Abstract
The reaction of azomethine ylide generated in situ from ninhydrin and sarcosine/thiaproline with fluorinated cyclopent[b]indole dipolarophiles in refluxing dioxane and methanol afforded a novel class of fluorinated cyclopent[b]indole dispiroheterocycles via 1,3-dipolar cycloaddition. The crystal structures of 4′-[4-(trifluoromethyl)phenyl]-1′,5-dimethyl-2,3-dihydrodispiro[cyclopent[b]-indol-2,3′-pyrrolidine-2′,2″-indene]-1,1″,3″-trione and 4′-(4-fluorophenyl)-5-methyl-2,3-dihydrodispiro[cyclopent[b]indol-2,3′-pyrrolizidine-2′,2″-indene]-1,1″,3″-trione are reported. New compounds are investigated theoretically via DFT calculations utilizing M062X hybrid function with 6-311++G(d,p) basis sets in vacuum. Results from in vitro cytotoxicity screening are compared with those of standard drugs.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A large number of indole alkaloids from cyclopent[b]indole ring systems include structurally complex tremorgenic mycotoxins [1] such as paxilline, paspaline, lolitrems, janthitrems, and yuehchukenes [2]. From the review of literature, we realized that many alkaloids containing cyclopent[b]indole unit in their architecture have displayed a broad spectrum of pharmacological properties [3,4,5,6]. A significant reaction mechanism for constructing pyrrolidine and pyrrolizidine spiroheterocycles takes advantage of the azomethine ylide generated via 1,3-dipolar cycloaddition [7]. Some naturally occurring substances with highly pronounced biological properties are prolific for the production of spiro compounds [8]. Here, functionalized pyrrolidine, pyrrolizidine, and oxindole alkaloids represent a novel class of compounds with remarkable biological activities. The spirooxindole structure has been utilized in numerous pharmacological agents and natural alkaloids [9, 10]. Fluoroorganics play a vital role in the development of many useful compounds in the agrochemical, pharmaceutical and material science avenue. Fluorination of heterocycles helps in tuning the course of the reaction besides influencing the biological/pharmacological activity. Incorporation of a trifluoromethyl substituent has been shown to increase the phytotoxicity along with selectivity and arenes bearing a trifluoromethyl substituent comprise the largest subgroup of commercially promising pesticides and herbicides [11,12,13,14,15].
The synthesis of dispiro compounds containing indole and quinolines was reported by our group recently [16,17,18]. Herein we report the synthesis of novel fluorinated dispiroheterocycles from the reaction of azomethine ylide generated in situ from ninhydrin and sarcosine/thiaproline with fluorinated cyclopent[b]indole dipolarophiles [19,20,21] in refluxing dioxane and methanol to afford via 1,3-dipolar cycloaddition. The stereochemistry and stability of the fluorinated compounds are well documented through single crystal XRD studies as well as DFT calculations. Quantum calculation studies of molecular properties have been proven useful [22,23,24,25,26] and are here performed at the M062X/6-311++G(d,p) level of theory in vacuum. Optimized structural parameters, vibration frequencies, contour diagram of frontier molecular orbitals (FMOs), molecular electrostatic potential (MEP) maps, MEP contours, and non-linear optical (NLO) properties of compounds 4′-[4-(trifluoromethyl)phenyl]-1′,5-dimethyl-2,3-dihydrodispiro[cyclopent[b]indol-2,3′-pyrrolidine-2′,2″-indene]-1,1″,3″-trione (3) and 4′-(4-fluorophenyl)-5-methyl-2,3-dihydrodispiro[cyclopent[b]indol-2,3′-pyrrolizidine-2′,2″-indene]-1,1″,3″-trione (4) are calculated with Gaussian09 [27]. In evaluating the cytotoxicity of the newly synthesized compounds, two different cancer cell lines were utilized such as HeLa (cervix adenocarcinoma), and MCF-7 (breast cancer).
