Abstract
An efficient organocatalytic approach for the synthesis of 1,3-oxathiolanes and 1,3-thiazolidines is reported. In this methodology, conjugated base of nitromethane was employed as a potential organocatalyst to promote cyclization reaction between strained heterocyclic compounds and heterocumulenes at ambient conditions.
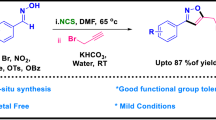
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Three-membered heterocyclic rings are valuable building block due to their ability to function as carbon electrophiles [1, 2]. These substrates undergo nucleophilic attack with heterocumulenes either at C-2 or C-3 position to afford corresponding heterocyclic compounds [3–6]. The extent of regioselectivity depends mainly on operating conditions and ring substituents [7, 8]. Furthermore, most of these nucleophilic ring opening (NRO) transformations rely on catalysts such as metal catalysts [9, 10] or organocatalysts to accomplish the desired transformation [11]. The advantages of organocatalysts include their lack of sensitivity to moisture and oxygen, low cost, and low toxicity, although their catalytic efficiency is usually lower than in metal-catalyzed processes in terms of turnover number. 1,3-Oxathiolane and 1,3-thiazolidine derivatives are found in a wide range of bioactive compounds [12, 13]. In recent years, several different methods for the synthesis of these heterocycles have been reported [14–17]. Hou has also disclosed a concise procedure for the construction of 1,3-thiazolidines using organophosphines [18]. These results encouraged us to examine alternative conditions for the synthesis of 1,3-thiazolidines and 1,3-oxathiolanes in the presence of Et3N in nitromethane (MeNO2).
Results and discussion
The reaction was initially examined using CS2 (1) and 2-methyloxirane (2a) in the presence of pyridine as a base. Stirring in MeNO2 at ambient conditions for 6 h gave 5-methyl-1,3-oxathiolane-2-thione (3a) in 69 % yield. To develop the reaction conditions a variety of bases and solvents were examined (Table 1). No reaction took place in the absence of a base even at higher temperatures. Among the bases examined, Et3N gave the best result. The yield was almost unchanged by the reducing amount of Et3N to 10 mol % (Table 1). Other solvents, such as THF, MeCN, DMF, hexane, and H2O were also examined; however, no transformation occurred in the absence of MeNO2 (Table 1). It could be deduced that MeNO2 involves in reaction progress beyond acting as the solvent. Finally, optimal results for this transformation were obtained when 10 mol % of Et3N in MeNO2 (2 cm3) was used, furnishing 3a in 94 % yield. The trans-structure of product was determined by the coupling constant of the two bridgehead hydrogens (3a, J = 11.4 Hz).

We next sought to explore the scope of the reaction (Table 2) using a wide range of oxiranes. Ring opening of alkyl-substituted oxiranes proceeded with good yields (Table 2). However, no reaction took place in the presence of cyclopentene and cyclooctene oxide as the oxirane sources due to the high strain energy of the product. Oxirane derived from cyclohexene gave excellent yield while cyclopentene oxide only afforded the product in moderate yield (Table 2). In the presence of alkyl-substituted oxiranes, the attack occurred exclusively at the terminal position, though styrene oxide gave benzylic-attacked product in high yield (Table 2). Isothiocyanates 4 were also examined and exhibited similar reactivity pattern to that observed with CS2. In all cases, the corresponding 1,3-oxathiolane-2-imine was obtained in good yield together with 3–8 % of hydrolyzed isothiocyanate. These products could be easily removed due to their different solubility from 5 (Table 2).

The reaction condition described above could also be extended to those with aziridines 6 (Table 3), providing corresponding heterocycles 7 or 8 in acceptable yields. However, aziridines 6 required longer reaction times to accomplish the transformation.

