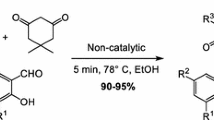
Abstract
Sodium acetate-catalyzed multicomponent reaction of aldehydes, 3-methyl-2-pyrazoline-5-one, and malononitrile initiated by grinding in mortar in the presence of small quantities of water results in the fast (15 min) and efficient formation of substituted pyrano[2,3-c]pyrazoles in 90–95 % yields. The developed fast multicomponent approach to the substituted pyrano[2,3-c]pyrazoles—the known pharmacologically active substances such as antibiotics, enzyme inhibitors, and anticancer drugs––is beneficial from the viewpoint of diversity-oriented large-scale processes and represents fast efficient and environmentally benign synthetic concept for multicomponent reactions strategy.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, the demand for clean and efficient chemical synthesis has become of serious importance and the elimination of volatile organic solvents in organic synthesis is now one of the most important goals in ‘green chemistry’ [1]. Among the proposed solutions, solvent-free conditions are most popular and it is often claimed that the best solvent is no solvent [2]. The implication of the solvent-free process in base-activated multicomponent reactions is highly promising as it allows for the combination of the synthetic virtues of the conventional multicomponent strategy with the ecological benefits and convenience of the solvent-free procedure.
The concept of “privileged medicinal scaffolds” has become one of the guiding principles in drug discovery [3, 4]. Privileged scaffolds commonly consist of rigid ring, including hetero ring, systems that present appended residues in well-defined orientations required for target recognition [4, 5].
Pyrano[2,3-c]pyrazole scaffold has a broad spectrum of biological activities [6–11]. Substituted pyrano[2,3-c]pyrazoles are known as different pharmacologically active substances, including antibiotics [6–8], enzyme inhibitors [9–11], and anticancer drugs [7, 8]. The methods of pyrano[2,3-c]pyrazoles synthesis have been long documented and consist of two main groups: (1) two-step synthesis [12–14] and (2) ‘one-pot’ multicomponent process. The multicomponent process usually includes Knoevenagel condensation of aldehyde and malononitrile, Michael reaction of formed Knoevenagel adduct with 3-methyl-2-pyrazolin-5-one, and final cyclization step to appropriate pyrano[2,3-c]pyrazole [15–21]. The main disadvantages of the known processes are large volumes of toxic solvents [15–18], 10–20 mol % of expensive or complex catalysts (indium(III) chloride [19], H14[NaP5W30O110] [20], ethylenediammonium diformate in PEG600 [21]) and specific reaction conditions (sonication [19] and microwave irradiation [20]). The four component methods starting from ethyl acetoacetate, hydrazine hydrate, malononitrile, and aldehydes having the same types of disadvantages are also known [22–25]. Besides, the known multicomponent methods are usually characterized by long and complex isolation stage.
Thus, the all known procedures for the synthesis of pyrano[2,3-c]pyrazoles have their merits, but the essence of fast facile and convenient multicomponent solventless methodology is yet not known and should to be developed.
Recently, we have accomplished solvent-free cascade and multicomponent assembling of 2-amino-4H-chromene scaffold from salicylaldehydes and malononitrile [26], salicylaldehydes and cyanoacetates [27], and both from salicylaldehyde, malononitrile or cyanoacetate, and nitroalkanes [28].
Considering our results on the solvent-free transformation of C–H acids and salicylaldehydes as well as the certain biomedical application of pyrano[2,3-c]pyrazoles mentioned above, we were prompted to design a convenient fast and facile solvent-free methodology for the efficient synthesis of substituted pyrano[2,3-c]pyrazoles based on multicomponent reaction of aldehydes, 3-methyl-2-pyrazoline-5-one, and malononitrile in connection with demands of ‘green chemistry’.
Results and discussion
As it follows from introduction we were prompted to design a fast convenient and facile solvent-free methodology for the efficient synthesis of functionalized pyrano[2,3-c]pyrazole system based on multicomponent reaction of aldehydes 1a–1g, 3-methyl-2-pyrazolin-5-one, and malononitrile. Thus, in the present study we report our results on multicomponent transformation of aldehydes 1a–1g, 3-methyl-2-pyrazolin-5-one, and malononitrile into substituted pyrano[2,3-c]pyrazoles 2a–2g under solvent-free conditions by grinding in mortar (Scheme 1; Tables 1, 2).