Results and discussion
Chemistry
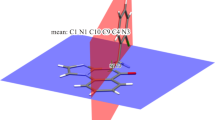
The 1,3-dipolar cycloaddition reaction of azomethine ylide, generated in situ via decarboxylative condensation of sarcosine with ninhydrin (2) to 5-methyl-2-[4-(trifluoromethyl)phenylmethylene]-1,2,3,8-tetrahydrocyclopent[b]indol-1-one (1a) in dioxane and methanol under reflux condition afforded a new class of regioselective dispiro substituted 1-oxo-cyclopent[b]indole compound 3 (Scheme 1). The FT-IR spectrum of the compound 3 showed strong bands at 3279, 1709, and 1646 cm−1, which were due to the presence of NH, ninhydrin C=O, and cyclopent[b]indole C=O groups, respectively. The 1H NMR spectrum of 3 showed one proton singlet at δ = 11.29 ppm for cyclopent[b]indole NH proton. In particular, a triplet at 4.65 ppm with J = 9.2 Hz was due to pyrrolidine ring C4–H proton, which confirmed the product. The methylene protons of pyrrolidine ring appeared as a two doublets at 3.88 (J = 9.2 Hz) and 3.48 (J = 9.2 Hz) ppm. These results also confirmed the regiochemistry of the cycloadduct. Further, two doublets at 3.68 (J = 17.6 Hz), and 2.76 (J = 17.6 Hz) ppm were due to methylene protons of the cyclopent[b]indole ring and two three proton singlets at 2.30 and 2.28 ppm were due to two methyl group protons (Fig. 1). Its 13C NMR spectrum showed the presence of 30 carbons including the three carbonyl carbons at δ = 200.12, 197.15, and 188.99 ppm for two carbonyl groups of ninhydrin and one carbonyl group of cyclopent[b]indole ring system and two peaks at 81.82 and 71.91 ppm due to two spiro carbons. The elemental analysis was found to be C31H23F3N2O4 and its HR mass was further supported by the appearance of the peak at m/z = 551.1601 (M++Na) and also in good agreement with the proposed structure. Based on the above spectral evidence and elemental analysis data supported the structure as 4′-[4-(trifluoromethyl)phenyl]-1′,5-dimethyl-2,3-dihydrodispiro[cyclopent[b] indol-2,3′-pyrrolidine-2′,2″-indene]-1,1″,3″-trione (3). The structure of the compound 3 was confirmed via single crystal X-ray diffraction studies (Fig. 2). Further, IR, NMR spectra, and geometrical parameters of molecule 3 was calculated at the M062X/6-311++G(d,p) level of theory in the gas phase. Good agreement was found between experimental and calculated results in the Supplementary Material (Tables S3–S7 and Figures S1–S6).

The formation of regioisomer 3 could be explained by in view of the secondary orbital interaction of the orbital of the carbonyl group of dipolarophile 1a with those of the azomethine ylide (2a, generated from the decarboxylative condensation of ninhydrin with sarcosine) via endo selectivity. Therefore, the observed regioisomer 3 via path A is more favorable because of the secondary orbital interaction [28,29,30] which is not possible in path B for getting other isomer 3′ as shown in Scheme 2.

Similarly, the 1,3-dipolar cycloaddition of azomethine ylide generated in situ from the reaction of ninhydrin and thiaproline to 5-methyl-2-(4-fluorophenylmethylene)-1,2,3,8-tetrahydrocyclopent[b]indol-1-one (1b) in dioxane and methanol under reflux condition afforded dispirothiapyrrolizidine 4 in good yields in a regioselective manner (Scheme 3). The compound 4 was unambiguously characterized by means of FT-IR, 1H and 13C NMR spectral, and elemental analysis data. The structure of compound 4 was further confirmed via single crystal X-ray diffraction studies (Fig. 3).

Frontier molecular orbitals, molecular electrostatic potential maps and contours
Frontier molecular orbitals which are highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbitals (LUMO) play an important role in chemical interaction, reaction, etc. Selected parts of molecular orbital energy diagram of molecules 3 and 4 are represented in Fig. S7. In these diagrams, degeneracy tolerance is determined as 0.005 a.u. Contour map diagrams of investigated compounds 3 and 4 of HOMO and LUMO are represented in Figs. S8 and S9, respectively. There are red and green lobes in these contour plots. These regions are active in interaction with appropriate molecules. If molecule gives electrons, red and green lobes are active in interaction. However, there is no electron in LUMO. If molecule accepts electrons, these electrons will localize on red and green regions on structures.
