Abstract
A series of ten novel benzoxazole analogues of combretastatin A-4 were designed, synthesized, and evaluated for their anticancer activity against three human cancer cell lines. Four of the synthesized compounds exhibited superior potency against different tumour cell lines. One of them showed more potent than control drug, particularly A549 and MCF-7 cell lines.
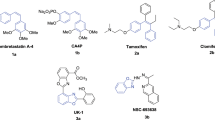
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is widely known that cancer is one leading the cause of death in developed countries [1], maybe induced by a plethora of both external and internal factors, including genetic mutations. Chemotherapy has still been an important fundament for cancer treatment to destroy the cancer cells without any harmful effect on the normal cells and it has achieved significant success through the discovery of various new drugs. Accordingly, a number of types of therapeutic attack has been investigated [2–6]. Drugs that perturb microtubule/tubulin dynamics are used widely in cancer chemotherapy [7]. Despite of this progress, the discovery of most potent anti-cancer agents is a challenging issue in cancer chemotherapy for the future generations.
Combretastatin-A4 (CA-4, 1, Fig. 1), is a natural product, and was first isolated by Pettit et al. from the bark of the South African willow tree Combretum caffrum in 1989 [8]. Combretastatin-A4 displays nanomolar activity against many cancer cell lines leading to arrest in the G2/M phase [8–10]. The mechanism of action of CA-4 involves reversible, high affinity binding in the colchicine site of tubulin [11]. Structure–activity relationship studies on CA-4 derivatives have established that pharmacophore structure for binding to tubulin is the cis-orientation of the two ethenyl-bridged aromatic rings which one of them bearing 3,4,5-trimethoxy substituents [12, 13]. Its water soluble prodrug CA-4P (2) is currently under phase II/III clinical evaluation in the USA as an agent used in combination treatment for various multidrug resistant solid tumours [14]. CA-4 and combretastatin-A4 phosphate (CA-4P, 2), are selectively cytotoxic to rapidly proliferating tumor vasculature than normal blood vessels resulting in reduced blood flow to tumor and eventual hemorrhagic necrosis [15, 16]. Hence, combretastatin-A4 is an attractive lead compound for the development of new anticancer agents [17, 18].
Similarly, benzoxazole ring occurs in a number of natural products such as salvianen and pseudosalvianen [19, 20]. Derivatives of benzoxazole have gained much importance because of its wide applications in medicinal sector. Benzoxazoles are important fragments in medicinal chemistry because of their wide range of biological activities, such as anticancer activities [21–23], and they serve as a topoisomerase-I poison [24] and show antibacterial [25], antifungal [26], antimicrobial [27], and anti-measles virus activities [28].
Both the combretastatin-A4 and benzoxazole are act as anti-cancer agents. In the view of synthesis of more potent anticancer drugs, we are planned to synthesize new benzoxazole derivatives of combretastatin-A4 and screened their anticancer activity against a panel of human cancer cell lines like A549, MCF-7, and Colo-205.
Results and discussion
Chemistry
The synthetic methods for the preparation of target compounds 8a–8j are summarized as follows (Scheme 1). Acrylic acid 6 was synthesized by means of a Perkin reaction, as previously reported [29]. Reaction of 4-hydroxy-3-methoxybenzaldehyde (4) with 3,4,5-trimethoxyphenylacetic acid (3) in the presence of acetic anhydride and triethyl amine at 140 °C for 24 h afforded the acetate of corresponding acrylic acid 5. Deprotection of 5 in mild basic (K2CO3) conditions afforded pure acrylic acid 6. This compound 6 undergoes cyclization with substituted 2-aminophenols 7a–7j in presence of polyphosphoric acid (PPA) at 170 °C for 6 h to afford pure compounds 8a–8j in good yield. The synthesis of cyclized benzoxazoles fused combretastatin from o-aminophenols and acrylic acids was novelty of this method.

