Abstract
Pseudorabies virus (PRV) is an important pathogen that can cause harm to the pig population. Since 2011, there have been a number of large-scale outbreaks of pseudorabies on Chinese farms where animals had been vaccinated with the Bartha-K61 vaccine. In order to understand the epidemiological trend and genetic variations of PRV in Guangxi province, China, 819 tissue samples were collected from swine farms where PRV infection was suspected from 2013 to 2019, and these were tested for infectious wild strains of PRV. The results showed a positive rate of PRV in Guangxi province of 28.21% (231/819). Thirty-six wild-type PRV strains were successfully isolated from PRV-positive tissue samples, and a genetic evolutionary analysis was performed based on the gB, gC, gD, gE, and TK genes. Thirty of the PRV strains were found to be closely related to the Chinese variant strains HeN1-China-2012 and HLJ8-China-2013. In addition, five PRV strains were genetically related to Chinese classical strains, and one isolate was a recombinant of the PRV variant and the vaccine strain Bartha-K61. Amino acid sequence analysis showed that all 36 PRV strains had characteristic variant sites in the amino acid sequences of the gB, gC, gD, and gE proteins. Pathogenicity analysis showed that, compared to classical PRV strains, the PRV variant strains were more pathogenic in mice and had a lower LD50. Taken together, our results show that wild-type PRV infections are common on pig farms in Guangxi province of China and that the dominant prevalent strains were those of the PRV variants. The PRV variant strains also had increased pathogenicity in mice. Our data will provide a useful reference for understanding the prevalence and genetic evolution of PRV in China.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pseudorabies (PR), also known as Aujeszky disease, is an acute infectious disease caused by pseudorabies virus (PRV). It is one of the important infectious diseases endangering the pig industry, and it has caused huge economic losses to the farming communities in China [1]. PRV is a linear double-stranded DNA virus belonging to the family Orthoherpesviridae and subfamily Alphaherpesvirinae [2]. A variety of animals can be infected with PRV, but pigs are the only known natural reservoir of the virus [3]. PRV infection mainly affects the respiratory, nervous, and reproductive systems [4]. Clinically, its symptoms in pregnant sows are often manifested as abortions, stillbirths, and the production of mummified fetuses. It can also infect the fetus through vertical transmission, and piglets infected with PRV usually show neurological signs as well as diarrhea and vomiting [5, 6]. In addition, several cases of cross-species transmission of PRV to humans have been reported in China in recent years. Researchers have successfully isolated PRV strains from the cerebrospinal fluid of patients, and these were determined by genetic evolutionary analysis to be closely related to PRV variants prevalent in China [7, 8]. These data suggested that PRV can cross the species barrier and be transmitted to humans, thus showing its importance for public health.
PR was first reported in China in the 1950s, and it subsequently became widespread in the country, leading to considerable economic losses. In 1979, China introduced a weak strain of Barth-K61 from Hungary and successfully developed a lyophilized vaccine, which effectively controlled outbreaks of PR in the pig population [9]. However, since 2011, there have been some large outbreaks of PR on vaccine-immunized pig farms in China. Genetic evolutionary analysis showed that the newly discovered strains had either sequential or single amino acid insertions or deletions and mutations in the main protective antigens when compared to the classical strains [10, 11]. This led to an increase in the virulence of the PRV strains, making the traditional Bartha-K61 vaccine inadequate to provide complete protection against the newly evolved PRV mutants [12,13,14]. Different PRV strains differ in their genetic characteristics and biological properties, and systematic genotyping is essential. The gC gene is one of the major virulence genes and has the highest mutation rate among the glycoprotein-encoding genes [15]. Sequencing of the gC gene can be used to distinguish PRV genotypes. Phylogenetic analysis based on the gC gene has shown that PRV can be classified into two major genotypes [16]. Genotype I PRV strains are mainly prevalent in Europe and North America, and PR has been eliminated in Canada, Mexico, and the United States. Genotype II PRV strains are prevalent in Asian countries, mainly in China [17]. Due to the significant differences in pathogenicity and genetic background of the currently prevalent strains in China, genotype II PRV strains are further divided into two subtypes: the classical and variant strains [16]. The morbidity and mortality rates have increased significantly in China, and this has been accompanied by the widespread prevalence of PRV variant strains. This has, in turn, caused great losses to the Chinese pig industry [18, 19].
To understand the situation regarding wild-type PRV infections in Guangxi Province, China, in this study, a total of 819 tissue samples were collected from swine farms where PRV infection was suspected from 2013-2019. The total positive rate of PRV was determined, and the wild-type PRV strains were isolated using cultured Vero cells. The gB, gC, gD, gE, and TK genes of the isolated strains were amplified and sequenced in order to study their genetic evolution and pathogenicity. Our results will lead to a better understanding of the evolution of PRV in China, and this study will provide a reference for the future clinical prevention and control of these viruses.