Molecular electrostatic potential (MEP) maps are related to electron mobility and density on molecule surface. These maps can be used to predict the active sites on molecules. MEP map and contour of investigated molecules 3 and 4 are calculated at same level of theory and represented in Fig. S10 and S11. The colors between red and green in MEP maps are related to electrophilic reactivity. Additionally, the colors between green and blue in MEP maps are related to nucleophilic reactivity. According to MEP maps in Fig. S11, electrophilic active regions are in the vicinity of oxygen atoms while the environment of nitrogen atoms is appropriate for nucleophilic attack.
Non-linear optical (NLO) properties
Calculated quantum chemical descriptors, including the total static dipole moment (μ), the average linear polarizability (α), the anisotropy of the polarizability (Δα) and first hyperpolarizability (β) characterize NLO properties. These parameters are calculated via following Eqs. (1)–(4) and results are given in Table S8.
It can be observed from Table S8, mentioned parameters of studied compounds 3 and 4 are calculated at the same level of theory. NLO properties can be affected from polarizability, anisotropy of the polarizability and hyperpolarizability. NLO properties increase with increasing these parameters. Numerical values of related parameters for urea and compounds 3 and 4 are plotted the column graphs and these are represented in Figs. S12 and S13, respectively. Related parameters are important in determination of NLO activation, urea is used as reference substance [31,32,33,34]. Therefore, urea is optimized at the same level of theory. The activity ranking is given as follow: compound 4 > compound 3 > urea (in µ, α, Δα, and β). According to Figs. S12, S13 and Table S8, investigated compounds 3 and 4 are more suitable for NLO applications compared to standard reference urea.
In vitro cytotoxicity
The in vitro cytotoxicity of 4′-[4-(trifluoromethyl)phenyl]-1′,5-dimethyl-2,3-dihydrodispiro[cyclopent[b]indol-2,3′-pyrrolidine-2′,2″-indene]-1,1″,3″-trione and 4′-(4-fluorophenyl)-5-methyl-2,3-dihydrodispiro[cyclopent[b]indol-2,3′-pyrrolizidine-2′,2″-indene]-1,1″,3″-trione was evaluated by the MTT method [35] against a pair of selected human breast cancer cell line (MCF-7) and cervix adenocarcinoma cell line (HeLa) by means of a colorimetric assay (MTT assay). This assay method measures mitochondrial dehydrogenase activity as an indication of cell viability after an exposure period of 24 h in the respective concentration range of 5–100 µM. Upon increasing the concentration of the compound, the results of MTT assay revealed a better growth inhibitory effect in a dose-dependent manner against HeLa and MCF-7 cells with IC50 values generally in the micromolar concentrations. Hence, compound 3 exhibits cytotoxicity in human breast cancer cells (MCF-7) 116.07 µM compared to cervix adenocarcinoma cells (HeLa) 94.67 µM. But compound 4 exhibits higher cytotoxicity in human breast cancer cells (MCF-7) 27.35 µM compared to cervix adenocarcinoma cells (HeLa) 68.49 µM. These results disclose that the newly synthesized compounds 3 and 4 have significantly lower IC50 values when compared to the conventional chemotherapeutic drug 5-fluorouracil. Further comparing with the IC50 values of other drugs such as cisplatin and methotrexate under similar conditions show that the IC50 values for the synthesized compounds are significantly lower than their conventional counter parts, suggesting enhanced cytotoxic efficacy of these compounds (Table 1). Among the newly synthesized dispiroheterocyclic compounds, compound 4 showed stronger cytotoxic activity against HeLa and MCF-7 cell lines compared to compound 3 and standard drugs. This might be due to the presence of pyrrolizidine ring and the ninhydrin, oxindole groups, which enhanced the cytotoxic activity.
