Abstract
In this study graphene oxide was functionalized by divinylsulfone and allyl acetoacetate conducting a simple route. This novel functionalized graphene oxide [graphene oxide grafted with allyl acetoacetate (GO-GAA)] was characterized by X-ray diffractometry, field emission scanning electron microscopy, and transmission electron microscopy analyses. Thereafter the adsorption property of GO-GAA for Cd2+ ion removal from aqueous media was investigated. The effect of several factors such as pH and temperature on the Cd2+ adsorption was also studied. It was found that the adsorption isotherm follows the Langmuir–Freundlich model and the maximum adsorption capacity was 71 mg/g for GO-GAA toward Cd2+ at 20 °C at pH 7. The fast adsorption kinetics which takes less than 2 min was following the pseudo-first-order model. The synthesized nanoadsorbent also showed a good reusability, even after ten cycles of adsorption–desorption using HNO3 as regenerating agent. Furthermore applicability of synthesized nanoadsorbent for Cd2+ removal from industrial wastewater and well water samples inoculated with cadmium was investigated and over 82 % efficiency was observed.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium is a toxic metal and exposure of human to cadmium affects his vital organs (kidney, liver, and lung) and systems (cardiovascular, immune, and reproductive); since it can efficiently retained in the human body [1]. The toxicological effects of cadmium are indicated by symptoms like kidney damage and destruction of red blood cells [2]. Therefore, removal of cadmium from water resources and industrial wastewaters is an issue of significant importance.
There are various techniques for remediation of metal-contaminated waters and wastewaters such as chemical reduction, precipitation, ion exchange, coagulation, electrochemical treatment and adsorption. Two main factors determine the treatment method: the concentration of pollutant and the cost of treatment [2–4]; and hence due to low operational cost and ease of operation, adsorption by low cost adsorbents is the method of choice for heavy metals removal from contaminated waters with relatively low concentrations of pollutants [5].
In the last decade carbon-based nanomaterials have attracted much attention as effective adsorbents [6]; and particularly graphene as a molecular sheet of graphite has been significantly considered because of its remarkable physical, chemical and biological characteristics [7]. High specific surface area and strong adsorption capacity next to safety, stability, low solubility in common solvents, and recyclability make graphene-based compounds applicable for environmental protection and detection [8], as well as for chemical reactions catalysis [8, 9]. However, application of functionalized graphene oxide as nanoadsorbent in heavy metals removal remains essentially unexplored.
Recently significant progress in graphene chemistry especially in modification of graphene surface has been achieved. Different strategies may be followed for incorporation of different atoms or organic groups into graphene; but the most frequently used strategy involves initial Hummers’ oxidation followed by organic reactions leading to specific functional outcomes [10, 11].
In this research poly allyl acetoacetate-grafted graphene oxide which is a graphene derivative with well-dispersion advantage over graphene was synthesized and used as adsorbent for cadmium removal and its adsorption performance was studied thoroughly. Although a few studies have been reported for cadmium removal using graphene-based materials [12–16], to the best of our knowledge, there is no report on the synthesis of allyl acetoacetate-grafted graphene oxide and its application as a high capacity nanoadsorbent for cadmium ion removal.
Results and discussion
Synthesis and characterization
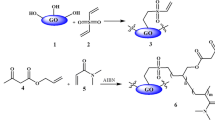
Graphene oxide (GO) was first synthesized from natural graphite via modified Hummers’ method. Chemical modification of GO was then carried out through a multi-step reaction of GO with divinylsulfone, then allyl acetoacetate and N,N-dimethylacrylamide (DMAA) in the presence of azobisisobutyronitrile (AIBN) as radical initiator to obtain graphene oxide grafted with allyl acetoacetate (GO-GAA).
XRD patterns for GO and GO-GAA are shown in Fig. 1. The primary peak of GO-GAA at 2θ ~ 13° confirmed that the distance between the GO layers was increased by grafting. The relatively small broad peak around 2θ ~ 22° attributes to the amorphous nature of polymer and indicates the better exfoliation of GO-GAA rather than GO [17].
The FT-IR spectra for GO (pure) and GO-GAA (functionalized) are depicted in Fig. 2. In the former the broad adsorption band located at about 3393 cm−1 is characteristic of O–H stretching vibration and the adsorption peak around 1580 cm−1 is attributed to physically adsorbed water and the adsorption peaks of COOH, COC, and COH functional groups can be observed at 1686, 1056, and 1236 cm−1, respectively [18].
On the other hand, the characteristic absorbance at about 2930 cm−1 indicates the presence of aliphatic hydrocarbons of GO-GAA. The broad bands at 2500–3500 cm−1 in both GO and GO-GAA spectra can be assigned to the OH stretching vibrations (for C–OH and OC–OH). Moreover, the sharp bands around 1738–1638 cm−1 for C=O stretching vibrations can be recognized obviously.
