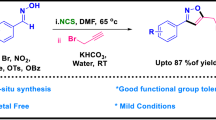
Abstract
Efficient and environmentally benign procedures have been reported for the synthesis of 1,4-disubstituted 1,2,3-triazoles by reaction of aryl azides with aldehydes in task-specific basic ionic liquid [DBU-Bu]OH under ultrasonic irradiation and in hydrated ionic liquid tetrabutylammonium hydroxide (Bu4NOH) under conventional heating. These protocols represent simple, general, and efficient approaches in terms of good yields, operational simplicity, easy workup, and shorter reaction time for the synthesis of 1,4-disubstituted 1,2,3-triazoles under metal-free conditions.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The synthesis of heterocycles is of great importance because of ever increasing demand for novel biologically active compounds. The laborious processes of lead discovery and optimization have resulted in the continuous search for simple and efficient methodologies for generating libraries of compounds for biological screening. 1,4-Disubstituted 1,2,3-triazoles are an important class of nitrogen-containing heterocyclic compounds because of their extensive biological and photo-physical properties. Heterocycles having 1,4-disubstituted 1,2,3-triazole units show diverse biological activities such as anticancer [1], anti-HIV [2], antimicrobial [3], antiviral [4], antiproliferative [5], antibiotic [6], insecticidal [7], and fungicidal [8] They are also attractive connecting units because of their stability to metabolic degradation and are capable of hydrogen bonding that can improve the solubility of drugs [9, 10]. The synthesis of 1,4-disubstituted 1,2,3-triazoles is often reported by Cu(I)-catalyzed azide–alkyne cycloaddition (CuAAC) reaction [11, 12]. This protocol requires Cu(I) as catalyst which may be difficult to remove from reaction mixture. Thus, this reaction is not useful for the labeling of biomolecules in living cells because of the cytotoxicity of the copper catalyst. The base-catalyzed reaction of aryl azides with enolizable aldehydes for the synthesis of 1,4-disubstituted 1,2,3-triazoles is a potentially powerful but less explored method. The two procedures that have been reported for this reaction so far in the literature involve certain disadvantages such as the need of column chromatography for product purification, lower yield of products, and use of toxic organic solvents [13, 14].
Green chemistry demands [15] the development of alternative chemical processes that involve use of nontoxic solvents and alternative sources of energy. The use of ultrasonic irradiation has advantages over conventional heating such as less energy consumption, simple experimental procedure, increased reaction rates, clean reactions, and increased product selectivity [16], resulting in higher yields. Ionic liquids (ILs) are emerging solvents of interest as alternatives to conventional organic solvents because of their unique physical properties [17–22]. They have also been referred to as “designer solvents” as their physical and chemical properties can be adjusted by a careful choice of cation and anion. DBU is a strong organic base and has been extensively applied in the base-induced reactions with excellent catalytic activity. However, the separation of DBU from the product mixture is generally difficult. The DBU-based ILs (DBU-ILs) overcome this drawback and exhibit the similar basicity to DBU accompanied with the general features of ILs [23]. Hydrated IL Bu4NOH has emerged as mild, nonmetallic, nonvolatile, inexpensive, and environmentally compatible, homogenous catalyst in various organic reactions [24–26]. Moreover, Bu4NOH is easily available and possesses high basicity.
In continuation of our research interest [27–30] for synthesis of differently substituted 1,2,3-triazoles and inspired by importance of ionic liquid [DBU-Bu]OH and hydrated ionic liquid Bu4NOH, we wish to report two new protocols for the synthesis of 1,4-disubstituted 1,2,3-triazoles.
Results and discussion
We present herein efficient and environmentally benign methodologies for the synthesis of 1,4-disubstituted 1,2,3-triazoles by 1,3-dipolar cycloaddition of various aryl azides with enolizable aldehydes (Scheme 1) in task-specific ionic liquid [DBU-Bu]OH under ultrasonic irradiation at room temperature (method A) and in hydrated ionic liquid Bu4NOH (40 %) under conventional heating in aqueous media (method B).

