Abstract
The voltammetric behavior of 5-nitroindazole was investigated at polished (p-AgSAE) and at mercury meniscus-modified (m-AgSAE) silver solid amalgam electrodes (inner diameter 0.5 mm) by direct current voltammetry and by differential pulse voltammetry. The results were compared with those obtained using hanging mercury drop electrode. Optimum conditions for differential pulse voltammetry determination of 5-nitroindazole were found in Britton-Robinson buffer of pH 8. The reaction mechanism was investigated using direct current voltammetry and elimination voltammetry with linear scan. Differential pulse voltammetry with optimized parameters was applied for determination of the compound in model and real samples. The limits of detection were calculated as 0.14 × 10−6 mol dm−3 for m-AgSAE and as 0.47 × 10−6 mol dm−3 for p-AgSAE. The proposed method was successfully applied for determination of 5-nitroindazole in real samples of river water.
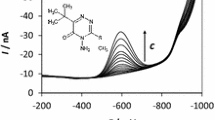
Graphical abstract

.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
5-Nitroindazole (5-NI, Fig. 1) belongs to the wide group of nitrated polycyclic aromatic hydrocarbons (NPAHs), which are formed during combustion processes [1]. NPAHs are intensively studied, because they have potential mutagenic and/or carcinogenic properties. NPAHs are ubiquitous environmental pollutants that have been detected in all environmental matrices [2]. The interest in these substances is obvious, because some of them are directly emitted to the atmosphere by diesel and petrol engines. Diesel engine exhaust belongs to the most important sources of NPAHs (more than 50 NPAHs were identified in the air [3]). These compounds are responsible for mutagenicity of engine exhausts, many of them even in the absence of exogenous metabolic activation. 5-NI is indazole derivative (nitro group is in the position 5 on the ring). It is utilized as part of the fixative solution in photographs [4] and as anti-fog agent in the preparation of thin metal surfaces for electronics [5]. 5-NI and its derivatives show wide spectrum of activities like antiprotozoality [6], antimalariality [7], and cytotoxicity [8, 9]. Derivatives of 5-NI are also studied as potential anticancer drugs [10]. This substance is harmful after ingestion and inhalation. It also irritates skin and eyes mucous membranes. It is also a proven mutagen [11]. Structure of 5-NI has been investigated by mass spectrometry, infrared spectrometry, and NMR; however, pure analytical studies are not known. Voltammetric methods have been utilized for determination of similar NPAHs such as 6-nitrobenzimidazole [12, 13], 5-nitrobenzimidazole [14, 15], and 2-amino-6-nitrobenzothiazole [16].
Electrochemical methods, especially voltammetric ones, are widely used in the field of environmental analysis [17–25]. Voltammetry has been used for investigation of nitro-group containing compounds for a long time. It utilizes primarily the reduction of nitro groups at the aromatic or heterocyclic ring [26]. Mercury seems to be the best electrode material for electroanalytical determination of these compounds. Unfortunately, strict ecological and safety rules and general fears make it difficult to utilize this material. Carbon paste electrodes [27], boron-doped diamond electrodes [28], solid composite electrodes [29–31], and other different types of solid electrodes can serve as substitutes for liquid mercury. For our research, we have used solid amalgam electrodes (SAEs) [32–34] which represent an intermediate step between mercury and solid working electrodes, e.g., [26, 28, 35–38]. The silver solid amalgam electrodes (AgSAE) were prepared from silver amalgam which is formed by mixing silver powder with liquid mercury. AgSAEs can be classified according to the modification of their surface, as polished (p-AgSAE), film modified (MF-AgSAE), and mercury meniscus modified (m-AgSAE) [32, 39]. Amalgam electrodes exhibit some advantages such as high hydrogen overvoltage and wide range of working potentials. They are also mechanically stable, easily handled with the possibility of electrochemical regeneration of the working surface [40, 41]. AgSAEs have been already successfully used for the determination of many inorganic [42], organic [43–45], and bioactive compounds [46–50]. The results achieved by AgSAEs are usually fully comparable with those achieved using other electrodes or techniques.
The main aim of this work was to develop a reliable method for the determination of 5-NI. This method is based on cathodic voltammetric response provided by the nitro-group of 5-NI, and its practical applicability is verified on model samples of real river water.