The structures of the products were confirmed by spectroscopic analyses. For example, the 1H NMR spectrum of 3a showed characteristic (AB)X spin system for the CH2-CH H-atoms, together with a doublet for the methyl group. The 13C NMR spectrum of 3a exhibited 4 signals in agreement with the proposed structure. The characteristic signal at 227 ppm belongs to the C-S double bond.
Although the mechanistic details of the formation of the products are not known, a plausible rationalization is proposed in Scheme 1. It is conceivable that the reaction starts with the formation of intermediate 11, followed by addition of oxirane to generate ring-opened intermediate 12. Cyclization of this adduct leads to formation of the intermediate 13, which is converted to 3a by elimination of the conjugated base of MeNO2. However, another possibility could involve the attack of 10 to heterocumulenes.

In conclusion, we have described an organocatalytic approach for the synthesis of functionalized 1,3-oxathiolanes and 1,3-thiazolidines. The reaction was completely regio-selective and in all cases only one regio-isomer was detected in NMR analyses. Electronic and steric variations of the substrates showed no appreciable change in the efficiency of the transformation. Several functional groups such as methyl methacrylate, alkoxy, phenoxy, allyl, and halide could be well tolerated. Using this procedure, a simple etheric extraction would suffice to isolate the pure product.
Experimental
Epoxides, CS2, nitromethane, bases, and solvents were obtained from Merck and were used without further purification. N-Tosylaziridines were prepared using the literature procedures [19]. M.p.: Electrothermal-9100 apparatus. IR Spectra: Shimadzu IR-460 spectrometer. 1H and 13C NMR spectra: Bruker DRX-500 AVANCE instrument; in CDCl3 at 500.1 and 125.7 MHz, resp; δ in ppm, J in Hz. EI-MS (70 eV): Finnigan-MAT-8430 mass spectrometer. Elemental analyses (C, H, N) were performed with a Heraeus CHN-O-Rapid analyzer. The results agreed favorably with the calculated values.
General procedure for the preparation of 3, 5, 7, and 8
To a stirred solution of heterocumulene (1–3 mmol) and strained heterocycle (1 mmol), 0.01 g Et3N (10 mol %) in 3 cm3 MeNO2 was added. The mixture was stirred for 6–10 h at 25 °C. After completion of the reaction (monitored by TLC), 5 cm3 of cold diethyl ether was added to the reaction mixture. Separation of etheric layer and removing of solvent under vacuo gave the pure title products. All the known compounds gave satisfactory spectroscopic values and are analog to spectroscopic data reported in literature [14, 16, 18].
5-Methyl-5-propyl-1,3-oxathiolane-2-thione (3j, C7H12OS2)
The crude product was purified by cold etheric extraction affording 0.16 g (94 %) 3j. Yellow oil; IR (KBr): \(\bar v\) = 3038, 2981, 1626, 1541, 1347, 1336, 1108 cm−1; 1H NMR (500 MHz, CDCl3): δ = 0.95 (t, 3 J = 6.4 Hz, Me), 1.21 (s, Me), 1.29–1.38 (m, 2 CH2), 3.35 (d, 2 J = 11.4 Hz, CH), 3.49 (d, 2 J = 11.4 Hz, CH) ppm; 13C NMR (125.7 MHz, CDCl3): δ = 13.5 (CH2), 14.2 (Me), 23.5 (Me), 41.1 (CH2), 52.9 (CH2), 84.3 (C), 228.2 (C) ppm; EI-MS (70 eV): m/z (%) = 176 (M+, 5), 160 (22), 116 (45), 100 (63), 85 (100), 76 (78).
Hexahydro-3aH-cyclohepta[d][1,3]oxathiole-2-thione (3 l, C8H12OS2)
The crude product was purified by cold etheric extraction affording 0.10 g (56 %) 3 l. Yellow oil; IR (KBr): \(\bar v\) = 3041, 2971, 1631, 1547, 1326, 1309, 1107 cm−1; 1H NMR (500 MHz, CDCl3): δ = 1.35–2.18 (m, 10H), 2.91–2.94 (m, CH), 4.08–4.11 (m, CH) ppm; 13C NMR (125.7 MHz, CDCl3): δ = 23.2 (CH2), 26.5 (CH), 30.1 (CH2), 30.8 (CH2), 32.6 (CH2), 51.8 (CH), 82.1 (CH), 227.9 (C) ppm; EI-MS (70 eV): m/z (%) = 188 (M+, 9), 112 (68), 96 (41), 76 (79) 54 (100).