Conclusion
The 1,3-dipolar cycloaddition reaction of azomethine ylide generated in situ from ninhydrin and sarcosine/thiaproline with fluorinated cyclopent[b]indole dipolarophiles in refluxing dioxane and methanol afforded a novel class of fluorinated cyclopent[b]indole dispiroheterocycles. The crystal structures of 4′-[4-(trifluoromethyl)phenyl]-1′,5-dimethyl-2,3-dihydrodispiro[cyclopent[b]indol-2,3′-pyrrolidine-2′,2″-indene]-1,1″,3″-trione and 4′-(4-fluorophenyl)-5-methyl-2,3-dihydrodispiro[cyclopent[b]indol-2,3′-pyrrolizidine-2′,2″-indene]-1,1″,3″-trione were obtained. The NMR and IR frequencies were calculated and the scaled values have been compared with experimental FT-NMR and FT-IR spectra. The geometrical parameters compared well with experimental XRD values and the corresponding correlation coefficient values. The molecular electrostatic potential (MEP) analysis and electronic properties, such as HOMO and LUMO energies were obtained using the M062X method with 6-311+G(d,p) basis sets in vacuum, showing an excellent agreement between the experimental and calculated values. Calculated high NLO properties of compounds 3 and 4 indicate suitability for NLO applications. Compounds 3 and 4 have significantly lower IC50 values than the conventional chemotherapeutic drug 5-fluorouracil. Further comparison with the IC50 values of other drugs such as cisplatin and methotrexate under similar conditions shows again that the IC50 values for the synthesized compounds are significantly lower than their conventional counter parts, suggesting enhanced cytotoxic efficacy of these compounds.
Experimental
Melting points were determined on a Mettler FP 51 apparatus (Mettler Instruments, Switzerland). A Nicolet Avatar Model FT-IR spectrophotometer was used to record the IR spectra (4000–400 cm−1). 1H NMR and 13C NMR spectra were recorded on Bruker AV 400 [400 MHz (1H) and 100 MHz (13C)] spectrometers using tetramethylsilane (TMS) as an internal reference. The chemical shifts are expressed in parts per million (ppm). Coupling constants (J) are reported in hertz (Hz). The terms J o and J m refer to ortho-coupling constant and meta-coupling constant. The terms s, d, t, dd refer to singlet, doublet, triplet and doublet of doublet, respectively, b s refers to a broad singlet. Microanalyses were performed on a Vario EL III model CHNS analyzer (Vario, Germany) at the Department of Chemistry, Bharathiar University. X-ray diffraction measurements were performed on a Bruker-Nonius FR590 Kappa CCD diffractometer at 130 K and a Bruker APEX II at 100 K, using monochromatic Mo Kα radiation. CIF files for compounds 3 and 4 have been deposited with the Cambridge Crystallographic Data Centre as CCDC number 1472338 and 1472337. Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge, CB2 1EZ, UK. [Fax: +44 (0) 1223 336033 or e-mail: deposit@ccdc.cam.ac.uk. The purity of the products was tested by TLC with plates coated with silica gel-G using petroleum ether and ethyl acetate in the ratio of 1:1 as developing solvents.
4′-[4-(Trifluoromethyl)phenyl]-1′,5-dimethyl-2,3-dihydrodispiro[cyclopent[b]indol-2,3′-pyrrolidine-2′,2″-indene]-1,1″,3″-trione (3, C31H23F3N2O4)
A mixture of 5-methyl-2-[4-(trifluoromethyl)phenylmethylene]-1,2,3,8-tetrahydrocyclopent[b]indol-1-one 1a (1.0 mmol), sarcosine (1.0 mmol), and ninhydrin 2 (1.0 mmol) was refluxed in 30 cm3 dioxane:methanol (1:1) for 5 h. Completion of the reaction was evidenced by TLC analysis. The solvent was removed in vacuo and crude product was subjected to silica gel column chromatography using petroleum ether:ethylacetate (90:10) as an eluant to yield the corresponding product 3. Yellow solid; yield: 369 mg (70%); m.p.: 226–228 °C; 1646; 1H NMR (400 MHz, DMSO-d 6): δ = 2.28 (s, 3H, N1′–CH3), 2.30 (s, 3H, C5–CH3), 2.76 (d, 1H, C3a–H, J = 18.00 Hz), 3.48 (d, 1H, C5a’–H, J = 9.20 Hz), 3.68 (d, 1H, C3b–H, J = 18.00 Hz), 3.88 (d, 1H, C5b′–H, J = 9.20 Hz), 4.65 (d, 1H, C4′–H, J = 9.20 Hz), 6.97–7.11 (m, 4H, C2‴–, C3‴–, C5‴–, C6‴–H), 7.16–7.20 (m, 2H, C6–, C7–H), 7.33 (s, 1H, C4–H), 7.58 (d, 1H, C5″–H, J = 7.60 Hz), 7.77–7.81 (m, 1H, C6″–H), 7.96-7.97 (m, 2H, C4″–, C7″–H), 11.29 (s, 1H, C8–NH) ppm; 13C NMR (100 MHz, DMSO-d 6): δ = 20.77, 24.18, 35.54, 47.27, 55.03, 71.91, 81.82, 113.32, 115.02, 115.23, 120.80, 122.23, 122.50, 122.60, 129.24, 129.59, 130.01, 132.53, 132.56, 136.08, 137.25, 137.87, 140.36, 140.40, 142.72, 143.77, 159.81, 162.23, 188.99, 197.45, 200.12 ppm; FT-IR (KBr): \(\bar{\nu }\) = 3279, 1709 cm−1; HRMS: 529.1797 (M+), 551.1601 (M++Na).