In Fig. 3 the FE-SEM and TEM images of GO-GAA are shown. The exfoliated morphology of GO-GAA with crumpled layers in FE-SEM image is in agreement with the sheet structure observed in TEM image of GO-GAA.
Adsorption kinetics
To realize the mechanism of Cd(II) adsorption on GO-GAA from aqueous solution, adsorption kinetics studies were performed and both pseudo-first order and pseudo-second order kinetic models were investigated [19]. The two models equations are, respectively, as follows:
where q e and q t are adsorbed Cd(II) in equilibrium and time t (min), respectively, and k (1/min) and k′ (g/mg min) are rate constants.
The results indicated that the adsorption kinetics is more compatible with pseudo-first order model rather than pseudo-second order by q e = 20.001 and k = 4.278 with R 2 = 0.999. The adsorption was performed by 0.05 g adsorbent in 10 mg/dm3 Cd2+ solution at 298 K and pH = 7.
Moreover, it should be noted that GO-GAA showed rapid adsorption toward Cd(II) so only 2 min is enough to reach the maximum removal of Cd(II) and this confirms a desirable accessibility of the chelating sites.
Adsorption isotherm
The adsorption experiments were performed in different concentrations of Cd2+ ion. According to better fit and easier comparison with other ions, the direct relationship between the retained and initial concentrations of cadmium ions was produced [20, 21]; and the experimental data were fitted by different adsorption isotherm models of Langmuir, Freundlich, Sips (Langmuir–Freundlich), and Dubinin–Radushkevich using initial cadmium concentrations. The determined parameters of each one are mentioned in Table 1.
The Langmuir equation is as follows:
In Langmuir model, q m (mg/g) is the maximum adsorption capacity attributed to the complete coverage of the surface layers and b is the Langmuir constant (1/mg). The high value of calculated q max (94.14 mg/g) can be related to the functional groups grafted into the GO motif. This isotherm is compatible with the homogeneous nature of the GO-GAA surface [22]. Much higher value of q max for GO-GAA than the pristine graphene oxide is to be expected because of bearing effective functionalities especially dicarbonyl groups which are effective chelating agents.
Freundlich equation which is an empirical equation is proposed for sorption on heterogeneous surface through a multilayer adsorption mechanism. It is characterized by the heterogeneity factor 1/n with empirical equation given as:
where K f is the Freundlich constant (mg/g) and K f and n [mg(1−1/n) g−1 dm(3/n)] are described as adsorption capacity and adsorption intensity, respectively. The value of n was calculated to be 4.3 and this is a desirable value since as a favorable adsorption, the Freundlich coefficient should take a place in the range of 1–10 [23].
Sips isotherm is a hybrid of the Langmuir and Freundlich isotherms and includes three parameters and is written as:
The Sips equation describes the saturation phenomenon of Langmuir (q = q m) at higher concentrations (bC ≫1) but at lower concentrations (bC ≪1) it reduces to the Freundlich isotherm.
The Dubinin–Radushkevich model is dependent on adsorption temperature and considers the adsorbent diameter to be comparable to the micropores. The D–R isotherm adsorption model is expressed as follows:
while epsilon is defined as
and energy of adsorption is calculated by
As given in Table 1, the experimental data match the Langmuir-like behavior satisfactorily but by using Langmuir-based three parameter adsorption isotherms like Sips isotherm, more compatibility could be obtained. Using the Dubinin–Radushkevich model which is dependent to adsorption temperature predicts adsorption energy of about 1.12 kJ/mg and this value (between 8 and 16 kJ/mol for chemisorption and below 8 for physiosorption) [24]; indicates the physiosorption mechanism of cadmium adsorption onto the modified graphene oxide. These results are in consistency with other works [25].
Optimization of adsorption conditions
The effect of pH on the adsorption amount using as-prepared adsorbent is shown in Fig. 4. As seen, the maximum of 99.8 % removal was obtained at pH = 7. It seems that in acidic pH, the H+ ions act as competitors for ionic cadmium to be attracted by chelating sites [18]; and by increasing of pH over neutrality the formation of Cd(OH)2 as a precipitate leads to decrease in adsorption by chelating sites.
The temperature dependency of metallic ions adsorption is well known; and by adsorption tests in different temperatures it was indicated in Fig. 5. This figure showed that the range of 20–25 °C was more suitable for adsorption of Cd (98 % removal). The adsorption increase in this temperature range is likely to be due to the increase of molecular mobility and diffusion of Cd2+ ions and more accessibility to the chelating sites [26]. It is noteworthy that at higher temperatures the adsorption efficiency decreases because of the excessive molecular mobility.