N-(4,5-Diphenyl-1,3-oxathiolan-2-ylidene)benzenamine (5e, C21H17NOS)
The crude product was purified by cold etheric extraction affording 0.31 g (94 %) 5e. Pale yellow solid; m.p.: 112-115 °C; IR (KBr): \(\bar v\) = 3025, 2967, 1610, 1547, 1330, 1323, 1113 cm−1; 1H NMR (500 MHz, CDCl3): δ = 4.41 (s, CH), 5.76 (s, CH), 7.11–7.35 (m, 15 CH) ppm; 13C NMR (125.7 MHz, CDCl3): δ = 44.9 (CH), 70.1 (CH), 123.1 (2 CH), 127.3 (CH), 127.6 (CH), 128.1 (2 CH), 128.5 (2 CH), 128.7 (CH), 129.5 (2 CH), 129.8 (2 CH), 131.1 (2 CH), 135.6 (C), 137.5 (C), 148.9 (C), 162.5 (C) ppm; EI-MS (70 eV): m/z (%) = 331 (M+, 2), 196 (53), 177 (37), 119 (62), 77 (100).
N-(5-Methyl-5-propyl-1,3-oxathiolan-2-ylidene)benzenamine (5j, C13H17NOS)
The crude product was purified by cold etheric extraction affording 0.22 g (92 %) 5j. Pale yellow oil; IR (KBr): \(\bar v\) = 3038, 2970, 1617, 1548, 1321, 1310, 1108 cm−1; 1H NMR (500 MHz, CDCl3): δ = 0.93 (t, 3 J = 6.1 Hz, Me), 1.24 (s, Me), 1.27–1.36 (m, 2 CH2), 3.32 (d, 2 J = 11.0 Hz, CH), 3.45 (d, 2 J = 11.0 Hz, CH), 6.91 (d, 3 J = 6.8 Hz, 2 CH), 7.11 (t, 3 J = 6.5 Hz, CH), 7.28 (t, 3 J = 6.4 Hz, 2 CH) ppm; 13C NMR (125.7 MHz, CDCl3): δ = 13.2 (CH2), 14.9 (Me), 24.2 (Me), 39.6 (CH2), 43.2 (CH2), 73.9 (C), 121.7 (2 CH), 126.1 (CH), 129.6 (2 CH), 147.3 (C), 164.8 (C) ppm; EI-MS (70 eV): m/z (%) = 235 (M+, 6), 119 (58), 117 (36), 85 (63), 77 (100).
N-[5-(Phenoxymethyl)-1,3-oxathiolan-2-ylidene]propan-2-amine (5p, C13H17NO2S)
The crude product was purified by cold etheric extraction affording 0.22 g (86 %) 5p. Pale yellow oil; IR (KBr): \(\bar v\) = 3025, 2970, 1644, 1548, 1340, 1314, 1109 cm−1; 1H NMR (500 MHz, CDCl3): δ = 1.13 (d, 3 J = 6.7 Hz, 2 Me), 3.11–3.15 (m, CH), 3.26-3.32 (m, CH2), 4.31–4.35 (m, CH2), 4.93-4.98 (m, CH), 6.89 (d, 3 J = 6.7 Hz, 2 CH), 7.05 (t, 3 J = 6.3 Hz, CH), 7.17 (d, 3 J = 6.8 Hz, 2 CH) ppm; 13C NMR (125.7 MHz, CDCl3): δ = 20.1 (2 Me), 36.2 (CH2), 48.9 (CH), 69.3 (CH2), 81.3 (CH), 114.1 (2 CH), 121.3 (CH), 129.8 (2 CH), 159.8 (C), 167.1 (C) ppm; EI-MS (70 eV): m/z (%) = 251 (M+, 7), 134 (45), 118 (52), 77 (100), 58 (69).