Results and discussion
Voltammetric behavior of 5-NI
Dependence on pH
The dependence of voltammetric behavior of 5-NI on pH was investigated using direct current voltammetry (DCV). First, the most suitable pH of the supporting electrolyte (Britton-Robinson buffer, BR buffer) was searched for. The used concentration of 5-NI for these purposes was 0.1 × 10−6 mol dm−3 for all three tested electrodes (m-AgSAE, for p-AgSAE, and for hanging mercury drop electrode (HMDE)). The tested pH range was from pH 2 to pH 12. Because the reduction of the nitro group was used for analytical purposes, only the cathodic polarization was studied. The reaction mechanisms of nitro compound reductions have been studied in many papers in last decades (e.g., [26, 51, 52]). Therefore, we could characterize the registered processes on the basis of well-known facts.
In the case of m-AgSAE, one well-developed peak was observed in the whole range of pH (at about −400 to −800 mV). This peak corresponds to irreversible four electron reduction of the nitro-group to the hydroxylamino group in acidic medium. In alkaline solutions, the mechanism is different; it consists of two steps. The first step includes a first irreversible uptake of one electron leading to the formation of nitro radical anion; the second step is a slow three electron irreversible reduction forming the hydroxylamino group [26, 53]. With increasing pH, the peak height decreased and the peak was shifted to more negative potential. In acidic (pH 2–5) and alkaline solutions (pH 9–12), the reproducibility of the signals was rather poor and the peak currents decreased with increasing pH. The current signals were better developed and reproducible at pH values from 6 to 8. Therefore, pH 8 was chosen as the optimum pH for voltammetric determination of 5-NI using m-AgSAE.
Afterwards, influence of pH on voltammetric behavior of 5-NI at p-AgSAE was investigated. We observed one peak corresponding to the reduction of nitro group in the whole tested pH range (2–12) (at about −400 to −800 mV). The best developed and reproducible peaks were obtained at pH 8.
The difference between reproducibility of measurements at pH 2–5 and 9–12 could be explained by different readiness to electron transfer and different adsorbability of protonated and unprotonated forms of 5-NI on the electrode surfaces which are formed in dependence on the pH value.
On both amalgam electrodes, a small peak at more positive potential area (about −200 mV) was observed in neutral and alkaline medium. It was proved that its appearance was connected with application of the regeneration potentials. It corresponded to the reversible two-electron reduction of nitroso group, which is formed at the electrode surfaces during application of negative regeneration potentials [53].
Finally, the voltammetric behavior of 5-NI at HMDE was studied in BR buffer (in the range of pH 2–12) (at about −400 to −800 mV). 5-NI yielded one cathodic peak corresponding to the reduction of nitro group in the whole pH range (similarly as described above). The best developed and the best reproducible peak was gained at pH 8 too.
The peak potentials were shifted toward more negative potentials with increasing pH. This can be explained by a preceding protonation of the nitro group, resulting in the decrease of the electron density at the nitro group which leads to easier electron acceptance at low pH values [16].
Regeneration of amalgam electrodes
Optimum regeneration potentials of amalgam electrodes were investigated by series of experiments. DCV measurements were carried out under found optimum pH value (pH 8) and under presence of 0.1 × 10−6 mol dm−3 concentration of 5-NI. The aim was to achieve the highest repeatability of current peaks. It was found that the best reproducibility was achieved for E reg1 = 0 mV and E reg2 = −1,200 mV. By application of these regeneration potentials the 20 times repeated registered current signals of 5-NI were almost constant (the values were normally distributed on both sides of their arithmetic mean, minimum, maximum values ± 2.7 %, none trend of data was statistical confirmed).
Because each measurement was realized using a new mercury drop at HMDE, the registered current could not be affected by the history of the electrode and none regeneration potential was applied.
Dependence on scan rate
The dependences of peak heights of reduction peaks of 5-NI on the scan rate were studied using DCV. The used concentration of 5-NI was equal (0.1 × 10−6 mol dm−3) for all investigated electrodes: m-AgSAE, p-AgSAE, and HMDE. The peak heights increased linearly with increasing square root of the scan rate (Eq. (1), (2)) in the range of scan rates from 0 to 160 mV s−1 at m-AgSAE and at HMDE:
where I P (nA) is a peak current, v (mV s−1) is a scan rate, and r is a correlation coefficient.
Therefore, it is possible to conclude that the above mentioned four-electron reduction processes at m-AgSAE and at HMDE are controlled by diffusion. On the contrary, the peak height was almost independent of scan rate at p-AgSAE (r amounted to −0.38 for linear dependence and −0.46 for square root dependence).