The optimum reaction conditions for the proposed reaction following method A were achieved by investigating the model reaction of 2-phenylacetaldehyde (1.0 mmol) and 4-nitrophenylazide (1.1 mmol) under different reaction conditions as given in Table 1. Reactions attempted in solvents such as ethanol, DMF, water, and DMSO in the absence of base catalyst under ultrasonic irradiation at room temperature did not yield any product even after 120 min (Table 1, entries 1–4). While reaction attempted in solvents such as ethanol, DMF, water, and DMSO in the presence of ionic liquid [DBU-Bu]OH (20 mol%) as catalyst under ultrasonic irradiation required longer reaction time and resulted in inferior yields of the product 1a (Table 1, entries 5–8). The best result was obtained when the reaction was performed under ultrasonic irradiation at room temperature using 20 mol% of [DBU-Bu]OH in the absence of any solvent. The reaction was completed in 10 min and gave 1-(4-nitrophenyl)-4-phenyl-1H-1,2,3-triazole (1a) in 94 % yield after a simple workup (Table 1, entry 9). Reaction repeated using 30 mol% of [DBU-Bu]OH, under otherwise identical conditions, did not have any considerable effect on reaction time and yield of product 1a, while reaction attempted using 10 mol% of [DBU-Bu]OH required longer time with inferior yield of product 1a (Table 1, entries 10, 11). The reaction performed at room temperature by normal stirring in the absence of ultrasonic irradiations was found to be incomplete after 120 min and afforded 65 % of product 1a (Table 1, entry 12). Thus, the reaction of 2-phenylacetaldehyde (1.0 mmol) and 4-nitrophenylazide (1.1 mmol) in the presence [DBU-Bu]OH (20 mol%) under ultrasonic irradiation was accepted as the most suitable conditions for further exploration (method A).
Under these optimized reaction conditions, the preparation of variously substituted aromatic azides was carried out with 2-phenylacetaldehyde using different aryl azides. All reactions proceeded smoothly for different aromatic azides and gave the corresponding 1-aryl-4-phenyl-1H-1,2,3-triazoles (1a–1m, Table 2, method A) in good yields and in short reaction times. This protocol was further extended by replacing 2-phenylacetaldehyde with aliphatic butyraldehyde. Thus, reaction in the presence of butyraldehyde (1.0 mmol) with 4-nitrophenylazide (1.1 mmol) gave 4-ethyl-1-(4-nitrophenyl)-1H-1,2,3-triazole (1n) in 87 % yield (Table 2, method A). Similarly, phenyl azide underwent successful condensation with butyraldehyde to give corresponding product 1o in 85 % yield (Table 2, method A).
We further decided to explore this reaction by using hydrated Bu4NOH in aqueous medium under conventional heating (method B). Therefore, model reaction of 2-phenylacetaldehyde (1.0 mmol) and 4-nitrophenylazide (1.1 mmol) was attempted in hydrated Bu4NOH at different temperatures. The best results were obtained when this reaction was attempted at 100 °C using 20 mol% of hydrated Bu4NOH (Table 1, entry 15). The reaction was completed in 35 min yielding 82 % of 1-(4-nitrophenyl)-4-phenyl-1H-1,2,3-triazole (1a) after a simple workup. When this reaction was attempted at lower temperatures, 60 and 80 °C, under otherwise identical conditions, it required longer reaction times for completion and gave lower yields of product (Table 1, entries 13, 14). Therefore, reaction in hydrated Bu4NOH (20 mol%) at 100 °C was chosen as optimum system to prepare 1,4-disubstituted 1,2,3-triazoles 1b–1m in good yields by 1,3-cycloaddition of 2-phenylacetaldehyde under the optimized conditions (Table 2, method B).
Similarly, the reaction of butyraldehyde with 4-nitrophenylazide and phenyl azide under these conditions yielded 4-ethyl-1-(4-nitrophenyl)-1H-1,2,3-triazole (1n) and 4-ethyl-1-phenyl-1H-1,2,3-triazole (1o), respectively (Table 2, method B). The results indicated that reactions by both methods proceed fast and gave better yields with aryl azides having electron-withdrawing groups compared to azides with electron-donating groups. Comparison of the two methods reveals that method A generally affords slightly better yields of products.
The recyclability of ionic liquid [DBU-Bu]OH was also explored on the cycloaddition reaction of 4-nitrophenylazide with 2-phenylacetaldehyde. Water was added after completion of the reaction. The ionic liquid dissolved in water and the solution was filtered to isolate the solid product. Water was then evaporated to recover ionic liquid which was dried under reduced pressure. The recovered ionic liquid was reused to study its catalytic activity in the subsequent runs. Thus, the preparation of compound 1a was repeated three times using each time the recovered ionic liquid, and a progressive decrease in yield was so observed, from 94 to 93, 90, and 85 % yield.