4,5-Diphenyl-3-tosylthiazolidine-2-thione (7a, C22H19NO2S3)
The crude product was purified by cold etheric extraction affording 0.38 g (89 %) 7a. Pale yellow solid; m.p.: 113–115 °C; IR (KBr): \(\bar v\) = 3035, 2978, 1637, 1548, 1341, 1308, 1111 cm−1; 1H NMR (500 MHz, CDCl3): δ = 2.35 (s, Me), 4.42 (s, CH), 5.28 (s, CH), 7.08–7.28 (m, 10 CH), 7.33 (d, 3 J = 6.5 Hz, 2 CH), 7.85 (d, 3 J = 6.7 Hz, 2 CH) ppm; 13C NMR (125.7 MHz, CDCl3): δ = 23.5 (Me), 54.3 (CH), 68.7 (CH), 126.1 (CH), 126.8 (CH), 127.4 (2 CH), 127.8 (2 CH), 128.1 (2 CH), 128.4 (2 CH), 129.1 (2 CH), 130.0 (2 CH), 135.2 (C), 140.2 (C), 141.7 (C), 145.7 (C), 201.7 (C) ppm; EI-MS (70 eV): m/z (%) = 425 (M+, 5), 270 (26), 170 (69), 155 (52), 91 (42), 77 (100).
4-Methyl-4-pentyl-3-tosylthiazolidine-2-thione (7e, C16H23NO2S3)
The crude product was purified by cold etheric extraction affording 0.29 g (82 %) 7e. Pale yellow oil; IR (KBr): \(\bar v\) = 3037, 2967, 1638, 1551, 1344, 1312, 1108 cm−1; 1H NMR (500 MHz, CDCl3): δ = 1.09 (t, 3 J = 6.9 Hz, Me), 1.28 (s, Me), 1.41–1.63 (m, 3 CH2), 1.81 (t, 3 J = 6.7 Hz, CH2), 2.31 (s, Me), 3.41 (d, 2 J = 11.3 Hz, CH), 3.54 (d, 2 J = 11.3 Hz, CH), 7.33 (t, 3 J = 6.9 Hz, 2 CH), 7.82 (d, 3 J = 6.5 Hz, 2 CH) ppm; 13C NMR (125.7 MHz, CDCl3): δ = 15.1 (Me), 21.3 (Me), 22.4 (CH2), 24.0 (CH2), 25.6 (Me), 35.7 (CH2), 37.4 (CH2), 56.1 (CH2), 84.1 (C), 126.9 (2 CH), 131.4 (2 CH), 135.8 (C), 142.1 (C), 202.0 (C) ppm; EI-MS (70 eV): m/z (%) = 357 (M+, 10), 202 (21), 170 (69), 155 (57), 113 (100), 77 (43).
3-Tosylthiazolidine-2-thione (7f, C10H11NO2S3)
The crude product was purified by cold etheric extraction affording 0.26 g (96 %) 7f. Pale yellow oily solid; IR (KBr): \(\bar v\) = 3025, 2961, 1642, 1553, 1344, 1310, 1109 cm−1; 1H NMR (500 MHz, CDCl3): δ = 2.26 (s, Me), 3.46 (t, 3 J = 7.1 Hz, CH2), 4.60 (t, 3 J = 7.0 Hz, CH2), 7.31 (d, 3 J = 6.7 Hz, 2 CH), 7.81 (d, 3 J = 6.2 Hz, 2 CH) ppm; 13C NMR (125.7 MHz, CDCl3): δ = 24.4 (Me), 36.2 (CH2), 60.1 (CH2), 127.0 (2 CH), 130.0 (2 CH), 135.9 (C), 141.0 (C), 201.1 (C) ppm; EI-MS (70 eV): m/z (%) = 273 (M+, 1), 197 (36), 170 (100), 155 (69), 118 (41), 91 (58).
