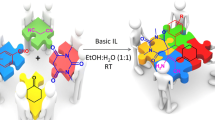
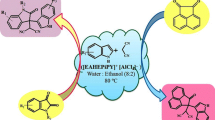
Abstract
An efficient, novel, task-specific ionic liquid, [2-hydroxymethylpyridinium butanesulfonic acid]HSO4 has been designed for the rapid synthesis of spiro-1,2,4-triazolidine-3-thiones from isatins and thiosemicarbazides in water as an universal solvent at room temperature. The hydroxymethyl functionality tethered to pyridinium nucleus facilitates the reaction in water. This is the first method proceeded at room temperature for synthesis of spiro-1,2,4-triazolidine-3-thiones. The novelty of the method is synthesis of novel task-specific ionic liquid, energy efficiency, atom economy, wide substrate scope, shorter reaction time, high yield of products, and use of water along with ionic liquid are greener aspects of present method.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Growing awareness about environmental hazards associated with use of volatile organic solvents and conventional corrosive catalysts for organic transformations has diverted the scientific community towards development of eco-benign methods for organic synthesis comprising maximum principles of green chemistry. In this perspective universal solvent, water has attracted attention due to its supreme advantages over organic solvents viz environmentally benign, abundant, inexpensive, and non-flammability [1]. By utilizing its unique physical and chemical properties, it would be possible to realize reactivity or selectivity that cannot be attained in organic solvents [2]. These are the reasons, why nature adopted it as medium in which life processes occur and life evolves [3]. To exploit the remarkable advantages of water, numerous reactions have been carried out in water [4]. The hydrogen bonding and hydrophobic effect of water is believed to be responsible for drastic acceleration of reaction rate in water even when the reactants are sparingly soluble or insoluble in it [5].
Another important aspect of green chemistry is use of eco-benign catalysts in organic synthesis. Ionic liquids (ILs) have marched far beyond the border as a solvent, playing a significant role in controlling the reaction as catalysts due to their high polarity and the ability to solubilise both inorganic and organic compounds, which can result in the enhancement of the rate of the reaction [6]. Thus an intriguing line in the development of eco-friendly methodologies is being fueled by basic paradigm shift from use of traditional catalysts to ionic liquids [7]. In addition, the properties of ionic liquids can be altered by varying the nature of anions and cations and thus they can be customized as task-specific reagents for a particular reaction. Nowadays, the applications of Brønsted acidic ionic liquids as catalytic materials have become popular in the field of catalysis. Thus, task-specific ionic liquids (TSILs) synthesized with the standpoint of combining strong acidic property of traditional mineral acids and structural or physicochemical features of a specific functionality assisting the reaction are being extensively utilized for promoting organic transformations [8–10]. In this stance, the hydroxyl functionalized acidic ionic liquids are found superior due to the capacity of hydroxyl group to form hydrogen bonds with substrate and thereby assisting the organic reaction in water [11]. Thus we envisioned that use of universal solvent, water and IL as catalyst is perfect combination for the synthesis of bioactive heterocycles.
Amongst various classes of heterocycles, synthesis of triazoles have gained much attention owing to their wide range of applications in pharmaceuticals, agrochemicals, dyes, photographic materials, corrosion inhibition, etc. [12]. Triazole derivatives have been found to exhibit biological activities such as antiviral, antiepileptic, antiallergic [13], anticancer [14, 15], anti-HIV [16], as well as antibacterial activities against Gram-negative bacteria and β-adrenergic receptor agonist [17, 18]. 1,2,4-Triazole nucleus have been incorporated into a wide range of therapeutically important drugs including anti-inflammatory, CNS stimulants, sedatives, antianxiety, antimicrobial, antimigraine (rizatriptan, Fig. 1a) and antimycotic agents (fluconazole, Fig. 1b). An anticonvulsant drug, estazolam (Fig. 1c) and antiviral drug, ribavirin (1-(D-ribofuranosyl)-1H-1,2,4-triazole-3-carboxamide) also possess 1,2,4-triazole nucleus (Fig. 1d) [19].