The plausible mechanism of 1,3-dipolar cycloaddition reaction of aryl azides with aldehyde is shown in Scheme 1. The initial reaction of [DBU-Bu]OH or Bu4NOH with aldehyde generates enolate A, which then undergoes 1,3-dipolar cycloaddition with aryl azide selectively to furnish the adduct 1,2,3-triazoline B with regeneration of ionic liquid. 1,2,3-Triazoline B further undergoes elimination of water to yield 1,4-disubstituted 1,2,3-triazoles 1a–1o.

Conclusion
We have reported efficient and environmentally benign methodologies for the synthesis of 1,4-disubstituted 1,2,3-triazoles by reaction of various aryl azides with aldehydes containing α-methylene group using task-specific basic ionic liquid [DBU-Bu]OH under ultrasonic irradiation at room temperature (method A) and in hydrated Bu4NOH under conventional heating at 100 °C (method B). These two new protocols offer several advantages in terms of operational simplicity, recyclability of ionic liquid, easy workup, short reaction time, and high yield of products. In general, method A was found to be slightly superior to method B in terms of yield of product formed.
Experimental
Silica gel 60 F254 (precoated aluminum plates) from Merck was used to monitor reaction progress. Melting points were determined on a melting point apparatus. The NMR (1H and 13C) spectra were recorded on JOEL JNM ECX-400P at 400 MHz and 100 MHz, respectively. The chemical shift values are recorded on δ scale, and the coupling constants (J) are in Hz. Ultrasonic bath (54 kHz, 300 W, 3 dm3 capacity) of Thoroughclean Ultrasonic Pvt. Ltd. (India) was used for reactions under ultrasonic irradiation. Different aryl azides and [DBU-Bu]OH were prepared by reported procedure [29].
General procedure for the synthesis of 1-aryl-4-phenyl/alkyl-1H-1,2,3-triazoles 1a–1o
Method A: A mixture of 2-phenylacetaldehyde/butyraldehyde (1.0 mmol), aryl azide (1.1 mmol), and task-specific basic ionic liquid [DBU-Bu]OH (20 mol%) was placed in a 50-cm3 round-bottomed flask. The reaction mixture was sonicated for appropriate time until the complete disappearance of starting materials. The progress of the reaction was monitored by TLC using ethyl acetate: petroleum ether (20:80, v/v) as eluent. After completion of the reaction as indicated by TLC, 10 cm3 water was added to the reaction mixture. The precipitate formed was collected by filtration at pump and washed with water. The product thus obtained was recrystallized from ethanol to yield pure product (Table 2, method A) in high yields.
Method B: A mixture of 2-phenylacetaldehyde/butyraldehyde (1.0 mmol), aryl azide (1.1 mmol), and hydrated Bu4NOH (20 mol%) was placed in a 50-cm3 round-bottomed flask. The mixture was stirred at 100 °C for appropriate time as reported in Table 2, method B. The progress of the reaction was monitored by TLC using ethyl acetate: petroleum ether (20:80, v/v) as eluent. After completion of the reaction as indicated by TLC, 10 cm3 water was added to the reaction mixture. The precipitate formed was collected by filtration at pump and washed with water. The product thus obtained was recrystallized from ethanol to yield pure product (Table 2, method B) in high yields.
1-(3-Chloro-4-fluorophenyl)-4-phenyl-1H-1,2,3-triazole (1h, C14H9ClFN3)
White solid; 1H NMR (400 MHz, CDCl3): δ = 8.01 (s, 1H, triazolyl), 7.79-7.60 (m, 3H, Ar–H), 7.51-7.38 (m, 5H, Ar–H) ppm; 13C NMR (100 MHz, CDCl3): δ = 158.53, 147.98, 133.23, 132.80, 128.3, 127.18, 125.34, 124.38, 123.45, 122.70, 121.23, 116.34 ppm; HRMS (ESI): m/z = 273.3532 ([M + H]+).
