mol dm−3) as eluent was prepared gravimetrically and spiked with 1 ng g−1 of Mn and Co. The subboiled water as eluent was spiked with 1 ng g−1 of Mn and Co.
For corrections of suppression effects and for the quantification of recoveries, a nine-point-calibration with the same multi-elemental mixture as the test solution was gravimetrically prepared in diluted nitric acid (w = 2%) covering the range of 0.05 ng g−1 and 150 ng g−1. All calibration standards were spiked with 1 ng g−1 of Mn and Co. Furthermore, the performance of the multi-elemental analysis was monitored with in-house quality control solution prepared in nitric acid (c = 3 mol dm−3), diluted nitric acid (w = 2%) and subboiled water. The quality control solutions were spiked with 1 ng g−1 of Mn and Co.
Offline multi-elemental analysis
To correct suppression effects of the different eluents and for quantification of recoveries, multi-elemental composition of the different eluents and the multi-elemental test solution were determined in standard liquid set-up using the ICP-QMS (NexION 2000B, PerkinElmer) coupled to an ESI SC-2 DX FAST autosampler. General instrumental settings are described in Supplementary Table 1.
Online analysis of Ca/matrix separation
Multi-elemental online analysis of Ca/matrix separation was carried out by connecting an autosampler probe (i.d. 1 µm, ESI, Omaha, USA) using a 10-cm-long PFA Capillary Extension (i.d. 1 µm, ESI) to a pump tubing (i.d. 1.1 µm, red/red, PerkinElmer, Ontario, Canada), which was clamped into a peristaltic pump (part. no. 3642, Ismatec, Wertheim, Germany). The sample was taken up and transported at a flow rate of 500 mm3 min−1 via a PFA capillary (i.d. 1 µm and ¼-28 nut, ESI) into the PFA column (ESI). The column was filled with 200 mm3 unbranched DGA Resin (part. no. DN-B25-S, TrisKem International). Sampling was performed manually. The column run-off was directly connected to an ICP-QMS (NexION 2000B, PerkinElmer) equipped with a sea-spray glass nebulizer (max. uptake 2 cm3 min−1, PerkinElmer).
The multi-element composition was monitored online in a time-resolved sequence using the ICP-QMS (NexION 2000B, PerkinElmer). General instrumental settings are described in Supplementary Table 1.
Procedure of the Ca/matrix separation
Ca/matrix separation was performed following the separation procedure of Feng et al. [21] using unbranched DGA Resin (TrisKem International): A conditioning step using nitric acid (c = 3 mol dm−3) was followed by a sample loading step using nitric acid (c = 3 mol dm−3), and a matrix wash step using nitric acid (c = 3 mol dm−3). Ca was finally eluted using subboiled water. The elution volumes were adapted according to the resin volume used. The corresponding elution steps and parameters are shown in Table 4.
Transient multi-elemental signal was recorded by online analysis for the Ca/matrix separation (transient signal) of a blank sample containing nitric acid (c = 3 mol dm−3, 500 mm3). Further, the transient multi-elemental signal was recorded by online analysis for the Ca/matrix separation (transient signal) of a multi-elemental test solution prepared in nitric acid (c = 3 mol dm−3, 500 mm3). The multi-elemental test solution contained approx. 750 ng total Ca as major matrix element and < 10 ng of Na, Mg, Al, K, Ti, Cr, Fe, Ni, Cu, Zn, Rb, Sr, Mo, Cd, In, Ba, Tl, Pb, and U as minor elements.
Data reduction–internal normalization
Elution steps were identified and defined by 55Mn and 59Co raw intensities. Blank correction of each elution step (loading, matrix wash, Ca elution) was performed by subtracting the average intensities of the corresponding blank elution step. In the case of loading with nitric acid (c = 3 mol dm−3) and Ca elution with subboiled water, only data points recorded after the signal reached baseline (141.1 s, see “Results and discussion”) were used.
After blank subtraction, the intensities of the transient signals (elements of interest) were normalized point-by-point with the corresponding 59Co signal intensity, applied as an internal standard. Point-by-point mass fractions (ng g−1) during the transient signal were quantified using the nine-point-calibration of the offline multi-elemental measurement, which was also normalized to 59Co signal intensity. Cumulative mass fractions for all elements under investigation were calculated as the integral over each elution step. To evaluate the recovery, the overall integrated mass fractions were calculated as the sum of the sample load, the matrix wash and the Ca elution (Note: Only an elution time of 1.5 min corresponding to an elution volume of 750 mm3 for Ca elution was used) and compared to the mass fractions of the loaded sample.