These versatile applications of triazole have been motivating the modern scientific community to explore efficient and green methodologies for their synthesis. In spite of being medicinally important moiety, scanty reports are available for the synthesis of spiro-1,2,4-triazolidinones. None of them are energy efficient as these methods required high temperature or reflux condition [20, 21]. Hence an efficient method for synthesis of spiro-1,2,4-triazolidinones operable at ambient temperature is highly warranted.
Results and discussion
In continuation with our work on 1,2,4-triazolidine-3-thiones [20, 22, 23], herein we reported ionic liquid catalysed synthesis of spiro-1,2,4-triazolidine-3-thiones from isatins and thiosemicarbazides in water as a solvent at room temperature (Scheme 1).

Initially, we focused our attention towards synthesis of task-specific ionic liquid which could facilitate the reaction in water. It is evident that reaction in the water can be accelerated with the help of mediator such as hydroxyl functionality which enhances water solubility of reactants facilitating smooth reaction [24]. So, in order to synthesize the catalyst assisting reaction in water, the hydroxyl functionality should have to be incorporated in the catalyst and hence we chosen pyridine-2-methanol as a precursor for synthesis of TSIL. The main advantage of this precursor is, its inherent hydroxyl functionality which avoids the step of tethering hydroxyl functionality to core cation and thus minimized the steps in catalyst synthesis implementing the principles of green chemistry. Literature survey reveals that reaction of aldehydes or ketones with thiosemicarbazide facilitated by acidic catalysts [22, 23]. In light of this, we have carried out reaction of 2-pyridinemethanol with 1,4-butanesultone in toluene to form zwitterions which then treated with equivalent quantity of conc. sulfuric acid to form the desired hydroxyl functionalized Brønsted acidic task-specific ionic liquid, [2-HMPyBSA]HSO4 (Scheme 2). The structure of the synthesized ionic liquid was confirmed by IR, 1H NMR, 13C NMR, and mass spectroscopy. The spectroscopic data obtained are in full agreement with the structure of IL.

After this preliminary success the attention was engrossed towards optimization of reaction conditions for synthesis of 1,2,4-triazolidine-3-thiones. In the pilot experiment the reaction of isatin (1 mmol) and thiosemicarbazide (1 mmol) at room temperature in water was selected as a model reaction. On comparison of the catalytic activity of synthesized TSIL with other acidic and basic catalysts (Table 1, entries 2–9), [2-HMPyBSA]HSO4 was found to be superior. It is worth mentioning that the reaction under catalyst-free conditions failed to furnish desired product even after prolong reaction time (Table 1, entry 1). Basic catalysts viz K3PO4 and K2CO3 led formation of desired product in low yield (Table 1, entries 2 and 3). We observed the increase in the rate of reaction with some Lewis and Brønsted acidic catalysts as well as in acidic ionic liquids (Table 1, entries 4–12). Gratifyingly in presence of [2-HMPyBSA]HSO4 best result was obtained in very short time (Table 1, entry 13). This may be due to the synergic effect of strong acidic site and hydroxyl functionality in the ionic liquid.
Motivated by these results, the effect of amount of catalyst on the reaction rate and yield was investigated by lowering the amount of [2-HMPyBSA]HSO4 to 20 and 10 mol% (Table 1, entries 14 and 15) and observed that 20 mol% of [2-HMPyBSA]HSO4 was enough to promote the reaction efficiently. Low yield of the product was detected with further lowering the amount of catalyst.
We also compared efficiency of present method with reported methods for synthesis of spiro-1,2,4-triazolidinones from isatin and thiosemicarbazide (Table 2). The comparison study accentuates the greener aspect of present methodology as it is feasible at ambient temperature, thus achieved energy efficiency. Tables 1 and 2 accustoms [2-HMPyBSA]HSO4 as a perfect choice of catalyst for present reaction.
It is worthy to note that the work-up involves only filtration and a simple washing with EtOH to afford highly pure desired product. The melting point of the product as well as IR, 1H, 13C NMR, and MS analysis data were in harmony with the reports in the literature.
To verify the generality of the protocol, we screened a wide range of substrates under optimum conditions as exemplified in Table 2. Gratifyingly, we observed that reaction tolerated diverse functional groups such as nitro, methoxy, methyl, and halogens on oxindole ring of isatin. Both thiosemicarbazide and 4-methyl-3-thiosemicarbazide undergo smooth reactions with isatins furnishing good yields of product (Table 3, entries a–j).