First, to evaluate the synthetic potential of the procedure proposed and to optimize the general conditions, the solvent-free transformation of aldehyde 1a, 3-methyl-2-pyrazolin-5-one, and malononitrile was studied under usual stirring conditions in mortar (Table 1). Solvent-free reaction of benzaldehyde (1a), 3-methyl-2-pyrazolin-5-one, and malononitrile in mortar with grinding without catalysts resulted in formation of [(5-hydroxy-3-methyl-1H-pyrazol-4-yl)(phenyl)methyl]malononitrile (3) in 45–71 % yield (Scheme 2; Table 1, entries 1–3).

Recently, we have found that Knoevenagel condensation of isatins with malononitrile which was performed by grinding at room temperature in the absence of any catalysts but in the presence of 1–5 equivalents of water resulted in formation of substituted (2-oxo-1,2-dihydro-3H-indol-3-ylidene)malononitriles in 89–99 % yields as result of the ‘on water’ reaction [29]. Thus, the next experiments were carried out in the presence of 0.5–2.0 cm3 of water (Table 1, entries 4–6). Addition of small quantity of water led to increasing yield of [(5-hydroxy-3-methyl-1H-pyrazol-4-yl)(phenyl)methyl]malononitrile (3) up to 80–90 % (Table 1, entries 4–6). However, under solvent-free conditions in the presence of catalytic quantities of NaOH or NaOAc, pyrano[2,3-c]pyrazole 2a was obtained in 65 and 70 % yields, respectively (Table 1, entries 7, 8). The best 95 % yield of pyrano[2,3-c]pyrazole 2a was achieved when the reaction was carried in the presence of 20 mol% of NaOAc and with addition of small quantity of water, reaction time 15 min (Table 1, entry 11). Under the optimal conditions thus found, aldehydes 1a–1g, 3-methyl-2-pyrazolin-5-one, and malononitrile were transformed into corresponding substituted pyrano[2,3-c]pyrazoles 2a–2g in 90–95 % yields (Table 2).
With the above results taken into consideration and the mechanistic data on solvent-free cascade process for the transformation of salicylaldehydes and malononitrile into substituted 2-amino-4H-chromenes [26], the following mechanism for the solvent-free cascade transformation of aldehydes 1a–1g, malononitrile, and 3-methyl-2-pyrazolin-5-one into substituted pyrano[2,3-c]pyrazoles 2a–2g is proposed. The initiation step of the catalytic cycle begins with the deprotonation of a molecule of malononitrile by the action of sodium acetate, which leads to the formation of malononitrile anion A (Scheme 3). The following process represents a typical multicomponent reaction. Knoevenagel condensation of the anion A with aldehyde 1 takes place with the elimination of a hydroxide anion and formation of Knoevenagel adduct 4 [30]. The subsequent hydroxide-promoted Michael addition of 3-methyl-2-pyrazolin-5-one to electron-deficient arylidenemalononitrile 4 results in formation of anions B and C. Cyclization of anion C with subsequent protonation of anion D leads to the formation of corresponding pyrano[2,3-c]pyrazoles 2 with the regeneration of malononitrile anion A at the last step of the catalytic cycle (Scheme 3).

The role of water in our multicomponent process is to accelerate all singular organic reactions. This effect of using water was known earlier [31]. Recently it was shown that several uni- and bimolecular reactions are greatly accelerated when carried out in vigorously stirred aqueous suspensions [32]. The experiments were performed with one or two liquids, water-insoluble reaction partners or, occasionally, a mixture of one liquid and one solid. Although the absence of detailed-kinetic experiments, the yields of pure products after varying reaction times convincingly demonstrate that the rates are higher than those under solvent-free (“neat”) or homogeneous conditions [32].
Thus, sodium acetate as catalyst can produce under grinding conditions in the presence of small quantities of water, a fast (15 min) and selective multicomponent transformation of aldehydes, 3-methyl-2-pyrazolin-5-one, and malononitrile into substituted pyrano[2,3-c]pyrazoles in 90–95 % yields. This new process opens an efficient and convenient multicomponent way to create substituted pyrano[2,3-c]pyrazoles, the promising compounds for different biomedical applications. This catalytic procedure utilizes simple equipment; it is easily carried out and is valuable from the viewpoint of environmentally benign diversity-oriented large-scale processes. This efficient and fast approach to substituted pyrano[2,3-c]pyrazoles represents a new synthetic concept for multicomponent reactions which integrate solvent-free and ‘on water’ reaction procedures, and allows for the combination of the synthetic virtues of solvent-free MCR with ecological benefits of ‘on water’ reactions and convenience of sodium acetate-catalyzed procedure.