Elimination voltammetry with linear scan
Elimination voltammetry with linear scan (EVLS) was used for elucidation of electrode processes occurring at the surface of all tested electrodes. The elimination curves were calculated according to the Eqs. (3)–(9) in Ref. [54]. EVLS revealed that the controlling processes differed at each of the tested electrodes.
In the case of HMDE, three sets of scan rates were evaluated: from 20 to 160 mV s−1, from 40 to 320 mV s−1, and from 80 to 620 mV s−1.
It was proved that the registered peak corresponds to the reduction in adsorbed state in all three sets of scan rates. However, in the case of middle and high scan rate sets, it was revealed that simultaneously this reduction in adsorbed state is preceded by a kinetically controlled reaction. The counter peaks behind the elimination peak are smaller than it would correspond to the theoretical value of the ratio peak vs. counter peak in the case of reduction process in adsorbed state (i.e., 3:4 [55]). Therefore, the adsorption was relatively weaker at the mercury surface. However, it is well known that the four electron reaction cannot be realized in one step. Some of the possible variants of aryl (Ar) nitro compound reduction are discussed in [26, 56, 57] (e.g., reduction can take place via the dihydroxylamine ArN(OH)2, which dehydrates to yield the nitroso compound (ArNO), which is reduced to ArNHOH; or ArNO2 could be reduced to \({\text{ArNO}}_{{}}^{ \cdot - }\) (one electron step), which can be further reduced to ArNHOH (three electron step)). Therefore, some of these reactions realized in the first step can be kinetically controlled and they can precede the reduction realized in weakly adsorbed state.
On the other hand, the reaction processes observed at the surfaces of both of the tested amalgam electrodes are different
At m-AgSAE in any scan rate set at pH 8, the recorded voltammetric peak corresponded to the diffusion-controlled process. Any significant influence of adsorption had not been confirmed. This indicates that the application of adsorptive accumulation cannot improve the signal intensity. Nevertheless, the more positively registered elimination peaks are relatively broader, what indicates that this process consists of more sub-steps as mentioned above in the case of HMDE.
EVLS brought new information on reaction processes in the case of p-AgSAE. The registered voltammetric peak was split by EVLS into two peaks. Both reduction processes were controlled by diffusion. These processes were not affected by adsorption. However, the more negative voltammetric peak corresponded to the reduction preceded by a kinetically controlled reaction. In this case, the reactions occurring at the surface of p-AgSAE were slower and the second registered voltammetric signal (the more negative voltammetric peak) depended on the slower “generation” of compound(s) in the first step.
The EVLS confirmed that the electrode material (mercury, silver amalgam) and its state (liquid, solid) are crucial for the realized processes. The results showed that the registered reduction peak corresponded to the diffusion controlled process which is only partly influenced by adsorption. It is in fair agreement with the results described in the chapter “Dependence on scan rate”.
Log–log analysis
To explain the above achieved results, we applied log–log analysis. The results are summarized in Eq. (3) for m-AgSAE, in Eq. (4) for p-AgSAE, and in Eq. (5) for HMDE.
The slope calculated for HMDE confirmed that the prevailing controlling process is diffusion (in correspondence with the chapter “Dependence on scan rate”). Nevertheless, this value was not equal to 0.5. Hence, small contribution of adsorption was confirmed (in correspondence with results of EVLS).
The non-applicability of this log–log analysis was proved in the case of p-AgSAE, because the analyzed signal consisted of two signals (as it was proved by EVLS) which depended on applied scan rate in different ways. Therefore, logarithm of the peak height was almost (statistically) independent of logarithm of the scan rate.
Similarly, low value of the slope of this dependence in the case of m-AgSAE can be explained by the fact that the evaluated signal is composed of a few sub-steps which depended on applied scan rate in different ways.
Determination of 5-NI in model solution
Differential pulse voltammetry (DPV) was used for 5-NI determination in model solutions. The effect of the initial potential changes was studied in the potential range from +200 to −400 mV vs. Ag/AgCl/3 M KCl. The best reproducible and the best developed peak for all three tested electrodes was obtained using E in = +100 mV.
The effect of accumulation potential (E acc) on the cathodic peak height was tested from +100 to −500 mV vs. Ag/AgCl/3 M KCl. The concentration of 5-NI in the tested solution amounted to 0.1 × 10−6 mol dm−3 and scan rate of 10 mV s−1 was applied for all measurements. Nevertheless, no significant effect of either E acc or accumulation time (t acc) was observed. However, negligible adsorptive accumulation of the determined compound could be realized during scan between E in and E p.