This result encouraged us to explore the plausible mechanism of the transformation which is represented in Scheme 3. Initially, the protonation of carbonyl oxygen of isatin 1 by acidic functionality of ionic liquid resulted in enhancement of electrophilic character of carbonyl carbon that facilitates nucleophilic attack of –NH2 of thiosemicarbazide 2 leading to formation of Schiff’s base 5. Consequent intramolecular nucleophilic attack of thioamidic –NH2 of thiosemicarbazide moiety furnished the desired product 4. It is hypothesized that the hydroxyl functionality of IL 3 assists the reaction throughout the course by forming hydrogen bonds thereby bringing the reactants together for smooth reaction.

Finally, from economical point of view, recyclability of the catalyst is highly advisable. Hence recyclability study of TSIL for model reaction was carried out (Fig. 2). The product formed after completion of reaction was separated by simple filtration and the filtrate was washed 2 times with 10 cm3 of diethyl ether and used for subsequent cycle. It was noteworthy that the filtrate was used for five cycles without any extensive loss in catalytic activity and for consecutive five cycles, 86, 84, 83, 82, and 82 % yield of the product was obtained, respectively.
Conclusion
In conclusion, the present manuscript puts forward room temperature synthesis of spiro-1,2,4-triazolidine-3-thiones from isatins and thiosemicarbazides in presence of catalytic amount of novel, reusable TSIL, [2-HMPyBSA]HSO4 in water. This experimental method offers promising green development in terms of energy efficiency, water as a solvent, shorter reaction time, 100 % carbon economy, only water as a by-product, reusability of TSIL, wide substrate scope, mild reaction conditions, good to excellent yields of the desired products, easy isolation of pure product avoiding tedious column chromatography and operational simplicity.
Experimental
Various isatins (Sigma-Aldrich) and thiosemicarbazides (Alfa Aesar) were used as received. IR spectra were recorded on PerkinElmer Spectrum 2 FT-IR spectrometer, NMR spectra were recorded on Bruker AC-300 spectrometer (300 MHz for 1H NMR and 75 MHz for 13C NMR) in DMSO-d 6 using TMS as an internal standard and δ values are expressed in ppm. Mass spectra were recorded on a Shimadzu LCMS 2020.
Synthesis of the ionic liquid [HMPBSA]HSO4
To the solution of pyridine-2-methanol in toluene, 1,4-butanesultone was added drop wise with stirring for 5 min and the reaction mixture was stirred for 12 h at 80 °C. The generated white precipitate was collected by suction filtration, washed with ethyl acetate and dried in oven at 60 °C for 5 h. The obtained precipitate was reacted with equimolar quantity of sulfuric acid at 60 °C in toluene for another 7 h and the final product [HMPBSA] HSO4 was obtained as a viscous liquid after separating the two phases. The product was washed with ethyl acetate to get pure product. IR (ATR): \(\bar{v}\) = 3355, 3089, 2935, 1630, 1583, 1509, 1454, 1135, 1023, 863, 775 cm−1; 1H NMR (300 MHz, DMSO-d 6 ): δ = 1.68 (s, 2H), 1.95 (s, 2H), 2.40 (s, 1H), 2.60 (s, 2H), 4.52 (s, 2H), 4.95 (s, 2H), 5.40–5.70 (s, 2H), 8.02 (s, 1H), 8.18 (s, 1H), 8.58 (s, 1H), 9.03 (s, 1H) ppm; 1H NMR (300 MHz, DMSO-d 6 , D2O ex.): δ = 1.63–1.73 (m, 2H), 1.88–1.98 (m, 2H), 2.76–2.81 (t, 2H), 4.35–4.40 (t, 2H), 4.88 (s, 2H), 7.75–7.79 (t, 1H, J = 12 Hz), 7.99–8.02 (d, 1H, J = 9 Hz), 8.30–8.36 (t, 1H, J = 18 Hz), 8.61–8.63 (d, 1H, J = 6 Hz) ppm; 13C NMR (75 MHz, DMSO-d 6 ): δ = 21.05, 28.57, 49.89, 56.49, 59.11, 126.59, 123.78, 145.88, 154.22, 156.14 ppm; MS (ESI): m/z = 246 (cation), 97 (anion).