In vitro cytotoxicity
The compounds 8a–8j were evaluated for their anticancer activity in selected human cancer cell lines like Colo-205 (colon), A-549 (lung), and MCF-7 (breast) by employing the sulforhodamine B (SRB) assay [30] and the results are summarized in Table 1. The GI50 range of all these compounds <0.1–27.30 µM and control drug <0.1–0.13 µM range. Among them, compounds 8b, 8c, 8d, and 8j were showed more potent anticancer activity. In which compound 8d was showed most active than control particularly A549 and MCF-7 cell lines.
Conclusion
In summary, a series of benzoxazole fused combretastatin-A4 analogs 8a–8j have been synthesized. All of the target compounds were evaluated for their in vitro anticancer activities on human cancer cell lines. Among these compounds, compounds 8b, 8c, 8d, and 8j were exhibited superior potency against different tumour cell lines. In which one compound 8d was showed more potent than control drug, particularly A549 and MCF-7 cell lines.
Experimental
All chemicals and reagents were obtained from Aldrich (Sigma-Aldrich, St. Louis, MO, USA), Lancaster (Alfa Aesar, Johnson Matthey Company, Ward Hill, MA, USA) and were used without further purification. Compounds 5 and 6 were prepared according to literature [29]. Reactions were monitored by TLC, performed on silica gel glass plates containing 60 F-254, and visualization on TLC was achieved by UV light or iodine indicator. 1H and 13C NMR spectra were recorded on Gemini Varian-VXR-unity and Bruker UXNMR/XWIN-NMR (200 and 300 MHz) instruments. Chemical shifts (δ) are reported in ppm downfield from internal TMS standard. ESI spectra were recorded on Micro mass, Quattro LC using ESI+ software with capillary voltage 3.98 kV and ESI mode positive ion trap detector. Melting points were determined with an electrothermal melting point apparatus.
(E)-4-[2-(Benzo[d]oxazol-2-yl)-2-(3,4,5-trimethoxyphenyl)vinyl]-2-methoxyphenol (8a, C25H23NO6)
A mixture of 500 mg (E)-3-(4-hydroxy-3-methoxyphenyl)-2-(3,4,5-trimethoxyphenyl)acrylic acid (6, 1.388 mmol) and 152 mg 2-aminophenol (7a, 1.388 mmol) were dissolved in sufficient quantity of polyphosphoric acid (PPA). The mixture was heated slowly to 170 °C for 6 h, permitted to cool to room temperature, and quenched with excess of 10 % Na2CO3 solution and extracted with ethyl acetate. Then dried over anhydrous Na2SO4 and the crude product was purified by column chromatography with ethyl acetate/hexane (3:7) to afford pure compound 8a, 584 mg in 97 % yield. M.p.: 225–227 °C; 1H NMR (300 MHz, DMSO-d 6 ): δ = 3.76 (s, 6H), 3.82 (s, 3H), 3.90 (s, 3H), 6.34 (s, 2H), 6.75 (d, 1H, J = 8.2 Hz), 6.91 (d, 1H, J = 8.0 Hz), 6.99 (s, 1H), 7.31 (t, 2H), 7.39 (s, 1H), 7.51 (d, 2H, J = 8.1 Hz), 10.20 (s, 1H) ppm; 13CNMR (75 MHz, DMSO-d 6 ): δ = 54.67, 56.12, 59.89, 110.21, 111.45, 113.87, 117.39, 117.98, 122.67, 125.50, 125.86, 126.54, 128.88, 130.64, 130.86, 137.68, 142.48, 147.76, 149.49, 149.89, 154.76, 159.87 ppm; MS (ESI): m/z = 434 ([M + H]+).