4-[2-(Trimethylsilyl)ethyl]-3-tosylthiazolidine-2-thione (7g, C15H23NO2S3Si)
The crude product was purified by cold etheric extraction affording 0.35 g (95 %) 7g. Pale yellow solid; m.p.: 98-100 °C; IR (KBr): \(\bar v\) = 3051, 2973, 1644, 1551, 1346, 1312, 1112 cm−1; 1H NMR (500 MHz, CDCl3): δ = 0.04 (s, 3 Me), 0.70 (t, 3 J = 6.8 Hz, CH2), 1.72–1.76 (m, CH2), 2.35 (s, Me), 3.46–3.49 (m, CH2), 5.12–5.16 (m, CH), 7.32 (d, 3 J = 6.5 Hz, 2 CH), 7.81 (d, 3 J = 6.7 Hz, 2 CH) ppm; 13C NMR (125.7 MHz, CDCl3): δ = 0.3 (3 Me), 13.4 (CH2), 24.4 (Me), 27.0 (CH2), 41.2 (CH2), 65.1 (CH), 126.1 (2 CH), 130.3 (2 CH), 136.8 (C), 142.6 (C), 198.6 (C) ppm; EI-MS (70 eV): m/z (%) = 373 (M+, 5), 218 (22), 170 (100), 155 (67), 91 (43).
4-[(Benzyloxy)methyl]-3-tosylthiazolidine-2-thione (7 h, C18H19NO3S3)
The crude product was purified by cold etheric extraction affording 0.35 g (90 %) 7 h. Pale yellow solid; m.p.: 119–121 °C; IR (KBr): \(\bar v\) = 3051, 2972, 1647, 1551, 1342, 1308, 1106 cm−1; 1H NMR (500 MHz, CDCl3): δ = 2.31 (s, Me), 3.26–3.38 (m, CH2), 4.48–4.71 (m, 2 CH2), 5.31–5.35 (m, CH), 7.11–7.25 (m, 5 CH), 7.32 (d, 3 J = 7.1 Hz, 2 CH), 7.81 (d, 3 J = 6.7 Hz, 2 CH) ppm; 13C NMR (125.7 MHz, CDCl3): δ = 24.1 (Me), 36.9 (CH2), 68.3 (CH), 73.7 (CH2), 80.4 (CH2), 126.2 (2 CH), 127.0 (CH), 128.3 (2 CH), 129.9 (2 CH), 131.4 (2 CH), 135.8 (C), 136.3 (C), 142.1 (C), 201.1 (C) ppm; EI-MS (70 eV): m/z (%) = 393 (M+, 2), 238 (37), 170 (72), 155 (69), 91 (100), 77 (59).
N-(4,5-Diphenyl-3-tosylthiazolidin-2-ylidene)benzenamine (8a, C28H24N2O2S2)
The crude product was purified by cold etheric extraction affording 0.41 g (85 %) 8a. Pale yellow solid; m.p.: 134–136 °C; IR (KBr): \(\bar v\) = 3046, 2981, 1623, 1548, 1338, 1305, 1109 cm−1; 1H NMR (500 MHz, CDCl3): δ = 2.32 (s, Me), 4.62 (s, CH), 5.38 (s, CH), 7.08-7.31 (m, 15 CH), 7.35 (d, 3 J = 6.7 Hz, 2 CH), 7.83 (d, 3 J = 6.9 Hz, 2 CH) ppm; 13C NMR (125.7 MHz, CDCl3): δ = 24.0 (Me), 45.1 (CH), 69.6 (CH), 121.2 (2 CH), 126.0 (CH), 127.1 (2 CH), 128.0 (2 CH), 128.2 (CH), 128.5 (CH), 128.7 (2 CH), 129.0 (2 CH), 129.5 (2 CH), 130.3 (2 CH), 132.5 (2 CH), 135.2 (C), 140.8 (C), 142.1 (C), 143.1 (C), 149.4 (C), 164.2 (C) ppm; EI-MS (70 eV): m/z (%) = 484 (M+, 2), 330 (42), 329 (11), 170 (72), 155 (52), 77 (100).