Optimized parameters for DPV were investigated and were applied for determination of the studied compound. DPV with pulse width of 80 ms, pulse height of −50 mV for cathodic scans and with scan rate of 10 mV s−1, was used for determination of the tested compound using all three working electrodes. The optimum pH of BR buffer, used as the supporting electrolyte, was found to be eight for all three working electrodes. DPV was applied for measurements with m-AgSAE with the initial potential E in = +100 mV and final potential E fin = −1,200 mV. The potential range for DPV in the case of p-AgSAE was from E in = +100 mV to E fin = −1,200 mV and in the case of HMDE was E in = +100 mV and E fin = −1,500 mV.
The linear dynamic range (LDR) at m-AgSAE was found to be from 0.2 × 10−6 mol dm−3 to 2 × 10−6 mol dm−3 (Fig. 2) and can be described by Eq. (6) with r = −0.9972 (c in 10−6 mol dm−3):
Some other chemometric parameters were calculated using direct signal method according to IUPAC [58]: limit of decision (CC) 0.07 × 10−6 mol dm−3, limit of detection (LOD) 0.14 × 10−6 mol dm−3, and limit of quantification (LOQ) 0.2 × 10−6 mol dm−3. These parameters were achieved without application of any t acc. It is possible to suppose that these reached chemometric parameters in model samples seem to be sufficient for 5-NI determination in real samples (e.g., real water samples, soil solutions).
The LDR corresponding to the peak at p-AgSAE was found to be from 1 × 10−6 mol dm−3 to 10 × 10−6 mol dm−3 and can be described by Eq. (7) with r = −0.998 (Fig. 3).
The intercept in the Eq. (7) could be omitted on the level of significance α = 0.05. Other chemometric parameters were calculated using direct signal method according to IUPAC [58]: CC 0.23 × 10−6 mol dm−3, LOD 0.47 × 10−6 mol dm−3, and LOQ 0.71 × 10−6 mol dm−3.
The LDR at HMDE was found to be from 0.2 × 10−6 mol dm−3 to 10 × 10−6 mol dm−3, which can be described by Eq. (8) with r = −0.999.
The intercept in the Eq. (8) could be omitted on the level of significance α = 0.05. Other chemometric parameters were calculated using direct signal method according to IUPAC [58]: CC 0.06 × 10−6 mol dm−3, LOD 0.12 × 10−6 mol dm−3, and LOQ 0.18 × 10−6 mol dm−3.
Determination of 5-NI in the model solution was carried out by DPV with optimized parameters within LDRs of the tested electrodes. The validation parameters and the corresponding standard deviations (S.D.) of five repeated determinations realized within one day were calculated for some selected concentrations using m-AgSAE, p-AgSAE, and HMDE. The obtained data are shown in Table 1. The validation parameters, evaluated for ten repeated determinations realized within 1 week, are summarized in Table 2.
Accuracies of determinations of 5-NI, realized within 1 day, on different concentration levels achieved using m-AgSAE, p-AgSAE, and HMDE are summarized in Table 3 (5 repeated determinations were realized on each concentration level).
The determinations realized on the concentration levels given in Table 3 were repeated within 1 week. The achieved accuracies of ten determinations on each concentration level with all investigated electrodes were evaluated and summarized in Table 4.
It is evident that all three used working electrodes provided correct, accurate, and reproducible results within 1 day as well as within 1 week (α = 0.05).
Determination of 5-NI in real sample solutions
5-NI was also determined in real samples, because the tested working electrodes with optimized parameters (applying DPV) were proved as sensitive tools for voltammetric determination of this compound in model solutions. The declared content of 5-NI in analyzed river water (2 × 10−6 mol dm−3) was compared with obtained results. Procedure of preparation of the sample solution was described in the chapter “Preparation and analysis of real sample solutions” of this manuscript. The standard addition method was used for determination of 5-NI. For each determination, three standard additions were added at least. Every determination was five times repeated and particular RSDD(5) was calculated. The obtained results are summarized in Table 5. It is evident that the found amounts of 5-NI are consistent with its declared content. All three used working electrodes provided correct, accurate, and reproducible results. From the statistical point of view, they are equivalent (α = 0.05).
Conclusions
Voltammetric behavior of 5-NI at amalgam electrodes has not been reported up to now. Therefore, we have developed a reliable and simple method for determination of 5-NI in various environmental matrices using DPV at AgSAEs.