(E)-2-Methoxy-4-[2-(6-methoxybenzo[d]oxazol-2-yl)-2-(3,4,5-trimethoxyphenyl)vinyl]phenol (8b, C26H25NO7)
The compound 8b was prepared following the method described for the preparation of the compound 8a, employing 500 mg (E)-3-(4-hydroxy-3-methoxyphenyl)-2-(3,4,5-trimethoxyphenyl)acrylic acid (6, 1.388 mmol) and 249 mg 2-amino-5-methoxyphenol hydrochloride (7b, 1.388 mmol), and the crude product was purified by column chromatography with ethyl acetate/hexane (3:7) to afford pure compound 8b, 541 mg in 84 % yield. M.p.: 221–123 °C; 1H NMR (300 MHz, DMSO-d 6 ): δ = 3.70 (s, 3H), 3.81 (s, 6H), 3.88 (s, 3H), 3.92 (s, 3H), 6.31 (s, 2H), 6.75–6.84 (m, 2H), 6.92 (d, 1H, J = 8.0 Hz), 6.98–7.03 (m, 1H), 7.10 (d, 1H, J = 8.1 Hz), 7.36 (d, 1H, J = 9.0 Hz), 7.48 (s, 1H), 10.14 (s, 1H) ppm; 13C NMR (75 MHz, DMSO-d 6 ): δ = 54.94, 55.68, 56.10, 60.06, 94.78, 109.59, 111.90, 113.78, 117.77, 120.72, 125.80, 126.65, 128.90, 129.79, 130.89, 135.94, 138.67, 147.75, 149.70, 151.87, 154.55, 156.80, 161.50 ppm; MS (ESI): m/z = 464 ([M + H]+).
(E)-2-Methoxy-4-[2-(5-methoxybenzo[d]oxazol-2-yl)-2-(3,4,5-trimethoxyphenyl)vinyl]phenol (8c, C26H25NO7)
The compound 8c was prepared following the method described for the preparation of the compound 8a, employing 500 mg (E)-3-(4-hydroxy-3-methoxyphenyl)-2-(3,4,5-trimethoxyphenyl)acrylic acid (6, 1.388 mmol) and 193 mg 2-amino-4-methoxyphenol (7c, 1.388 mmol), and the crude product was purified by column chromatography with ethyl acetate/hexane (3:7) to afford pure compound 8c, 546 mg in 87 % yield. M.p.: 219–121 °C; 1H NMR (300 MHz, DMSO-d 6 ): δ = 3.73 (s, 3H), 3.80 (s, 6H), 3.84 (s, 3H), 3.88 (s, 3H), 6.29 (s, 2H), 6.72 (d, 1H, J = 8.0 Hz), 6.79 (s, 1H), 6.86 (d, 1H, J = 9.0 Hz), 7.06–7.18 (m, 2H), 7.40 (d, 1H, J = 8.1 Hz), 7.49 (s, 1H), 10.23 (s, 1H) ppm; 13C NMR (75 MHz, DMSO-d 6 ): δ = 54.89, 55.76, 56.46, 60.23, 103.54, 105.49, 108.39, 111.78, 113.98, 116.78, 124.91, 126.76, 128.87, 130.43, 130.67, 138.85, 143.59, 145.65, 147.96, 149.60, 152.93, 154.39, 161.70 ppm; MS (ESI): m/z = 464 ([M + H]+).
(E)-2-Methoxy-4-[2-(5,6-dimethoxybenzo[d]oxazol-2-yl)-2-(3,4,5-trimethoxyphenyl)vinyl]phenol (8d, C27H27NO8)
The compound 8d was prepared following the method described for the preparation of the compound 8a, employing 500 mg (E)-3-(4-hydroxy-3-methoxyphenyl)-2-(3,4,5-trimethoxyphenyl)acrylic acid (6, 1.388 mmol) and 234 mg 2-amino-4,5-dimethoxyphenol (7d, 1.388 mmol), and the crude product was purified by column chromatography with ethyl acetate/hexane (4:6) to afford pure compound 8d, 564 mg in 82 % yield. M.p.: 229–231 °C; 1H NMR (300 MHz, DMSO-d 6 ): δ = 3.71 (s, 3H), 3.79 (s, 6H), 3.83 (s, 3H), 3.87 (s, 3H), 3.93 (s, 3H), 6.27 (s, 2H), 6.71 (s, 1H), 6.81–6.86 (m, 2H), 6.89 (d, 1H, J = 8.1 Hz), 7.12 (s, 1H), 7.46 (s, 1H), 10.09 (s, 1H) ppm; 13C NMR (75 MHz, DMSO-d 6 ): δ = 55.56, 55.98, 56.32, 56.49, 59.97, 95.89, 103.34, 110.98, 114.56, 116.89, 125.45, 126.21, 128.92, 129.76, 130.88, 137.23, 138.34, 144.85, 146.69, 148.35, 148.95, 154.56, 161.32 ppm; MS (ESI): m/z = 494 ([M + H]+).