The Limits of Detection and Quantification (LOD, LOQ) was calculated according to DIN ISO 11843-2 [37]. For each elution step, the LOD was defined as 3 × SD of the corresponding baseline blank signal. (Note: In the evaluation of the multi-elemental test solution, all values below LOD were set to zero.) Mass fractions, recoveries, LODs and LOQs, including expanded relative uncertainties (Urel (k = 2)) are summarized in Supplementary Table 2.
Data reduction–dynamic correction of suppression factors
Elution steps were identified and defined by 55Mn and 59Co raw intensities. Blank correction of each elution step (loading, matrix wash, Ca elution) was performed by subtracting the average intensities of the corresponding blank elution step. In the case of loading with nitric acid (c = 3 mol dm−3) and Ca elution with subboiled water, only data points after reaching the baseline (141.1 s, see “Results and discussion”) were used.
After blank subtraction, the intensities of the transient signals (elements of interest) were dynamically corrected for suppression effects in the sample loading and the Ca elution step. Therefore, a linear gradient of the mean suppression factors from one eluent to the other was applied. The time required for the gradient was estimated based on the elution profiles of 55Mn and 59Co signal intensities reaching baseline intensities (see “Results and discussion”). To enable a quantification without internal normalization, a nine-point-calibration based on total mass (accounted by sample volume (500 mm3) and the density of diluted nitric acid (w = 3%)) plotted against signal intensity was a set-up from the offline multi-elemental measurement. Point-by-point total masses (ng) during the transient signal were quantified using the slope of this nine-point-calibration (mass vs. intensity). Cumulative masses for all elements under investigation were calculated as the integral over each elution step. To evaluate the recovery, the overall integrated total masses were calculated as the sum of the sample load, the matrix wash and the Ca elution (Note: Only an elution time of 1.5 min corresponding to an elution volume of 750 mm3 for Ca elution was used) and compared to the total mass of the loaded sample. The mass of the loaded sample was quantified by the nine-point-calibration (accounted by sample volume (500 mm3) of the test solution and density of the nitric acid (c = 3 mol dm−3)). The LOD and LOQ for the total mass were defined as 3 × SD and 10 × SD, respectively, of the baseline blank signal from the corresponding eluent. Total masses, recoveries, LODs and LOQs, including expanded relative uncertainties (Urel (k = 2)) are summarized in Supplementary Table 3.
Uncertainty calculations
The total combined uncertainty for each elemental total mass (mspl) was calculated using a simplified Kragten approach [38] using Eq. (1) as the model equation. The precision of the measured raw intensity of the sample (Ispl), the estimated correction factor for the suppression effect (f(I)sup) and the slope of the calibration curve (k) were identified as the main contributors to the uncertainty.
References
Horwitz EP, McAlister DR, Bond AH, Barrans RE (2005) Solvent Extr Ion Exch 23:219
Eichrom Technologies (2020) DGA resins. Eichrom Technologies, LLC. https://www.eichrom.com/eichrom/products/dga-resins/. Accessed 16 Jun 2020
Bertelsen ER, Jackson JA, Shafer JC (2020) Solvent Extr Ion Exch 38:251
McLain DR, Tsai Y, Graczyk DG, Canaday JL, Steeb JL (2018) J Radioanal Nucl Chem 317:1439
Shao Y, Yang G, Tazoe H, Ma L, Yamada M, Xu D (2018) J Environ Radioact 192:321