The voltammetric behavior of 5-NI was investigated using two modifications of silver solid amalgam electrodes, i.e., m-AgSAE, and p-AgSAE, and using HMDE for comparison in the present paper. It was found that this compound provided cathodic signal with all three electrodes in a wide range of pH. This reduction response was dependent on pH and the highest and the best reproducible DPV cathodic peak of 5-NI was obtained in slightly alkaline medium. Therefore, BR buffer of pH 8 was utilized for determination of this nitro compound using DPV at m-AgSAE, p-AgSAE, and HMDE under the found optimal working conditions. Some chemometric parameters were calculated (LOD (m-AgSAE) = 0.14 × 10−6 mol dm−3, LOD (p-AgSAE) = 0.47 × 10−6 mol dm−3, LOD (HMDE) = 0.12 × 10−6 mol dm−3). The achieved LODs at m-AgSAE and HMDE were almost the same and LOD at p-AgSAE was a bit higher (0.74 × 10−6 mol dm−3). Determinations of a few different concentration levels of 5-NI were five times repeated within 1 day and the obtained RSD of five repeated determinations (RSDD(5) < 3.0 %) confirmed a good reproducibility of the developed determinations with the tested amalgam electrodes and with the mercury electrode as well. Similar results were reached by repetition of measurements performed within 1 week (RSDD (10) < 4.4 %).
The statistical parameters (LOD, LOQ), achieved using m-AgSAE in case of 5-NI, were very close to those achieved using HMDE. Similarly, the sensitivity of this electrode was better than the sensitivity obtained using p-AgSAE. Therefore, it is possible to conclude that m-AgSAE could be used as substitution for HMDE. However, the utilization of p-AgSAE is not excluded too.
The applicability of the proposed method was verified by the determination of 5-NI in a real sample of river water. It was proved that the results achieved with the tested working amalgam electrodes were (from the statistical point of view, α = 0.05) equal to those achieved with HMDE, and they were consistent with the declared content of 5-NI. It can be concluded that the tested modifications of amalgam electrodes are suitable tools for voltammetric determination of 5-NI.
Experimental
The stock solution of 5-NI (10 × 10−3 mol dm−3) was prepared by dissolving 4.5 mg of solid substance 5-nitroindazole (Sigma-Aldrich, Czech Republic) in 250 cm3 of deionized water using ultrasonic bath (P-LAB, Czech Republic). It was stored in dark and cold. BR buffer solutions of pH values from 2 to 12 were prepared by mixing the proper amounts of alkaline component of 0.2 mol dm−3 NaOH (Lachema, Czech Republic) and of acidic components consisting of 0.04 mol dm−3 H3PO4, 0.04 mol dm−3 H3BO3, and 0.04 mol dm−3 CH3COOH (all Lachema, Czech Republic). All the other chemicals used were of analytical grade purity. For all the measurements, deionized water from Milli-Q-Gradient, Millipore, Prague, Czech Republic (conductivity <0.05 µS cm−1) was used.
Voltammetric measurements were executed with the computer-controlled Eco-Tribo Polarograph (Polaro-Sensors, Prague, Czech Republic), equipped by MultiElChem 3.1 software for Windows XP/7/8 (J. Heyrovský Institute of Physical Chemistry of the AS CR, v.v.i., Czech Republic). The AgSAE as the working electrode was utilized in two types of surface modifications: m-AgSAE with the working surface 0.382 ± 0.025 mm2 (level of significance, α < 0.05), and p-AgSAE with the surface 0.196 ± 0.015 mm2 (α < 0.05). HMDE with the surface 0.721 ± 0.022 mm2 (α < 0.05); the inner diameter of the capillary about 55 µm (all from Eco-Trend Plus, Prague, Czech Republic) was utilized as the working electrode for the sake of comparison.
Ag/AgCl/3 M KCl was used as the reference and platinum wire as the auxiliary electrode (both from Elektrochemicke detektory, Turnov, Czech Republic). The measurements were performed at laboratory temperature (25 ± 2 °C). Oxygen was removed from the measured solutions by bubbling nitrogen (purity class 4.6; Messer Technogas, Prague, Czech Republic) for 5 min. The values of pH were measured using pH-meter Jenway 3505 (Bibby Scientific Limited, UK).