(E)-2-Methoxy-4-[2-(3,4,5-trimethoxyphenyl)-2-(6-nitrobenzo[d]oxazol-2-yl)vinyl]phenol (8e, C25H22N2O8)
The compound 8e was prepared following the method described for the preparation of the compound 8a, employing 500 mg (E)-3-(4-hydroxy-3-methoxyphenyl)-2-(3,4,5-trimethoxyphenyl)acrylic acid (6, 1.388 mmol) and 213 mg 2-amino-5-nitrophenol (7e, 1.388 mmol), and the crude product was purified by column chromatography with ethyl acetate/hexane (2:8) to afford pure compound 8e, 572 mg in 86 % yield. M.p.: 234–236 °C; 1H NMR (300 MHz, DMSO-d 6 ): δ = 3.76 (s, 6H), 3.83 (s, 3H), 3.89 (s, 3H), 6.30 (s, 2H), 6.83 (d, 1H, J = 8.1 Hz), 6.99 (d, 1H, J = 9.2 Hz), 7.10 (s, 1H), 7.47 (s, 1H), 7.70 (d, 1H, J = 8.1 Hz), 7.98 (d, 1H, J = 8.2 Hz), 8.48 (s, 1H) ppm; 13C NMR (75 MHz, DMSO-d 6 ): δ = 55.43, 56.48, 61.34, 94.41, 111.67, 113.78, 117.54, 118.99, 121.34, 126.12, 126.48, 129.34, 130.67, 131.23, 137.89, 140.34, 146.88, 147.86, 149.56, 150.21, 153.67, 160.34 ppm; MS (ESI): m/z = 479 ([M + H]+).
(E)-4-[2-(5-Chloro-6-nitrobenzo[d]oxazol-2-yl)-2-(3,4,5-trimethoxyphenyl)vinyl]-2-methoxyphenol (8f, C25H21ClN2O8)
The compound 8f was prepared following the method described for the preparation of the compound 8a, employing 500 mg (E)-3-(4-hydroxy-3-methoxyphenyl)-2-(3,4,5-trimethoxyphenyl)acrylic acid (6, 1.388 mmol) and 262 mg 2-amino-4-chloro-5-nitrophenol (7f, 1.388 mmol), and the crude product was purified by column chromatography with ethyl acetate/hexane (2:8) to afford pure compound 8f, 623 mg in 88 % yield. M.p.: 239–241 °C; 1H NMR (200 MHz, DMSO-d 6 ): δ = 3.88 (s, 6H), 3.93 (s, 3H), 3.95 (s, 3H), 6.36 (s, 2H), 6.83 (d, 1H, J = 8.1 Hz), 6.98 (d, 1H, J = 8.9 Hz), 7.16 (s, 1H), 7.51 (s, 1H), 7.98 (s, 1H), 8.54 (s, 1H) ppm; 13C NMR (75 MHz, DMSO-d 6 ): δ = 55.78, 56.34, 61.12, 105.65, 110.98, 113.66, 116.90, 121.50, 125.41, 128.99, 130.67, 130.89, 131.76, 137.78, 140.43, 146.23, 147.89, 148.89, 150.32, 153.94, 160.78 ppm; MS (ESI): m/z = 513 ([M + H]+).