Effect of other metal ions on Cd(II) adsorption
According to Table 2, the cadmium adsorption studies in binary solutions of different metal ions as competitors revealed that compared to 88 % removal of cadmium from cadmium solution; different metal ions only decrease the cadmium removal slightly (only below 13 %); and they also can be adsorbed nearly completely (over 99 % for Pb, Mn, and Mg). The little effect of other ions on cadmium removal in addition to their removal indicate that the as prepared adsorbent not only can adsorb cadmium even in presence of other metal ions; but also has this capability toward other metal ions due to its non-selective character. Although according to high adsorption of metal ions in binary solutions, using this adsorbent for fast and simultaneous removal of metal ions (such as cadmium and lead) is a promising idea; but we focused on cadmium removal as a case study to investigate the adsorbent behavior.
Reusability of synthesized adsorbent
As depicted in Fig. 6, by regeneration of the adsorbent using HNO3 (1 M) and reuse it, less than 3 % decrease in its efficiency was seen after the second run. Although after 4 runs about 30 % decrease in removal efficiency was observed; but it remains constant even up to ten times of regeneration and reuse. Therefore, it can be concluded that synthesized adsorbent can be successfully recycled and reused for several times.
Performance of synthesized adsorbent
Table 3 shows the comparison of our sorbent with previously reported sorbents in terms of the langmuir maximum adsorption capacities for Cd(II) removal. As evidenced the synthesized sorbent capacity for cadmium removal is comparable and in some cases higher than other graphene based sorbents in the literature.
Application of synthesized GO-GAA sorbent for wastewater treatment
Real samples including industrial wastewater of North Pars Gas Field (Parse-Jonobi) and a well water sample from Tehran city were inoculated with cadmium for study of industrial applicability of synthesized GO-GAA nanoadsorbent. As shown in Table 4 the results prove the applicability of the present synthesized sorbent for Cd(II) decontamination from real industrial and natural samples.
Conclusion
The graphene oxide was synthesized and then functionalized by allyl acetoacetate for increasing Cd2+ sorption capacity. Based on the Langmuir isotherm analysis the maximum of the adsorption capacity was determined to be 94.1 mg/g for Cd(II) ions at 25 °C confirming favorable adsorption compared to other reported adsorbents. Additionally the newly synthesized nanoadsorbent has some interesting advantages over existing adsorbents such as high adsorption capacity, good reusability, high stability, and rapid adsorption regarded to other carbon-based adsorbents.
Experimental
All of chemicals were purchased from Merck (Darmstadt, Germany). GO was prepared using modified Hummers’ method from flake graphite (Merck, Darmstadt, Germany) [27]. Transmission electron microscopy (TEM) was carried out using a Zeiss-EM10c-100 kV instrument. FE-SEM images of the nanostructures were taken by a Zeiss-Sigma instrument operating at an accelerating voltage of 300 kV. X-ray diffractograms were obtained by D8, Advance Bruker, AXS. Atomic absorption spectrophotometry mercury/hydride system (AAS-MHS) was used for cadmium determination.
Synthesis of GO
Briefly for 1 g graphite, the natural graphite flakes and 50 cm3 H2SO4 were mixed in a round-bottom flask and stirred for half an hour at 5 °C. Thereafter 6 g of KMnO4 was added slowly during 1 h and the mixture was stirred for 24 h at 5 °C [28]. Afterwards 150 cm3 deionized water was poured into the mixture and stirred for another 15 min at room temperature. In the next step, 50 cm3 H2O2 (37 %) was added and after 15 min, the mixture was placed in a centrifuge cell for 20 min at 8000 rpm. The resulted precipitate was washed for five times using HCl (5 % wt) under centrifugation (20 min, 6000 rpm). After neutralization by deionized water, to obtain a fully oxidized graphene, the mixture of 1 dm3 deionized water and 1 g dried powder was sonicated for 1 h and centrifuged at 8000 rpm. Subsequently the mixture was filtered and dried under reduced pressure at 70 °C.
Synthesis of GO grafted with ally acetoacetate (GO-GAA)
GO (3 g) was mixed with 15 cm3 divinylsulfon in 15 cm3 EtOH and stirred for 3 h at room temperature. The mixture was filtered and the filtrate was washed with 15 cm3 EtOH. The filtrate was dispersed in 35 cm3 EtOH and placed in a round bottom flask under N2 atmosphere. Then 10 cm3 allyl acetoacetate and 2 cm3 N,N-dimethylacrylamide were added to the mixture and stirred for 20 min at 65 °C and after that, 0.1 g azobisisobutyronitrile was added as an initiator. The mixture was allowed to stir for 8 h at 65 °C. Then, the mixture was filtered and washed with 30 cm3 EtOH and dried at room temperature.