Voltammetric measurements
For first set of studies of voltammetric behavior of 5-NI at m-AgSAE, p-AgSAE and at HMDE, cyclic voltammetry (CV) and DCV were applied. Dependences of the obtained signals on pH and on scan rate have been studied. DPV with pulse width of 80 ms, pulse height of −50 mV for cathodic scans and with scan rate of 10 mV s−1, was used for determination of the tested compound using all three working electrodes.
Purified nitrogen was passed through the sample solution for a period of 5 min before each measurement, and then nitrogen atmosphere was maintained above the solution in the cell during the measurement. The heights of DPV peaks as well as of DCV waves (in the following text the term “peak(s)” was used for both techniques) were evaluated from the straight line connecting the minima before and after the peak (tangent to the curve joining the beginning and the end of the given peak).
The minimum number of standard additions was five. Each measurement of the current signals was implemented in cycles composed of five times repeated records under the same conditions. In some cases, the first curve somewhat differed from others. Therefore, “first” curves were omitted from evaluations. The achieved results were evaluated according to [58] and using QC Expert statistical software (Trilobyte, Czech Republic) and MS Excel (Microsoft Inc., Czech Republic). The limits of decision (CC), detection (LOD) and quantification (LOQ) and the parameters of the calibration curves (e.g., slope, intercept) were calculated as described in [38, 58].
Preparation of silver solid amalgam electrodes
AgSAE (Eco-Trend Plus, Prague, Czech Republic, diameter 0.5 mm) was constructed from drawn-out glass tube, the bore of which near the tip was filled with solid amalgam which was connected to an electric contact. This electrode was scraped on a soft emery paper and then polished on polishing polyurethane pad, using polishing kit (Electrochemical Detectors, Turnov, Czech Republic), consisting of Al2O3 suspension (particle size 1.1 mm) and soft polishing Al2O3 powder (particle size 0.3 mm). After finishing this step, the p-AgSAE was ready for its following use. Usually, the electrode surface should be polished once a week in case of daily measurements. m-AgSAE was prepared by immersing of p-AgSAE into a small volume of liquid mercury for 15 s (meniscus was created).
Before starting the work as well as after every pause longer than 1 h, the electrode surfaces of both amalgam electrodes were activated in the solution of 0.2 mol dm−3 KCl by applying −2,200 mV vs. Ag/AgCl/3 M KCl electrode for 300 s whilst stirring. The regeneration step of the electrode surface was inserted into the “design” utility of particular measuring methods used in the computer software MultiElChem 3.1. The regeneration was realized directly in the supporting electrolyte [38].
Elimination voltammetry with linear scan
EVLS was utilized for elucidation of the registered electrochemical processes on the surface of tested electrodes. This method was also used to increase the voltammetric signals, and to achieve higher resolution [59, 60]. This method is based on elimination of some contributions from the total registered current [54, 55, 61, 62]. It is assumed that the total current is the sum of partial currents (see Eq. (9)), such as the charging current I c, the diffusion-controlled current I d, the kinetic current I k and the s.c. irreversible current I ir [37, 38].
Simultaneously, it is assumed that each particular current can be expressed in the following form (Eq. (10)):
where I j represents a particular current, W j(v) represents a function of the scan rate, Y j(E) is a function of the potential, and v is the scan rate [50, 54, 55, 61, 62].
Preparation and analysis of real sample solutions
Real sample preparation was analogical to that described in [38]. River water was sampled from the river Labe near the floodgate Velký Osek less than two miles southwest of the village Velký Osek (East Bohemia, Czech Republic). It is located on the river on 912 km by European kilometrage (with zero at the mouth of the Labe into the North Sea). At this place, Labe can be contaminated by number of pollution sources (providers of emissions are, e.g., Synthesia Pardubice; Veolia Voda Czech Republic, Rybitví; ČEZ electrical power station Chvaletice; Lučební závody Kolín). Further, Labe can be contaminated from runoff of agricultural area too. Moreover, also some problematic substances are emitted from households, e.g., residues of drugs, hormonal contraception or substances used in cosmetics and cleaning products. River water was sampled into a plastic bottle. If the sample was not analyzed immediately after sampling (until 4 h), it was stored in a refrigerator (4 °C). The river water was spiked with an appropriate amount of stock solution of 5-NI and further used as a real sample matrix. The final concentration of 5-NI amounted to 2 × 10−6 mol dm−3. The gained solutions were filtered through a glass frit funnel of porosity S4. The procedure for the DPV determination of 5-NI in the real samples was as follows: 5 cm−3 of filtered sample spiked by 5-NI was diluted to 5 cm−3 with the BR buffer of the appropriate pH and, after deaeration with nitrogen, DP voltammograms were recorded.