(E)-2-Methoxy-4-[2-(3,4,5-trimethoxyphenyl)-2-(5,6-dinitrobenzo[d]oxazol-2-yl)vinyl]phenol (8 g, C25H21N3O10)
The compound 8 g was prepared following the method described for the preparation of the compound 8a, employing 500 mg (E)-3-(4-hydroxy-3-methoxyphenyl)-2-(3,4,5-trimethoxyphenyl)acrylic acid (6, 1.388 mmol) and 276 mg 2-amino-4,5-dinitrophenol (7 g, 1.388 mmol), and the crude product was purified by column chromatography with ethyl acetate/hexane (2:8) to afford pure compound 8 g, 629 mg in 87 % yield. M.p.: 245–247 °C; 1H NMR (200 MHz, DMSO-d 6 ): δ = 3.81 (s, 3H), 3.85 (s, 3H), 3.88 (s, 6H), 6.32 (s, 2H), 6.79–6.83 (m, 1H), 6.93 (s, 1H), 6.95–6.99 (m, 1H), 7.51 (s, 1H), 8.62 (s, 1H), 8.69 (s, 1H) ppm; 13C NMR (75 MHz, DMSO-d 6 ): δ = 55.43, 56.67, 61.78, 107.85, 112.45, 115.65, 115.89, 118.76, 126.34, 126.98, 129.89, 130.23, 130.87, 137.56, 138.34, 138.67, 146.61, 149.33, 149.66, 150.90, 154.89, 161.92 ppm; MS (ESI): m/z = 524 ([M + H]+).
(E)-4-[2-(5-Chlorobenzo[d]oxazol-2-yl)-2-(3,4,5-trimethoxyphenyl)vinyl]-2-methoxyphenol (8 h, C25H22ClNO6)
The compound 8 h was prepared following the method described for the preparation of the compound 8a, employing 500 mg (E)-3-(4-hydroxy-3-methoxyphenyl)-2-(3,4,5-trimethoxyphenyl)acrylic acid (6, 1.388 mmol) and 199 mg 2-amino-4-chlorophenol (7 h, 1.388 mmol), and the crude product was purified by column chromatography with ethyl acetate/hexane (2:8) to afford pure compound 8 h, 654 mg in 78 % yield. M.p.: 233–235 °C; 1H NMR (200 MHz, DMSO-d 6 ): δ = 3.79 (s, 3H), 3.82 (s, 3H), 3.86 (s, 6H), 6.29 (s, 2H), 6.83-6.87 (m, 1H), 6.90–6.97 (m, 2H), 7.11–7.17 (m, 1H), 7.39–7.49 (m, 3H), 7.64–7.68 (m, 1H) ppm; 13C NMR (75 MHz, DMSO-d 6 ): δ = 55.23, 57.41, 60.26, 105.96, 111.69, 114.56, 114.89, 117.67, 124.77, 126.51, 128.90, 130.45, 130.78, 136.81, 137.82, 138.89, 146.34, 148.68, 149.73, 150.39, 155.56, 160.89 ppm; MS (ESI): m/z = 604 ([M + H]+).
(E)-2-Methoxy-4-[2-(3,4,5-trimethoxyphenyl)-2-(5-methylbenzo[d]oxazol-2-yl)vinyl]phenol (8i, C26H25NO6)
The compound 8i was prepared following the method described for the preparation of the compound 8a, employing 500 mg (E)-3-(4-hydroxy-3-methoxyphenyl)-2-(3,4,5-trimethoxyphenyl)acrylic acid (6, 1.388 mmol) and 170 mg 2-amino-4-methylphenol (7i, 1.388 mmol), and the crude product was purified by column chromatography with ethyl acetate/hexane (2:8) to afford pure compound 8i, 562 mg in 90 % yield. M.p.: 216–219 °C; 1H NMR (200 MHz, DMSO-d 6 ): δ = 2.41 (s, 3H), 3.81 (s, 3H), 3.84 (s, 3H), 3.88 (s, 6H), 6.27 (s, 2H), 6.80–6.84 (m, 1H), 6.93–6.97 (m, 2H), 7.06-7.11 (m, 1H), 7.52 (s, 1H), 7.58–7.61 (m, 2H) ppm; 13C NMR (75 MHz, DMSO-d 6 ): δ = 21.45, 54.29, 56.67, 59.39, 107.45, 110.67, 114.12, 116.34, 120.68, 124.98, 126.78, 127.67, 128.89, 129.86, 130.11, 137.62, 140.19, 146.76, 147.99, 149.56, 154.23, 159.48 ppm; MS (ESI): m/z = 448 ([M + H]+).