Experimental
All melting points were measured with a Gallenkamp melting-point apparatus. 1H and 13C NMR spectra were recorded in DMSO-d 6 with a Bruker Avance II 300 spectrometer at ambient temperature. Chemical shift values are relative to Me4Si. IR spectra were recorded with a Bruker ALPHA-T FT-IR spectrometer in KBr pellets. Mass spectra (EI, 70 eV) were obtained directly with a Kratos MS-30 spectrometer. All chemicals used in this study were commercially available.
General procedure
A mixture of benzaldehyde 1 (2 mmol), 0.13 g malononitrile (2 mmol), 0.20 g 3-methyl-2-pyrazolin-5-one (2 mmol), and 0.02 g sodium acetate (0.4 mmol) in the presence of 1 cm3 of H2O was grinded thoroughly in mortar for 15 min. The resulting mixture was air dried to cause crystallization of the product. Crude solid was then put on filter, rinsed with water (2 × 2 cm3), and dried with water pump.
6-Amino-3-methyl-4-phenyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (2a)
Yield 95 %; m.p.: 244–245 °C (Ref. [15] m.p.: 244–245 °C and 1H NMR data).
6-Amino-3-methyl-4-(4-methylphenyl)-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (2b)
Yield 91 %; m.p.: 197–198 °C (Ref. [15] m.p.:197–198 °C and 1H NMR data).
6-Amino-4-(4-tert-butylphenyl)-3-methyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (2c, C18H20N4O)
Yield 93 %; m.p.: 230–231 °C; 1H NMR (300 MHz, DMSO-d 6 ): δ = 1.26 (s, 9H, 3 CH3), 1.80 (s, 3H, CH3), 4.54 (s, 1H, CH), 6.83 (s, 2H, NH2), 7.08 (d, J = 8.1 Hz, 2H, Ar), 7.33 (d, J = 8.1 Hz, 2H, Ar), 12.31 (s, 1H, NH) ppm; 13C NMR (75 MHz, DMSO-d 6 ): δ = 9.8, 31.2 (3C), 34.1, 35.7, 57.3, 97.7, 120.9, 125.1 (2C), 127.0 (2C), 135.5, 141.5, 148.8, 154.7, 160.9 ppm; IR (KBr): \(\bar{v}\) = 3,478, 3,242, 3,130, 2,965, 2,195, 1,638, 1,594, 1,488, 1,400, 1,054 cm−1; MS (EI, 70 eV): m/z (%) = 308 ([M]+, 43), 293 (9), 251 (21), 242 (74), 227 (37), 185 (15), 176 (98), 175 (100), 141 (15), 115 (16).
6-Amino-4-(4-methoxyphenyl)-3-methyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (2d)
Yield 90 %; m.p.: 224–225 °C (Ref. [15] m.p.: 225–226 °C and 1H NMR data).
6-Amino-4-(4-chlorophenyl)-3-methyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (2e)
Yield 92 %; m.p.: 250–252 °C (Ref. [15] 252–253 °C and 1H NMR data).
6-Amino-4-(3-bromophenyl)-3-methyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (2f)
Yield 91 %; m.p.: 224–225 °C (Ref. [15] m.p.: 223–224 °C and 1H NMR data).
6-Amino-4-(4-fluorophenyl)-3-methyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (2g)
Yield 93 %; m.p.: 222–223 °C (Ref. [15] m.p.: 223–224 °C and 1H NMR data).
[(5-Hydroxy-3-methyl-1H-pyrazol-4-yl)(phenyl)methyl]malononitrile (3)
Yield 90 %; m.p.: 256–258 °C (Ref. [15] m.p.: 258–259 °C and 1H NMR data).