N-(4-Benzyl-3-tosylthiazolidin-2-ylidene)benzenamine (8c, C23H22N2O2S2)
The crude product was purified by cold etheric extraction affording 0.38 g (89 %) 8c. Pale yellow solid; m.p.: 100-102 °C; IR (KBr): \(\bar v\) = 3046, 2978, 1644, 1542, 1346, 1312, 1110 cm−1; 1H NMR (500 MHz, CDCl3): δ = 2.34 (s, Me), 2.79–2.85 (m, 2 CH), 3.41–3.47 (m, 2 CH), 5.13–5.16 (m, CH), 7.08–7.29 (m, 10 CH), 7.31 (d, 3 J = 6.8 Hz, 2 CH), 7.83 (d, 3 J = 6.4 Hz, 2 CH) ppm; 13C NMR (125.7 MHz, CDCl3): δ = 24.3 (Me), 35.9 (CH2), 43.7 (CH2), 67.9 (CH), 121.8 (2 CH), 125.3 (CH), 126.7 (2 CH), 127.1 (CH), 127.6 (2 CH), 128.0 (2 CH), 129.8 (2 CH), 131.4 (2 CH), 135.0 (C), 136.3 (C), 142.3 (C), 149.0 (C), 164.7 (C) ppm; EI-MS (70 eV): m/z (%) = 422 (M+, 5), 267 (18), 155 (69), 91 (100), 77 (58).
N-(3-Tosylthiazolidin-2-ylidene)benzenamine (8f, C16H16N2O2S2)
The crude product was purified by cold etheric extraction affording 0.31 g (94 %) 8f. Pale yellow solid; m.p.: 86–88 °C; IR (KBr): \(\bar v\) = 3037, 2977, 1652, 1549, 1342, 1311, 1110 cm−1; 1H NMR (500 MHz, CDCl3): δ = 2.24 (s, Me), 3.49 (t, 3 J = 6.7 Hz, CH2), 4.72 (t, 3 J = 6.5 Hz, CH2), 7.10 (d, 3 J = 6.9 Hz, 2 CH), 7.18–7.29 (m, 3 CH), 7.32 (d, 3 J = 6.5 Hz, 2 CH), 7.81 (d, 3 J = 6.9 Hz, 2 CH) ppm; 13C NMR (125.7 MHz, CDCl3): δ = 24.1 (Me), 34.9 (CH2), 59.8 (CH2), 121.1 (2 CH), 126.0 (CH), 128.2 (2 CH), 130.2 (2 CH), 131.7 (2 CH), 135.8 (C), 142.2 (C), 149.5 (C), 164.9 (C) ppm; EI-MS (70 eV): m/z (%) = 207 (M+, 2), 170 (61), 155 (48), 91 (39), 77 (100).