(E)-2-Methoxy-4-[2-(3,4,5-trimethoxyphenyl)-2-(5,6-dimethylbenzo[d]oxazol-2-yl)vinyl]phenol (8j, C27H27NO6)
The compound 8j was prepared following the method described for the preparation of the compound 8a, employing 500 mg (E)-3-(4-hydroxy-3-methoxyphenyl)-2-(3,4,5-trimethoxyphenyl)acrylic acid (6, 1.388 mmol) and 190 mg 2-amino-4,5-dimethylphenol (7j, 1.388 mmol), and the crude product was purified by column chromatography with ethyl acetate/hexane (2:8) to afford pure compound 8j, 527 mg in 82 % yield. M.p.: 213–215 °C; 1H NMR (200 MHz, DMSO-d 6 ): δ = 2.36 (s, 3H), 2.39 (s, 3H), 3.76 (s, 3H), 3.79 (s, 3H), 3.83 (s, 6H), 6.28 (s, 2H), 6.78–6.80 (m, 1H), 6.84–6.90 (m, 2H), 7.28 (s, 1H), 7.43–7.48 (m, 3H) ppm; 13C NMR (75 MHz, DMSO-d 6 ): δ = 20.45, 54.34, 55.65, 60.07, 108.98, 110.78, 113.56, 116.61, 123.72, 124.55, 125.91, 128.87, 129.99, 130.89, 132.59, 137.83, 141.26, 145.63, 148.67, 149.39, 153.26, 159.23 ppm; MS (ESI): m/z = 462 ([M + H]+).
Procedure of the SRB-assay
All the synthesized new compounds 8a–8j were evaluated for their in vitro cytotoxicity against human cancer cell lines. A protocol of 48 h continuous drug exposure has been used and a sulforhodamine B (SRB) protein assay has been used to estimate cell viability or growth. The cell lines were grown in DMEM medium containing 10 % fetal bovine serum and 2 mM l-glutamine and were inoculated into 96 well microtiter plates in 90 cm3 at plating densities depending on the doubling time of individual cell lines. The microtiter plates were incubated at 37 °C, 5 % CO2, 95 % air, and 100 % relative humidity for 24 h prior to addition of experimental drugs. Aliquots of 10 cm3 of the drug dilutions were added to the appropriate microtiter wells already containing 90 cm3 of cells, resulting in the required final drug concentrations. For each compound four concentrations (0.1, 1, 10, and 100 µM) were evaluated and each was done in triplicate wells. Plates were incubated further for 48 h and assay was terminated by the addition of 50 cm3 of cold trichloroacetic acid (TCA) (final concentration 10 % TCA) and incubated for 60 min at 4 °C. The plates were washed five times with tap water and air dried. Sulforhodamine B (SRB) solution (50 cm3) at 0.4 % (w/v) in 1 % acetic acid was added to each of the cells, and plates were incubated for 20 min at room temperature. The residual dye was removed by washing five times with 1 % acetic acid. The plates were air dried. Bound stain was subsequently eluted with 10 mM trizma base, and the absorbance was read on an ELISA plate reader at a wavelength of 540 nm with 690 nm reference wavelengths. Percent growth was calculated on a plate by plate basis for test wells relative to control wells. The above determinations were repeated three times. Percentage growth was expressed as the (ratio of average absorbance of the test well to the average absorbance of the control wells) × 100. Growth inhibition of 50 % (GI50) was calculated from [(T i − T z)/(C − T z)] × 100 = 50, which is the drug concentration resulting in a 50 % reduction in the net protein increase (as measured by SRB staining) in control cells during the drug incubation, where T z = optical density at time zero, C = control growth, and T i = test growth in the presence of drug at the four concentration levels.