Materials and methods
Cells, animals, and statement of ethics
Vero and PK-15 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Gibco, Carlsbad, CA, USA) containing antibiotic/antimycotic solution (Hyclone, Logan, UT, USA) at 37°C in a 5% CO2 incubator. Specific-pathogen-free (SPF) BALB/c mice were purchased from Guangdong Provincial Medical Laboratory Animal Center (Guangzhou, China). Our study was approved by the Ethics Committee of Animal Experiments of Guangxi University (protocol number: GXU2019-025).
Sample collection and virus detection
From June 2013 to August 2019, 819 tissue samples, including brains, lungs, tonsils, lymph nodes, aborted fetuses, and fetal coats, were collected from Barth-K61-vaccinated swine farms with suspected PRV infections in 13 cities in Guangxi province of China. The samples were ground in sterile PBS under aseptic conditions. After three cycles of freezing and thawing, the supernatants were collected by centrifugation at 12,000 rpm for 5 min. Viral DNA was extracted using a Viral Genomics DNA Isolation Kit (Axygen Scientific, Silicon Valley, USA) according to the manufacturer's instructions. PRV-positive samples were identified by PCR, using the primer pairs targeting the gE gene (PRVE-F: 5'-CGGCTTCCACTCGCAGCTCTCTTCTC-3', PRVE-R: 5'-TGTGGGTCATCACGAGCACGTACAGC-3'). The PCR products were subjected to electrophoresis in 1.5% agarose gels, and these were visualized under UV light (Bio-Rad Inc., USA).
Virus isolation, identification, and viral titer determination
PRV-positive tissue supernatants were filtered through 0.22-µm filters. The filtrates were co-incubated with confluent monolayers of Vero cells in a constant-temperature cell culture incubator at 37℃ in an atmosphere of 5% CO2 for 1 h. The supernatants were then discarded, and the cells were maintained in DMEM containing 2% fetal bovine serum (FBS) and 100 U of penicillin and 100 μg of streptomycin per mL. The presence of a cytopathic effect (CPE) in the infected Vero cells was observed and recorded twice daily. When 80% of the cells showed stable CPE, the cell supernatant was collected and subjected to three freeze-thaw cycles. After three blind passages, the cell supernatants were used in plaque purification assays, and PCR was used for virus identification.
Vero cells were transferred to 96-well cell culture plates and cultured in a 5% CO2 cell incubator until they formed confluent monolayers. Several representative PRV strains isolated in this study and the vaccine strain Bartha-K61 were serially diluted tenfold with DMEM containing 2% FBS, and 101- to 1010-fold dilutions were added to the monolayers. An equal amount of DMEM containing 2% FBS was used as a negative control. The cell culture plates were kept in a 5% CO2 incubator at 37℃ for 96 h and observed for the development of CPE The median tissue culture infectious dose (TCID50) was calculated by the Reed-Muench method [20].
Plaque assay and multi-step growth curve
Virus preparations were frozen and thawed three times and then centrifuged at 10,000 rpm for 5 minutes. Viral plaque assays were performed using PK-15 cell monolayers in six-well plates. Serial tenfold dilutions of the virus sample were inoculated onto the PK-15 cells, which were then incubated at 37 °C for 1 h. Next, the cells were covered with a mixture of MEM containing 2% FBS and 1% low-melting-point agarose and incubated at 37 °C with 2% CO2 for 3 days. After careful removal of the medium, the cells were fixed with 4% paraformaldehyde for 6 h and then stained with a solution containing 0.5% crystal violet and 25% formaldehyde for 30 min. The plaques were visualized after washing the cells with tap water.
PK-15 cell monolayers in 12-well plates were infected with five different PRV strains (GXBB1776-2013, GXNN2020-2014, GXLB1918-2013, GXGG1901-2013, and Bartha-K61) at an MOI of 0.01. After incubation at 37°C for 1 h, the cells were washed twice with PBS and then cultured in DMEM containing 2% FBS. Three hundred μL of cell supernatant was collected at 6, 12, 24, 36, 48, 72, and 96 hours postinfection (hpi). The TCID50 of the virus was calculated by the Reed-Muench method [20], and three independent replicates of each experiment were used to determine the mean titer.
Gene amplification, sequencing, and phylogenetic analysis
Viral DNA was extracted from the virus preparations, and the full-length PRV gB, gC, gD, gE, and TK genes were amplified by PCR with the specific primer pairs shown in Table 1. The PCR products were then purified using a TIANgel Midi Purification Kit (Tiangen Biotechnique Inc., Beijing, China) and ligated into the pMD18-T vector (TAKARA, Shiga, Japan). Positive plasmids were identified and used for gene sequencing by Sangon Biotech Co., Ltd., Shanghai, China. The nucleotide sequences obtained in this study were submitted to the GenBank database under the accession numbers listed in Supplementary Table S1. The full-length sequences of gB, gC, gD, gE, and TK genes of 17 reference PRV strains were downloaded from the NCBI database, and the names, accession numbers, and geographical regions of origin of the reference strains are shown in Table 2. The nucleotide and deduced amino acid sequence of the isolates and reference strains were compared using DNASTAR Lasergene.v7.1, and phylogenetic trees were constructed by the maximum-likelihood (ML) method, using Molecular Evolutionary Genetics (MEGA; version 5.1) software.