N-[4-[2-(Trimethylsilyl)ethyl]-3-tosylthiazolidin-2-ylidene]benzenamine (8g, C21H28N2O2S2Si)
The crude product was purified by cold etheric extraction affording 0.38 g (89 %) 8g. Pale yellow solid; m.p.: 116–118 °C; IR (KBr): \(\bar v\) = 3048, 2970, 1648, 1544, 1343, 1311, 1116 cm−1; 1H NMR (500 MHz, CDCl3): δ = 0.08 (s, 3 Me), 0.79 (t, 3 J = 7.1 Hz, CH2), 1.70–1.74 (m, CH2), 2.32 (s, Me), 3.39–3.45 (m, CH2), 5.08–5.12 (m, CH), 7.13 (d, 3 J = 6.9 Hz, CH), 7.18 (t, 3 J = 7.0 Hz, 2 CH), 7.25 (d, 3 J = 6.4 Hz, 2 CH), 7.34 (d, 3 J = 6.8 Hz, 2 CH), 7.83 (d, 3 J = 6.5 Hz, 2 CH) ppm; 13C NMR (125.7 MHz, CDCl3): δ = 0.5 (3 Me), 10.8 (CH2), 24.1 (Me), 29.3 (CH2), 36.6 (CH2), 62.3 (CH), 120.3 (2 CH), 126.7 (CH), 128.0 (2 CH), 130.6 (2 CH), 131.4 (2 CH), 135.2 (C), 141.4 (C), 149.48 (C), 165.8 (C) ppm; EI-MS (70 eV): m/z (%) = 432 (M+, 2), 277 (21), 170 (45), 155 (78), 77 (100).
N-[4-[(Benzyloxy)methyl]-3-tosylthiazolidin-2-ylidene]benzenamine (8h, C24H24N2O3S2)
The crude product was purified by cold etheric extraction affording 0.39 g (87 %) 8h. Pale yellow solid; m.p.: 132–134 °C; IR (KBr): \(\bar v\) = 3052, 2972, 1652, 1551, 1344, 1310, 1109 cm−1; 1H NMR (500 MHz, CDCl3): δ = 2.34 (s, Me), 3.32–3.48 (m, CH2), 4.51–4.79 (m, 2 CH2), 5.26–5.29 (m, CH), 7.11–7.29 (m, 10 CH), 7.31 (d, 3 J = 6.7 Hz, 2 CH), 7.82 (d, 3 J = 6.3 Hz, 2 CH) ppm; 13C NMR (125.7 MHz, CDCl3): δ = 24.2 (Me), 34.5 (CH2), 69.9 (CH), 71.2 (CH2), 81.1 (CH2), 121.3 (2 CH), 127.1 (CH), 127.5 (2 CH), 128.1 (CH), 129.1 (2 CH), 129.6 (2 CH), 130.1 (2 CH), 131.4 (2 CH), 135.0 (C), 137.4 (C), 143.4 (C), 149.9 (C), 165.7 (C) ppm; EI-MS (70 eV): m/z (%) = 452 (M+, 2), 297 (18), 170 (70), 155 (51), 91 (100), 77 (62).
N-(4-Benzyl-3-tosylthiazolidin-2-ylidene)-4-methylbenzenamine (8k, C24H24N2O2S2)
The crude product was purified by cold etheric extraction affording 0.39 g (89 %) 8k. Pale yellow solid; m.p.: 101–103 °C; IR (KBr): \(\bar v\) = 3046, 2977, 1652, 1550, 1343, 1310, 1107 cm−1; 1H NMR (500 MHz, CDCl3): δ = 2.29 (s, Me), 2.32 (s, Me), 2.73–2.80 (m, 2 CH), 3.35–3.43 (m, 2 CH), 5.15–5.19 (m, CH), 7.10 (d, 3 J = 6.7 Hz, 2 CH), 7.15–7.28 (m, 7 CH), 7.33 (d, 3 J = 6.5 Hz, 2 CH), 7.85 (d, 3 J = 6.9 Hz, 2 CH) ppm; 13C NMR (125.7 MHz, CDCl3): δ = 23.2 (Me), 24.5 (Me), 36.5 (CH2), 40.3 (CH2), 68.1 (CH), 123.1 (2 CH), 126.0 (CH), 127.1 (2 CH), 127.5 (2 CH), 128.0 (2 CH), 130.6 (2 CH), 132.1 (2 CH), 135.1 (C), 135.7 (C), 139.2 (C), 141.6 (C), 148.3 (C), 165.2 (C) ppm; EI-MS (70 eV): m/z (%) = 436 (M+, 2), 128 (23), 170 (38), 155 (45), 91 (100), 77 (63).