References
Garcia M, Jemal A, Ward EM, Center MM, Hao Y, Siegel RL, Thun MJ (2007) Global cancer facts & figures. American Cancer Society, Atlanta
Hanahan D, Weinberg RA (2000) Cell 100:57
Stratton MR, Campbell PJ, Futreal PA (2009) Nature 458:719
Hanahan D, Weinberg RA (2011) Cell 144:646
Boyle FT, Costello GF (1998) Chem Soc Rev 27:251
Gibbs JB (2000) Science 287:1969
Lu Y, Chen YJ, Xiao M, Li W, Miller DD (2012) Pharm Res 29:2943
Pettit GR, Singh SB, Hamel E, Lin CM, Alberts DS, Garcia-Kendall D (1989) Experientia 45:209
Woods JA, Hadfield JA, Pettit GR, Fox BW, McGown AT (1995) Br J Cancer 71:705
Meng F, Cai X, Duan J, Matteucci MG, Hart CP (2008) Cancer Chemother Pharmacol 61:953
Lin CM, Ho HH, Pettit GR, Hamel E (1989) Biochemistry 28:6984
Hu L, Li ZR, Li Y, Qu J, Ling YH, Jiang JD, Boykin DW (2006) J Med Chem 49:6273
Cushman M, Nagarathnam D, Gopal D, Chakraborti AK, Lin CM, Hamel (1991) Eur J Med Chem 34:2579
Zweifel M, Jayson GC, Reed NS, Osborne R, Hassan B, Ledermann J, Shreeves G, Poupard L, Lu SP, Balkissoon J, Chaplin DJ, Rustin G (2011) J Ann Oncol 22:2036
Dark GG, Hill SA, Prise VE, Tozer GM, Pettit GR, Chaplin D (1997) J Cancer Res 57:1829
Grosios K, Holwell SE, McGown AT, Pettit GR, Bibby MC (1999) Br J Cancer 81:1318
Kanthou C, Tozer GM (2009) Int J Exp Pathol 90:284
Siemann DW, Chaplin DJ, Walicke PA (2009) Expert Opin Invest Drugs 18:189
Don M-J, Shen C-C, Lin Y-L, Syu WJ, Ding Y-H, Sun C-M (2005) J Nat Prod 68:1066
Viirre RD, Evindar G, Batey RA (2008) J Org Chem 73:345
Ueki M, Ueno K, Miyadoh S, Shibata K, Taniguchi M, Oi SJ (1993) Antibiotics 46:1089
Cheng CC, Liu DE, Chou TC (1993) Heterocycles 35:775
Kamal A, Suresh P, Ramaiah MJ, Mallareddy A, Kumar BA, Raju P, Gopal JV, Pushpavalli SNCVL, Lavanya A, Sarma P, Pal-Bhadra M (2011) Bioorg Med Chem 19:4589
Kim JS, Sun Q, Gatto B, Yu C, Liu LE, LoVoie EJ (1996) Bioorg Med Chem 4:621
Sun L-Q, Chen J, Bruce M, Deskus JA, Epperson JR, Takaki K, Johnson G, Iben L, Mahle CD, Ryan E, Xu C (2004) Bioorg Med Chem Lett 14:3799
Ertan T, Yildiz I, Tekiner-Gulbas B, Bolelli K, Temiz-Arpaci O, Yalcin I, Aki E, Ozkan S, Kaynak F (2009) Eur J Med Chem 44:501
Alper-Hayta S, Arisoy M, Temiz-Arpaci O, Yildiz I, Aki E, Oezkan S, Kaynak F (2008) Eur J Med Chem 43:2568
Sun A, Prussia A, Zhan W, Murray EE, Doyle J, Cheng L-T, Yoon JJ, Radchenko EV, Palyulin VA, Compans RW, Liotta DC, Plemper RK, Snyder JA (2006) J Med Chem 49:5080
Borrel C, Thoret S, Cachet X, Guénard D, Tillequin F, Koch M, Michel S (2005) Bioorg Med Chem 13:3853
Skehn P, Storeng R, Scudiero A, Monks J, McMohan D, Vistica D, Jonathan WT, Bokesch H, Kenney S, Boyd RM (1990) J Natl Cancer Inst 82:1107
Acknowledgments
We are grateful to CSIR-IICT, Hyderabad for providing analytical facilities.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bramhananda Reddy, N., Burra, V.R., Ravindranath, L.K. et al. Synthesis and biological evaluation of benzoxazole fused combretastatin derivatives as anticancer agents. Monatsh Chem 147, 593–598 (2016). https://doi.org/10.1007/s00706-016-1685-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-016-1685-y