References
Yosypchuk O, Karasek J, Vyskocil V, Barek J, Peckova K (2012) Sci World J. doi:10.1100/2012/231986
Kobeticova K, Buchlebova J, Lana J, Sochova I, Hofman J (2008) Ecotoxicol Environ Saf 71:650
Arey J, Zielinska B, Atkinson R, Winer AM, Ramdahl T, Pitts JN (1986) Atmos Environ 20:2339
Fujita Y (2012) Plating method, light-transmitting electrically-conductive film and light-transmitting electromagnetic wave shield film. USA Patent US 8097143 B2, Jan 17, 2012; (2006) Chem Abstr 146:51623
Adkins JL, Patel DP (1993) Water soluble antifoggant for powder developer solutions. USA Patent US 5272045 A, Dec 21, 1993; (1994) Chem Abstr 120:231870
Olea-Azar C, Cerecetto H, Gerpe A, Gonzalez M, Aran VJ, Rigol C, Opazo L (2006) Spectrochim Acta A 63:36
Alho MM, Garcia-Sanchez RN, Nogal-Ruiz JJ, Escario JA, Gomez-Barrio A, Martinez-Fernandez AR, Aran VJ (2009) Chem Med Chem 4:78
Aran VJ, Ochoa C, Boiani L, Buccino P, Cerecetto H, Gerpe A, Gonzalez M, Montero D, Nogal JJ, Gomez-Barrio A, Azqueta A, de Cerain AL, Piro OE, Castellano EE (2005) Bioorg Med Chem 13:3197
Upadhyay A, Srivastava SK, Srivastava SD (2010) Eur J Med Chem 45:3541
Cheptea C, Sunel V, Stan C, Dorohoi DO (2012) Rev Roum Chim 57:229
Rosenfeld RJ, Garcin ED, Panda K, Andersson G, Aberg A, Wallace AV, Morris GM, Olson AJ, Stuehr DJ, Tainer JA, Getzoff ED (2002) Biochemistry 41:13915
Deylova D, Barek J, Vyskocil V (2009) Collect Czech Chem Commun 74:1443
Deylova D, Barek J, Vyskocil V (2011) Collect Czech Chem Commun 76:1317
Deylova D, Barek J (2012) XXXII Moderni Elektrochemicke Metody 27
Deylova D, Yosypchuk B, Vyskocil V, Barek J (2011) Electroanalysis 23:1548
Deylova D, Vyskocil V, Barek J (2012) Electrochim Acta 62:335
Gal M, Hives J, Sokolova R, Hromadova M, Kolivoska V, Pospisil L (2009) Collect Czech Chem Commun 74:1571
Sokolova R, Degano I, Hromadova M, Bulickova J, Gal M, Valasek M (2010) Collect Czech Chem Commun 75:1097
Gal M, Hromadova M, Pospisil L, Hives J, Sokolova R, Kolivoska V, Bulickova J (2010) Bioelectrochemistry 78:118
Sokolova R, Degano I, Ramesova S, Bulickova J, Hromadova M, Gal M, Fiedler J, Valasek M (2011) Electrochim Acta 56:7421
Dytrtova JJ, Sestakova I, Jakl M, Navratil T (2009) Electroanalysis 21:573
Matelkova K, Ossberger K, Hudak J, Vatral J, Boca R, Linert W (2013) Monatsh Chem 144:937
Moradi S, Ajloo D, Lashkarbolouki T, Alizadeh R, Saboury AA (2013) Monatsh Chem 144:1499
Raman N, Sobha S, Selvaganapathy M (2012) Monatsh Chem 143:1487
Sakthivel A, Raman N, Mitu L (2013) Monatsh Chem 144:605
Peckova K, Barek J, Navratil T, Yosypchuk B, Zima J (2009) Anal Lett 42:2339
Svancara I, Zima J (2011) Curr Org Chem 15:3043
Barek J, Fischer J, Navratil T, Peckova K, Yosypchuk B, Zima J (2007) Electroanalysis 19:2003
Navratil T (2011) Curr Org Chem 15:2996
Navratil T, Kopanica M (2002) Chem Listy 96:111
Navratil T, Kopanica M (2002) Crit Rev Anal Chem 32:153
Novotny L, Yosypchuk B (2000) Chem Listy 94:1118
Novotny L, Havran L, Josypchuk B, Fojta M (2000) Electroanalysis 12:960
Mikkelsen O, Schroder K (2000) Anal Lett 33:3253
Fadrna R, Yosypchuk B, Fojta M, Navratil T, Novotny L (2004) Anal Lett 37:399