Aluicio-Sarduy E, Hernandez R, Valdovinos HF, Kutyreff CJ, Ellison PA, Barnhart TE, Nickles RJ, Engle JW (2018) Appl Radiat Isot 142:28
Maxwell SL, Culligan BK, Hutchison JB, Utsey RC, McAlister DR (2013) J Radioanal Nucl Chem 298:1977
McAlister DR, Horwitz EP (2018) Appl Radiat Isot 140:18
Maxwell SL, Culligan BA, Jones VD, Nichols ST, Noyes GW (2011) J Radioanal Nucl Chem 287:223
Grate JW, O’Hara MJ, Farawila AF, Douglas M, Haney MM, Petersen SL, Maiti TC, Aardahl CL (2011) Anal Chem 83:9086
Dolique I, Kabai E, Schuster M (2019) J Radioanal Nucl Chem 322:1423
Wang Z, Zheng J, Tagami K, Uchida S (2017) J Radioanal Nucl Chem 312:151
Kazi Z, Guérin N, Christl M, Totland M, Gagné A, Burrell S (2019) J Radioanal Nucl Chem 321:227
Sasaki Y, Zhu Z-X, Sugo Y, Kimura T (2007) J Nucl Sci Technol 44:405
Pourmand A, Dauphas N (2010) Talanta 81:741
Morgan JLL, Skulan JL, Gordon GW, Romaniello SJ, Smith SM, Anbar AD (2012) Proc Natl Acad Sci 109:9989
Heuser A, Eisenhauer A, Scholz-Ahrens KE, Schrezenmeir J (2016) Isot Environ Health Stud 52:633
Tanaka Y-K, Mikuni-Takagaki Y, Hidaka K, Wada-Takahashi S, Kawamata R, Hirata T (2019) Anal Sci 35:793
Eisenhauer A, Müller M, Heuser A, Kolevica A, Glüer CC, Both M, Laue C, Hehn UV, Kloth S, Shroff R, Schrezenmeir J (2019) Bone Rep 10:100200
Feng L, Zhou L, Yang L, Zhang W, Wang Q, Shuoyun T, Hu Z (2018) J Anal At Spectrom 33:413
Feng L-P, Zhou L, Yang L, DePaolo DJ, Tong S-Y, Liu Y-S, Owens TL, Gao S (2017) Geostand Geoanal Res 41:93
Retzmann A, Zimmermann T, Pröfrock D, Prohaska T, Irrgeher J (2017) Anal Bioanal Chem 409:5463
Zimmermann T, Retzmann A, Schober M, Pröfrock D, Prohaska T, Irrgeher J (2019) Spectrochim Acta Part B 151:54
Latkoczy C, Prohaska T, Watkins M, Teschler-Nicola M, Stingeder G (2001) J Anal At Spectrom 16:806
Galler P, Limbeck A, Boulyga SF, Stingeder G, Hirata T, Prohaska T (2007) Anal Chem 79:5023
Galler P, Limbeck A, Uveges M, Prohaska T (2008) J Anal At Spectrom 23:1388
Romaniello SJ, Field MP, Smith HB, Gordon GW, Kim MH, Anbar AD (2015) J Anal At Spectrom 30:1906
Bao Z, Zong C, Chen K, Lv N, Yuan H (2020) Int J Mass Spectrom 448:116268
Feng L, Hu W, Jiao Y, Zhou L, Zhang W, Hu Z, Liu Y (2020) J Anal At Spectrom 35:736
Inglis EC, Creech JB, Deng Z, Moynier F (2018) Chem Geol 493:544
Pourmand A, Prospero JM, Sharifi A (2014) Geology 42:675
Li C-F, Wang X-C, Guo J-H, Chu Z-Y, Feng L-J (2016) J Anal At Spectrom 31:1150
Pin C, Gannoun A (2019) J Anal At Spectrom 34:2136
Pin C, Gannoun A (2019) J Anal At Spectrom 34:310
Chu Z-Y, Wang M-J, Li C-F, Yang Y-H, Xu J-J, Wang W, Guo J-H (2019) J Anal At Spectrom 34:2053
Vogl J, Meyer C, Koenig M, Becker D, Noordmann J, Rienitz O, Mamakos A, Riccobono F (2015) J Anal At Spectrom 30:479
DIN ISO 11843-2:2006-06, Erkennungsfähigkeit—Teil 2: Verfahren im Fall der linearen Kalibrierung (ISO 11843-2:2000; Text Deutsch, Englisch) (2006) Beuth Verlag GmbH
Kragten J (1994) Analyst 119:2161
Acknowledgements
This work was funded by the Chemical Monthly fellowship (2018) of the Austrian Academy of Sciences (ÖAW) (grant number: Stipendium der Monatshefte für Chemie).
Funding
Open access funding provided by Montanuniversität Leoben.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Retzmann, A., Walls, D., Miller, K. et al. Assessing the potential of online ICP–MS analysis to optimize Ca/matrix separation using DGA Resin for subsequent isotopic analysis. Monatsh Chem 152, 401–410 (2021). https://doi.org/10.1007/s00706-021-02754-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-021-02754-2