Adsorption studies
The as-prepared adsorbent (0.05 g) was separately added to solutions of Cd2+ with different concentrations (10, 20, 50, 80, 100 ppm), and allowed to stir for 2 h at room temperature. The pH was controlled using a 0.1 M CH3COONa buffer. At the end of the contact time the final concentration of Cd(II) was determined by flame atomic absorption spectrometry. The adsorption capacity Q which indicates the amount of adsorbed Cd(II) per unit weight of adsorbent as mg/g was calculated as:
where C 0 and C e represent initial and equilibrium concentrations of Cd (mg/dm3), respectively, W represents the adsorbent weight (g), and V represents the total volume of Cd(II) solution (dm3).
To investigate the effect of other metal ions on the cadmium adsorption efficiency of prepared adsorbent, a series of adsorption studies were performed in optimized conditions (pH = 7.0, T = 25 °C) for different metal ions with initial concentration of 10 ppm for both the cadmium and the other metal ion.
References
Salmani MH, Davoodi M, Ehrampoush MH, Ghaneian MT, Fallahzadah MH (2013) Iran J Environ Health Sci Eng 10:16
Yavuz O, Guzel R, Aydin F, Tegin I, Ziyadanogullari R (2007) Pol J Environ Stud 16:467
Parham H, Zargar B, Shiralipour R (2012) J Hazard Mater 205–206:94
Wang J, Deng B, Chen H, Wang X, Zheng J (2009) Environ Sci Technol 43:5223
Rao K, Mohapatra M, Anand S, Venkateswarlu P (2010) Int J Eng Sci Technol 2:81
Singh SK, Singh MK, Kulkarni PP, Sonkar VK, Grácio JJ, Dash D (2012) ACS Nano 6:2731
Stankovich S, Piner RD, Chen X, Wu N, Nguyen ST, Ruoff RS (2006) J Mater Chem 16:155
Lü M, Li J, Yang X, Zhang C, Yang J, Hu H, Wang X (2013) Chin Sci Bull 58:2698
Khodabakhshi S, Marahel F, Rashidi A, Abbasabadi MK (2015) J Chin Chem Soc 62:389
Dreyer DR, Park S, Bielawski CW, Ruoff RS (2010) Chem Soc Rev 39:228
Liu Z, Zhou H, Huang Z, Wang W, Zeng F, Kuang Y (2013) J Mater Chem A 1:3454
Zhao G, Li J, Ren X, Chen C, Wang X (2011) Environ Sci Technol 45:10454
Deng X, Lü L, Li H, Luo F (2010) J Hazard Mater 183:923
Deng J-H, Zhang X-R, Zeng G-M, Gong J-L, Niu Q-Y, Liang J (2013) Chem Eng J 226:189
Wang Y, Liang S, Chen B, Guo F, Yu S, Tang Y (2013) PLoS One 8:e65634
Zhang W, Shi X, Zhang Y, Gu W, Li B, Xian Y (2013) J Mater Chem A 1:1745
García-Valdez O, Ledezma-Rodríguez R, Saldívar-Guerra E, Yate L, Moya S, Ziolo RF (2014) Polymer 55:2347
Yan L, Zhao Q, Jiang T, Liu X, Li Y, Fang W, Yin H (2015) RSC Adv 5:67372
Ho Y-S, McKay G (1999) Process Biochem 34:451
Coles CA, Yong RN (2006) Eng Geol 85:19
Yong RN (2000) Geoenvironmental engineering: contaminated soils, pollutant fate, and mitigation. CRC Press, Boca Raton
Langmuir I (1918) J Am Chem Soc 40:1361
Frendlich H (1906) J Phys Chem 57:385
Dawodu F, Akpomie G, Ogbu I (2012) Int J Multidisc Sci Eng 3:9
Fialova D, Kremplova M, Hynek D, Kopel P, Adam V, Kizek R (2013) Carbon nanoparticles modified by graphene for binding heavy metal ions. In: Zboril R (ed) Nanocon. TANGER Ltd, Czech Republic, p 443
Hu X-J, Liu Y-G, Zeng G-M, Wang H, Hu X, Chen A-W, Wang Y-Q, Guo Y-M, Li T-T, Zhou L, Liu S-H, Zeng X-X (2014) J Colloid Interface Sci 426:213
Khodabakhshi S, Karami B, Eskandari K, Hoseini SJ, Rashidi A (2014) RSC Adv 4:17891
Abbasabadi MK, Rashidi A, Khodabakhshi S (2016) J Nat Gas Sci Eng 28:87
Acknowledgments
We are grateful to Dr. A. Rashidi for his valuable advices.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aghdam, K., Alaei, E., Panahi, H.A. et al. Modification of graphene oxide by introduction of allyl acetoacetate functionality and its application as a novel nanoadsorbent in cadmium removal from water. Monatsh Chem 147, 1863–1869 (2016). https://doi.org/10.1007/s00706-016-1745-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-016-1745-3