References
Fray MJ, Bull DJ, Carr CL, Gautier ECL, Mowbray CE, Stobie A (2001) J Med Chem 44:1951
Velazquez S, Alvarez R, Perez C, Gago F, De C, Balzarin J, Camarasa MJ (1998) Antivir Chem Chemother 9:481
Genin MJ, Allwine DA, Anderson DJ, Barbachyn MR, Emmert DE, Garmon SA, Graber DR, Grega KC, Hester JB, Hutchinson DK, Morris J, Reischer RJ, Ford CW, Zurenko GE, Hamel JC, Schaadt RD, Stapert D, Yagi BH (2000) J Med Chem 43:953
Jordão AK, Ferreira VF, Souza TM, Faria GG, Machado V, Abrantes JL, Souza MC, Cunha AC (2011) Bioorg Med Chem 19:1860
Agalave SG, Maujan SR, Pore VS (2011) Chem Asian J 6:2696
Kume M, Kubota T, Kimura Y, Nakashimizu H, Motokawa K, Nakano M (1993) J Antibiot 46:177
Boddy IK, Briggs GG, Harrison RP, Jones TH, Omahony MJ, Marlow ID, Roberts BG, Willis RJ, Bardsley R, Reid J (1996) Pestic Sci 48:189
Buechel KH, Gold H, Frohberger PE, Kaspers H (1975) Fungicidal triphenyl-1,2,3-triazol-1-ylmethanes. German Patent DE 2407305, Aug 28, 1975; (1975) Chem Abstr 83:206290
Dalvie DK, Kalgutkar AS, Khojasteh-Bakht SC, Obach RS, Donnell JPO (2002) Chem Res Toxicol 15:269
Horne WS, Yadav MK, Stout CD, Ghadiri MR (2004) J Am Chem Soc 126:15366
Rostovtsev VV, Green LG, Fokin VV, Sharpless KB (2002) Angew Chem Int Ed 41:2596
Ornoe CW, Christensen C, Meldal M (2002) J Org Chem 67:3057
Ramachary DB, Shashank AB, Karthik S (2014) Angew Chem Int Ed 53:10420
Jia Q, Yang G, Chen L, Du Z, Wei J, Zhong Y, Wang J (2015) Eur J Org Chem 215:3435
Anastas PT, Kirchhoff MM (2002) Acc Chem Res 35:686
Mason TJ, Lorimer JP (1988) Sonochemistry: theory, application and uses of ultrasound in chemistry. Wiley, New York
Zhao DB, Fei ZF, Geldbach TJ, Scopelliti R, Dyson PJ (2004) J Am Chem Soc 126:15876
Noda A, Susan MABH, Kudo K, Mitsushima S, Hayamizu K, Watanabe MJ (2003) J Phys Chem B 107:4024
Xiang HF, Yin B, Wang H, Lin HW, Ge XW, Xie S, Chen CH (2010) Electrochim Acta 55:5204
Bond AH, Dietz ML, Rogers RD (1999) ACS Symp Ser 716:234
Fadeev AG, Meagher MM (2001) Chem Commun 3:295
Finan DA, Koval C, DuBois D, Noble RJ (2004) J Membr Sci 238:57
Ying AG, Wang LM, Deng HX, Chen JH, Chen XZ, Yeb WD (2009) Arkivoc 11:288
Khazaeia A, Veisib H, Safaeib M, Ahmadianb H (2014) J Sulfur Chem 35:270
Veisi H, Maleki B, Hameliana M, Ashra SS (2015) RSC Adv 5:6365
Otaka H, Ikeda J, Tanaka D, Tobe M (2015) Tetrahedron Lett 55:979
Singh H, Sindhu J, Khurana JM, Sharma C, Aneja KR (2014) Eur J Med Chem 77:145
Singh H, Kumari S, Khurana JM (2014) Chin Chem Lett 25:1336
Singh H, Sindhu J, Khurana JM (2013) J Iran Chem Soc 10:883
Singh H, Sindhu J, Khurana JM (2013) RSC Adv 3:22360
Marullo Anna FD, Rizzo C, Marullo RNS, Rizzo C, Noto R (2015) Ultrason Sonochem 23:317
Guo S, Lim MH, Huynh HV (2013) Organometallics 32:7225
Chen Z, Yan Q, Liu Z, Xu Y, Zhang Y (2013) Angew Chem Int Ed 52:13324
Ali A, Corrêa AG, Alves D, Zukerman-Schpector J, Westermann B, Ferreira MAB, Paixão MW (2014) Chem Commun 50:11926
Acknowledgments
H.S. and G.K. thank UGC-CSIR for grant of junior and senior research fellowships.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, H., Khanna, G., Nand, B. et al. Metal-free synthesis of 1,2,3-triazoles by azide–aldehyde cycloaddition under ultrasonic irradiation in TSIL [DBU-Bu]OH and in hydrated IL Bu4NOH under heating. Monatsh Chem 147, 1215–1219 (2016). https://doi.org/10.1007/s00706-015-1623-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-015-1623-4