4′-(4-Fluorophenyl)-5-methyl-2,3-dihydrodispiro[cyclopent[b]indol-2,3′-pyrrolizidine-2′,2″-indene]-1,1″,3″-trione (4, C31H23FN2O3S)
A mixture of 5-methyl-2-(4-fluorophenylmethylene)-1,2,3,8-tetrahydrocyclopent[b]indol-1-one 1b (1.0 mmol), 1,3-thiazolan-4-carboxylic acid (1.0 mmol), and ninhydrin 2 (1.0 mmol) was refluxed in 30 cm3 dioxane: methanol (1:1) for 5 h. Completion of the reaction was evidenced by TLC analysis. The solvent was removed in vacuo and crude product was subjected to silica gel column chromatography using petroleum ether: ethylacetate (90:10) as an eluant to yield the corresponding product 4. Yellow solid; yield: 365 mg (70%); m.p.: 212–214 °C; 1H NMR (400 MHz, DMSO-d 6): δ = 2.31 (s, 3H, C5–CH3), 2.91 (d, 1H, C3a–H, J = 18.00 Hz), 3.09–3.15 (m, 2H, C8′–H2), 3.64 (d, 1H, C6a′–H, J = 8.00 Hz), 3.76 (d, 1H, C3b–H, J = 18.00 Hz), 3.87 (d, 1H, C6b′–H, J = 8.00 Hz), 4.25 (d, 1H, C4′–H, J = 8.00 Hz), 4.67–4.72 (m, 1H, C5′–H), 6.99–7.13 (m, 4H, C2‴–, C3‴–, C5‴–, C6‴–H), 7.25–7.29 (m, 2H, C6–, C7–H), 7.35 (s, 1H, C4–H), 7.56 (d, 1H, C5″–H, J = 7.60 Hz), 7.77–7.81 (m, 1H, C6″–H), 7.96–7.99 (m, 2H, C4″–, C7″–H), 11.29 (s, 1H, C8–NH) ppm; 13C NMR (100 MHz, DMSO-d 6): δ = 20.76, 24.21, 35.32, 49.83, 51.46, 70.34, 76.54, 81.11, 113.31, 115.15, 115.35, 120.81, 122.10, 122.59, 129.31, 129.74, 130.16, 130.24, 131.15, 136.26, 137.10, 137.50, 139.72, 140.66, 142.82, 143.40, 159.97, 187.37, 196.71, 199.60 ppm; FT-IR (KBr): \(\bar{\nu }\) = 3264, 1710, 1634 cm−1.