N-(4-Benzyl-3-tosylthiazolidin-2-ylidene)propan-2-amine (8l, C20H24N2O2S2)
The crude product was purified by cold etheric extraction affording 0.29 g (75 %) 8l. Pale yellow solid; m.p.: 67–69 °C; IR (KBr): \(\bar v\) = 3046, 2977, 1652, 1544, 1349, 1311, 1108 cm−1; 1H NMR (500 MHz, CDCl3): δ = 1.15 (d, 3 J = 7.0 Hz, 2 Me), 2.32 (s, Me), 2.78–2.89 (m, 2 CH), 3.20–3.41 (m, 3 CH), 5.11–5.14 (m, CH), 7.07 (d, 3 J = 6.4 Hz, 2 CH), 7.19 (t, 3 J = 7.1 Hz, CH), 7.25 (d, 3 J = 6.7 Hz, 2 CH), 7.31 (d, 3 J = 6.5 Hz, 2 CH), 7.82 (d, 3 J = 6.7 Hz, 2 CH) ppm; 13C NMR (125.7 MHz, CDCl3): δ = 21.1 (2 Me), 24.2 (Me), 37.1 (CH2), 41.5 (CH2), 49.2 (CH), 67.3 (CH), 125.2 (CH), 126.7 (2 CH), 127.3 (2 CH), 128.1 (2 CH), 130.3 (2 CH), 135.8 (C), 137.5 (C), 142.1 (C), 164.7 (C) ppm; EI-MS (70 eV): m/z (%) = 388 (M+, 1), 233 (31), 170 (58), 155 (46), 91 (100), 77 (62).
References
Yudin AK (ed) (2006) Aziridines and Epoxides in Organic Synthesis. Wiley-VCH, Weinheim
Hu XE (2004) Tetrahedron 60:2701
ShenYM, Duan WL, Shi M (2003) J Org Chem 68:1559
Nozaki K, Nakano K, Hiyama T (1999) J Am Chem Soc 121:11008
Tascedda P, Dunach E (2000) Chem Commun 6:449
Hancock MT, Pinhas AR (2003) Tetrahedron Lett 44:5457
Evans DA, Downey CW, Hubbs JL (2003) J Am Chem Soc 125:8706
McCoull W, Davisb FA (2000) Synthesis 10:1347
Ferraris D, Cox C, Lectka T (1998) J Org Chem 63:4568
Khumtaveeporn K, Alper H (1995) Acc Chem Res 28:414
Fan RH, Hou XL, Dai LX (2004) J Org Chem 69:689
Evans DA, Downey CW, Hubbs JL (2003) J Am Chem Soc 125:8706
Murugana R, Anbazhaganb S, Narayanan S (2009) Eur J Med Chem 44:3272
Yavari I, Ghazanfarpour-Darjani M, Hossaini Z, Sabbaghan M, Hosseini N (2008) Synlett 6:889
Yavari I, Ghazanfarpour-Darjani M (2014) J Sulfur Chem 35:477
Samzadeh-Kermani A (2014) Monatsh Chem 145:611
Sriharshaa SN, Satishb S, Shashikantha S, Raveeshab KA (2006) Bioorg Med Chem 14:7476
Wu JY, Luo ZB, Dai LX, Hou XL (2008) J Org Chem 73:9137
Ando T, Kano D, Minakata S, Ryu N, Komatsu M (1998) Tetrahedron 54:13485
Acknowledgments
This work was supported by the Young researchers and elite club of IRAN (research grant). We thank Islamic Azad University for additional unrestricted support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghazanfarpour-Darjani, M., Khodakarami, A. Organocatalytic one-pot synthesis of functionalized 1,3-oxathiolanes and 1,3-thiazolidines. Monatsh Chem 147, 829–835 (2016). https://doi.org/10.1007/s00706-015-1543-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-015-1543-3