Synthesis of spiro-1,2,4-triazolidine-3-thiones 4
A mixture of isatin (1 mmol), thiosemicarbazide/4-methyl-3-thiosemicarbazide (1 mmol) was taken in 5 cm3 water containing 20 mol% of [2-HMPyBSA]HSO4 and stirred at room temperature for the time specified in Table 2. The progress of reaction was monitored by TLC. After completion of the reaction, the reaction mixture was filtered and washed with ethanol to yield corresponding product in pure form. Products were characterized by usual spectral techniques. (i.e. IR, 1H, 13C NMR, and MS).
5-Fluoro-5′-thioxospiro[indole-3,3′-[1,2,4]triazolidin]-2(1H)-one (4e, C9H7FN4OS)
Grey solid; m.p.: 279 °C; IR (ATR): \(\bar{v}\) = 3428, 3260, 3136, 3071, 1683, 1620, 1477, 1453, 1196, 1137, 1049, 863 cm−1; 1H NMR (300 MHz, DMSO-d 6 ): δ = 6.90–6.94 (q, 1H), 7.16–7.23 (m, 1H), 7.48–7.52 (q, 1H), 8.78 (s, 1H, –NH), 9.13 (s, 1H, –NH), 11.22 (s, 1H, –NH), 12.37 (s, 1H, –NH) ppm; 13C NMR (75 MHz, DMSO-d 6 ): δ = 108.40, 112.60, 118.0, 121.86, 131.91, 139.04, 157.13, 163.19, 179.23 ppm; MS (ESI): m/z = 239 (M+1), 237 (M−1).
5-Methoxy-4′-methyl-5′-thioxospiro[indole-3,3′-[1,2,4]triazolidin]-2(1H)-one (4f, C11H12N4O2S)
Yellow solid; m.p.: 265 °C; IR (ATR): \(\bar{v}\) = 3307, 3239, 1695, 1551, 1478, 1463, 1195, 1172, 1134, 1042, 1031 cm−1; 1H NMR (300 MHz, DMSO-d 6 ): δ = 3.08 (s, 3H), 3.76 (s, 3H), 6.84–6.86 (d, 1H, J = 6 Hz), 6.92–6.96 (q, 1H), 7.25–7.26 (d, 1H, J = 3 Hz), 9.28 (s, 1H, –NH), 11.4 (s, 1H, –NH), 12.59 (s, 1H, –NH) ppm; 13C NMR (75 MHz, DMSO-d 6 ): δ = 31.75, 56.05, 106.5, 112.39, 117.73, 121.15, 132.38, 136.28, 155.77, 163.21, 178.06 ppm; MS (ESI): m/z = 265 (M+1), 263 (M−1).
4′,5-Dimethyl-5′-thioxospiro[indole-3,3′-[1,2,4]triazolidin]-2(1H)-one (4 g, C11H12N4OS)
Yellow solid; m.p.: 258 °C; IR (ATR): \(\bar{v}\) = 3286, 3233, 3199, 1691, 1663, 1544, 1471, 1313, 1202, 1135, 1040 cm−1; 1H NMR (300 MHz, DMSO-d 6 ): δ = 2.30 (s, 3H), 3.75 (s, 3H), 6.81–6.83 (d, 1H, J = 6 Hz), 7.15–7.18 (d, 1H, J = 9 Hz), 7.46 (s, 1H), 9.25 (s, 1H, –NH), 11.12 (s, 1H, –NH), 12.56 (s, 1H, –NH) ppm; 13C NMR (75 MHz, DMSO-d 6 ): δ = 21.07, 31.78, 111.30, 120.47, 121.53, 131.82, 132.03, 132.15, 140.46, 163.16, 178.11 ppm; MS (ESI): m/z = 249 (M+1), 247 (M−1).