References
Corma A, Garcia H (2003) Chem Rev 103:4307
Sheldon RA (2000) Pure Appl Chem 72:1233
Evans BE, Rittle KE, Bock G, DiPardo RM, Freidinger FM, Whitter WL, Lundell GF, Veber DF, Anderson PS, Chang RSL, Lotti VG, Cerino DJ, Chen TB, Kling PJ, Kunkel KA, Springer JP, Hirshfield J (1988) J Med Chem 31:2235
Poupaert O, Carato P, Colacino E (2005) Curr Med Chem 12:877
Song Y, Zhan P, Zhang QZ, Liu XY (2013) Curr Pharm Design 19:1528
Mishriky N, Girgis AS, Asaad FM, Ibrahim YA, Sobieh UI, Fawzy NG (2001) Boll Chim Farm 140:129
Coen DM, Loregian A (2006) Antiviral methods and compositions. International Patent WO 2006019955, Feb 23, 2006
Coen DM, Loregian A (2007) Chem Abstr 147:398624
Foloppe N, Fisher LM, Howes R, Potter A, Robertson AGS, Surgenor AE (2006) Bioorg Med Chem 14:4792
Kaiser D, Terfloth L, Kopp S, Schulz J, de Laet R, Chiba P, Ecker GF, Gasteiger J (2007) J Med Chem 50:1698
La Motta C, Sartini S, Tuccinardi T, Nerini E, Da Settimo F, Martinelli A (2009) J Med Chem 52:964
Abdou S, Fahmy SM, Sadek KU, Elnagdi MH (1981) Heterocycles 16:177
Elnagdi MH, Abdel-Motaleb RM, Mustafa M, Zayed MF, Kamel EM (1987) J Heterocycl Chem 24:1677
Abdel-Latif FF (1990) Z Naturforsch B 45:1675
Sharanin YA, Sharanina LG, Puzanova VV (1983) J Org Chem USSR 19:2291
Sheibani H, Babaie M (2010) Synth Commun 40:257
Shanthi G, Subbulakshmi G, Perumal PT (2007) Tetrahedron 63:2057
Heravi MM, Ghods A, Derikvand F, Bakhtiari K, Bamoharram FF (2010) J Iran Chem Soc 7:615
Dandia A, Jain AK, Bhati DS (2011) Synth Commun 41:2905
Zhou JF, Tu SJ, Zhu HQ, Zhi SJ (2002) Synth Commun 32:3363
Thakur A, Tripathi M, Rajesh UC, Rawat DS (2013) RCS Advances 3:18142
Al-Matar HM, Khalil KD, Adam AY, Elnagdi MH (2010) Molecules 15:6619
Mecadon H, Rohman MR, Kharbangar I, Laloo BM, Kharkongor I, Rajbangshi M, Myrboh B (2011) Tetrahedron Lett 52:3228
Kshirsagar SW, Patil NR, Samant SD (2011) Synth Commun 41:1320
Darandale SN, Sangshetti JN, Shinde DB (2012) J Korean Chem Soc 56:328
Elinson MN, Medvedev MG, Ilovaisky AI, Merkulova VM, Zaimovskaya TA, Nikishin GI (2013) Mendeleev Commun 23:94
Elinson MN, Nasybullin RF, Ryzhkov FV, Zaimovskaya TA, Egorov MP (2014) Monatsh Chem 145:605
Elinson MN, Ilovaisky AI, Merkulova VM, Chizhov AO, Belyakov PA, Nikishin GI (2010) Tetrahedron 66:4043
Demchuk DV, Elinson MN, Nikishin GI (2011) Mendeleev Commun 21:224
Patai S, Israeli Y (1960) J Chem Soc: 2025
Lindström NM (ed) (2007) Organic reactions in water: principles, strategies and application. Blackwell, New York
Narayan S, Muldoon J, Finn MJ, Fokin VV, Kolb HC, Sharpless KB (2005) Angew Chem Int Ed 44:3275
Acknowledgments
The authors gratefully acknowledge the financial support of the Russian Foundation for Basic Research (Project No. 13-03-00096a).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elinson, M.N., Nasybullin, R.F., Ryzhkov, F.V. et al. Solvent-free and ‘on-water’ multicomponent assembling of aldehydes, 3-methyl-2-pyrazoline-5-one, and malononitrile: fast and efficient approach to medicinally relevant pyrano[2,3-c]pyrazole scaffold. Monatsh Chem 146, 631–635 (2015). https://doi.org/10.1007/s00706-014-1318-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-014-1318-2