References
Steyn PS, Vleggar R (1985) Fortschr Chem Org Naturst 48:1
Cheng KF, Cao GA, Yu YW (1994) Synth Commun 24:65
Harrison CA, Leineweber R, Moody CJ, Williams JMJ (1993) Tetrahedron Lett 34:8527
Smith AB, Leenay TL (1985) J Am Chem Soc 107:1769
Bergman J, Venemalm L (1987) Tetrahedron Lett 28:3741
Bergman J, Venemaln L (1992) Tetrahedron 48:759
Padwa A (1984) 1,3-Dipolar cycloaddition chemistry, vol 1 and 2. Wiley, New York
James DM, Kunze HB, Faulkner DJJ (1991) Nat Prod 54:1137
Trost BM, Brennan MK (2009) Synthesis:3003
Galliford CV, Scheidt KA (2007) Angew Chem Int Ed 46:8748
Dandia A, Khanna S, Joshi KC (1991) Ind J Chem 30B:469
Arya K, Rawat DS, Dhandia A, Sasai H (2012) J Fluor Chem 137:117
Filler R, Kobayashi Y (1982) Biomedical aspects of fluorine chemistry. Kodansha and Elsevier Biomedical, Tokyo
Filler R, Banks RE (1979) Organofluorine chemicals and their industrial applications. Horwood, London
Filler R, Kobayashi Y, Yagupolskii LM (1993) Organofluorine compounds in medicinal chemistry and biomedical applications. Elsevier, Amsterdam
Satheeshkumar R, Lincy E, Sameerkumar VB, Rajendra Prasad KJ (2017) ChemistrySelect 2:2626
Senthil Kumar G, Satheeshkumar R, Kaminsky W, Platts J, Rajendra Prasad KJ (2014) Tetrahedron Lett 55:5475
Yamuna E (2011) Synthetic studies on hetero-fused analogues of cyclohepta[b]indoles and carbazoles. Ph.D. thesis, Bharathiar University, Coimbatore, Tamil Nadu, India
Sangeetha V, Rajendra Prasad KJ (2002) Heterocycl Commun 8:65
Sangeetha V, Rajendra Prasad KJ (2003) Ind J Chem 42B:2109
Sangeetha V, Rajendra Prasad KJ (2004) Ind J Chem 43B:2231
Satheeshkumar R, Shankar R, Kaminsky W, Kalaiselvi S, Vijaya Padma V, Rajendra Prasad KJ (2016) J Mol Struct 1109:247
Satheeshkumar R, Sayin K, Kaminsky W, Rajendra Prasad KJ (2017) J Mol Struct 1128:279
Satheeshkumar R, Shankar R, Kaminsky W, Rajendra Prasad KJ (2016) ChemistrySelect 1:6823
Sayin K, Karakaş D (2014) J Mol Struct 1076:244
Sayin K, Karakaş D (2015) Spectrochim Acta A 144:176
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, revision D.01. Gaussian Inc, Wallingford, CT
Dandia A, Jain AK, Bhati DS (2011) Tetrahedron Lett 52:5333
Liu H, Dou G, Shi DJ (2010) Comb Chem 12:633
Lakshmi NV, Thirumurugan P, Perumal PT (2010) Tetrahedron Lett 51:1064
Sümeyye A, Davut A, Ömer T, Yusuf A (2017) Comput Theor Chem 1100:34
Prashant M, Elizabeth M, Subramaniyan C, Isaac HJ, Nagaiyan S (2017) Opt Mater 72:549
Amalanathan M, Femina JG, Dawn Dharma RS (2017) J Mol Struct 1141:400
Satheeshkumar R, Sayin K, Kaminsky W, Rajendra Prasad KJ (2017) Synth Commun. doi:10.1080/00397911.2017.1357185
Mosmann TJ (1983) Immunol Methods 65:55
Acknowledgements
Rajendran Satheeshkumar is grateful to the University Grant Commission (UGC-SAP), New Delhi, for BSR—Senior Research Fellowship, which is thankfully acknowledged. Dr. Karnam Jayarampillai Rajendra Prasad greatly acknowledged UGC-Emeritus fellowship for research. Dr. Werner Kaminsky thanks the Department of Chemistry, University of Washington, Seattle, USA, for access to the X-ray diffraction facility. Dr. Koray Sayin thanks to the High Performance and Grid Computing Center (TRUBA Resources), numerical calculations are performed at TUBITAK ULAKBIM.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Satheeshkumar, R., Sayin, K., Kaminsky, W. et al. Synthesis, spectroscopic, in vitro cytotoxicity and crystal structures of novel fluorinated dispiroheterocycles: DFT approach. Monatsh Chem 149, 141–147 (2018). https://doi.org/10.1007/s00706-017-2050-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-017-2050-5