4′-Methyl-5-nitro-5′-thioxospiro[indole-3,3′-[1,2,4]triazolidin]-2(1H)-one (4i, C10H9N5O3S)
Dark yellow solid; m.p.: > 300 °C; IR (ATR): \(\bar{v}\) = 3342, 3202, 3101, 1703, 1621, 1551, 1492, 1340, 1292, 1207, 1123, 1080, 1045 cm−1; 1H NMR (300 MHz, DMSO-d 6 ): δ = 3.09 (s, 3H), 7.11–7.14 (d, 1H, J = 9 Hz), 8.26–8.29 (d, 1H, J = 9 Hz), 8.54 (s, 1H), 9.57 (s, 1H, –NH), 11.84 (s, 1H, –NH), 12.35 (s, 1H, –NH) ppm; 13C NMR (75 MHz, DMSO-d 6 ): δ = 31.81, 79.33, 111.76, 121.26, 127.36, 130.19, 143.23, 147.60, 163.40, 177.95 ppm; MS (ESI): m/z = 278 (M−1).
References
Dicks AP (2009) Green Chem Lett Rev 2:9
Azizi N, Aryanasab F, Torkiyan L, Ziyaei A, Saidi MR (2006) J Org Chem 71:3634
Pore DM, Hegade PG, Gaikwad DS, Patil PB, Patil JD (2014) Lett Org Chem 11:131
Li C, Chen L (2006) Chem Soc Rev 35:68
Narayan S, Muldoon J, Finn MG, Fokin VV, Kolb HC, Sharpless KB (2005) Angew Chem Int Ed 44:3275
Bao W, Wang Z (2006) Green Chem 8:1028
Appell R, Gala D, Sanghvi YS (2011) Org Process Res Dev 15:898
Kotadia DA, Soni SS (2012) J Mol Catal A: Chem 353:44
Fang D, Zhou XL, Ye ZW, Liu ZL (2006) Ind Eng Chem Res 45:7982
Leng Y, Wang J, Zhu D, Ren X, Ge H, Shen L (2009) Angew Chem Int Ed 48:168
Holbrey JD, Turner MB, Reichert WM, Rogers RD (2003) Green Chem 5:731
Wamhoff H (1984) In: Katritzky AR, Rees CW (eds) Comprehensive Heterocyclic Chemistry, vol 5. Pergamon Press, Oxford, p 669
Buckle DR, Rockell CJM (1982) J Chem Soc Perkin Trans 1:627
Buckle DR, Outred DJ, Rockell CJM, Smith H, Spicer BA (1983) J Med Chem 26:251
Buckle DR, Rockell CJM, Smith H, Spicer BA (1986) J Med Chem 29:2269
Alvarez R, Velazquez S, Felix AS, Aquaro S, Clercq ED, Perno CF, Karlsson A, Balzarini J, Camarasa MJ (1994) J Med Chem 37:4185
Genin MJ, Allwine DA, Anderson DJ, Barbachyn MR, Emmert DE, Garmon SA, Graber DR, Grega KC, Hester JB, Hutchinson DK, Morris J, Reischer RJ, Ford CW, Zurenko GE, Hamel JC, Schaadt RD, Stapert D, Yagi BH (2000) J Med Chem 43:953
Brockunier LL, Parmee ER, Ok HO, Candelore MR, Cascieri MA, Colwell LF, Deng L, Feeney WP, Forrest MJ, Hom GJ, MacIntyre DE, Tota L, Wywratt MJ, Fisher MH, Weber AE (2000) Bioorg Med Chem Lett 10:2111
Küçükgüzel SG, Çıkla-Süzgün P (2015) Eur J Med Chem 97:830
Pore DM, Hegade PG, Mane MM, Patil JD (2013) RSC Adv 3:25723
Rathinam R, Appaswami L (2015) RSC Adv 5:51188
Patil PB, Patil JD, Korade SN, Kshirsagar SD, Govindwar SP, Pore DM (2015) Res Chem Intermed. doi:10.1007/s11164-015-2267-z
Patil JD, Pore DM (2014) RSC Adv 4:14314
Patil JD, Korade SN, Patil SA, Gaikwad DS, Pore DM (2015) RSC Adv 5:79061
Acknowledgments
The authors DMP and SNK thank the DST Fast Track Scheme, New Delhi for financial assistance [No. SB/FT/CS-154/2012].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Korade, S.N., Patil, J.D. & Pore, D.M. Novel task-specific ionic liquid for room temperature synthesis of spiro-1,2,4-triazolidine-3-thiones. Monatsh Chem 147, 2143–2149 (2016). https://doi.org/10.1007/s00706-016-1740-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-016-1740-8