Navratil T, Svancara I, Mrazova K, Novakova K, Sestakova I, Heyrovsky M, Pelclova D (2011) Mercury and mercury electrodes: the ultimate battle for the naked existence (A Consideration). In: Kalcher K, Metelka R, Svancara I, Vytras K (eds) Sensing in electroanalysis, vol 6. University Press Centre, Pardubice, p 23
Novakova K, Navratil T, Dytrtova JJ, Chylkova J (2013) Int J Electrochem Sci 8:1
Novakova K, Navratil T, Dytrtova JJ, Chylkova J (2013) J Solid State Electrochem 17:1517
Vyskocil V, Danhel A, Fischer J, Novotny V, Deylova D, Horakova E, Barek J, Yosypchuk B, Wang J (2010) Silver amalgam electrodes - a look back at the last five years of their development and applications. In: Vytras K, Kalcher K, Svancara I (eds) Sensing in electroanalysis, vol 5. University Press Centre, Pardubice, p 13
De Souza D, de Toledo RA, Mazo LH, Machado SAS (2005) Electroanalysis 17:2090
De Souza D, de Toledo RA, Suffredini HB, Mazo LH, Machado SAS (2006) Electroanalysis 18:605
Cizkova P, Navratil T, Sestakova I, Yosypchuk B (2007) Electroanalysis 19:161
Selesovska-Fadrna R, Navratil T, Vlcek M (2007) Chem Anal-Warsaw 52:911
Vankova L, Maixnerova L, Cizek K, Fischer J, Barek J, Navratil T, Yosypchuk B (2006) Chem Listy 100:1105
Danhel A, Peckova K, Cizek K, Barek J, Zima J, Yosypchuk B, Navratil T (2007) Chem Listy 101:144
Selesovska-Fadrna R, Fojta M, Navratil T, Chylkova J (2007) Anal Chim Acta 582:344
Fadrna R, Cahova-Kucharikova K, Havran L, Yosypchuk B, Fojta M (2005) Electroanalysis 17:452
Bandzuchova L, Selesovska R, Navratil T, Chylkova J (2011) Electrochim Acta 56:2411
Selesovska R, Bandzuchova L, Navratil T (2011) Electroanalysis 23:177
Selesovska R, Bandzuchova L, Navratil T, Chylkova J (2012) Electrochim Acta 60:375
Peckova K, Barek J, Zima J (2001) Chem Listy 95:709
Laviron E, Meunierprest R, Mathieu E (1994) J Electroanal Chem 371:251
Vyskocil V, Navratil T, Danhel A, Dedik J, Krejcova Z, Skvorova L, Tvrdikova J, Barek J (2011) Electroanalysis 23:129
Skopalova J, Navratil T (2007) Chem Anal-Warsaw 52:961
Trnkova L, Kizek R, Dracka O (2000) Electroanalysis 12:905
Laviron E, Meunierprest R, Lacasse R (1994) J Electroanal Chem 375:263
Laviron E, Vallat A, Meunierprest R (1994) J Electroanal Chem 379:427
Miller JN, Miller JC (2005) Statistics and chemometrics for analytical chemistry, 2nd edn. Pearson Education, Harlow
Trnkova L, Jelen F, Petrlova J, Adam V, Potesil D, Kizek R (2005) Sensors-Basel 5:448
Trnkova L, Novotny L, Serrano N, Klosova K, Polaskova P (2010) Electroanalysis 22:1873
Dracka O (1996) J Electroanal Chem 402:19
Trnkova L (2005) J Electroanal Chem 582:258
Acknowledgments
K. Nováková and T. Navrátil are grateful for the support of the Czech Science Foundation (project GA ČR No. P208/12/1645) and of University of Pardubice (grant No. SGSFChT_2014006). J. Barek and V. Vyskočil thank for support to the Ministry of Education, Youth and Sports of the Czech Republic (project LH 13002 Program KONTAKT II).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nováková, K., Hrdlička, V., Navrátil, T. et al. Determination of 5-nitroindazole using silver solid amalgam electrode. Monatsh Chem 146, 761–769 (2015). https://doi.org/10.1007/s00706-014-1346-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-014-1346-y