Pathogenicity in mice
Six-week-old SPF BALB/c mice (n = 105) were randomly divided into four experimental groups (25 in each group) and one negative control group (n = 5). Each of the test groups received 103 to 107 TCID50 of the virus. Each mouse was injected subcutaneously in the groin with 0.2 mL of the virus preparation. At the same time, five mice were designated as negative controls and injected with an equivalent volume of DMEM. After inoculation, the mice were observed each day for clinical symptoms and death, and the median lethal dose (LD50) of the virus strains were calculated by the Reed-Muench method.
Statistical analysis
The chi-square test was used for statistical analysis (SPSS v 22.0, IBM, Chicago, IL, USA). P < 0.05 was considered statistically significant.
Results
Prevalence of wild -type PRV infection in Guangxi province
During the period of June 2013-August 2019, a total of 819 tissue samples were collected from various swine farms in Guangxi Province where PRV infections were suspected and tested using a PCR assay. Two hundred thirty-one positive samples were identified, with an overall positivity rate of 25.27% (231/819). The PRV positivity rates for each year were 25.27% (23/91), 26.15% (34/130), 17.94% (28/156), 32.91% (52/158), 20.33% (37/182), 55.41% (41/74), and 57.14% (16/28), respectively, from 2013 to 2019 (Table 3). These data showed an increasing trend of infection in recent years, suggesting that wild-type PRV infection is common on swine farms in this region of the country.
Virus isolation and in vitro growth characteristics
To investigate the epidemiological characteristics of wild-type PRV strains in Guangxi province, tissue sample supernatants from the 231 samples that tested positive for PRV were inoculated onto Vero cells for virus isolation. After three blind passages in Vero cells, the cells showed a typical CPE, including rounding, shedding, and aggregation (Fig. 1A). These cell supernatants were collected and used for multiple rounds of plaque purification and PCR measurements. In total, 36 wild-type PRV strains were successfully isolated in this study. The titers of 16 of these isolates were determined in Vero cells. As shown in Table 4, the viral titers ranged from 105.7 to 107.7 TCID50/0.1 mL, indicating that the wild-type PRV strains isolated in this study grew well in Vero cells.
In vitro biological characteristics of the isolated PRV strains. (A) Vero cells and PK-15 cells were infected with strain GXNN2020-2014 and strain GXLB1918-2013, respectively, and cytopathic effects were observed and recorded at 24 hpi under an inverted microscope. (B) Plaque morphology of PRV strains GXNN2020-2014 (a), GXBB1776-2013 (b), GXGG1901-2013 (c), GXLB1918-2013 (d), and Bartha-K61 (e) grown in PK-15 cells. Uninfected cells were used as a negative control (f). (C) Growth kinetics of the eight isolates. PK-15 cells were infected with PRV isolates at an MOI of 0.01, cell supernatants were collected at different time points after infection, and the virus titer at each time point was determined as the TCID50. The values represent the average of three independent experiments.
To investigate possible differences in the replication characteristics of the isolates, five of them were selected, and the multi-step growth kinetics and plaque morphology of the viruses were analyzed in PK-15 cells. The results showed that these five isolates had similar growth characteristics and exhibited similar plaque phenotypes in PK-15 cells (Fig. 1B-C).
Phylogenetic analysis based on the gB, gC, gD, gE, and TK genes
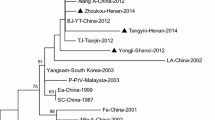
The gB, gC, gD, gE, and TK genes of the 36 PRV isolates were amplified and sequenced. Separate phylogenetic trees were constructed based on the gB, gC, gD, gE, and TK genes. As shown in Fig. 2, 35 of the isolates belonged to genotype II based on their gB and gC genes. In the phylogenetic trees, 30 of them clustered with variant strains isolated in China since 2012, and the remaining five were classified as classical PRV strains. In addition, one strain (GXLB2401-2015) belonged to the clade of genotype I (Fig. 2A-C). However, in the phylogenetic tree based on the gD gene, all 26 PRV strains isolated in this study clustered with the genotype II PRV strains, 21 of which were classified as variant strains and five of which were classical strains (Fig. 2B). In the phylogenetic tree based on the gE gene, all 36 isolates belonged to genotype II. Of these, 30 were classified as variant PRV strains, and six were classical PRV strains (Fig. 2D). However, in the phylogenetic tree based on the TK gene, no obvious genotypic or evolutionary trends were found with respect to the time and place of sample collection, and they could only be divided into two large clades according to their genotype (Fig. 2E). Interestingly, the isolate GXLB2401-2015 was classified as a genotype I strain when the gB and gC genes were analyzed, but it was clustered with genotype II strains in the phylogenetic tree based on the gE gene (Fig. 2A). This suggests that strain GXLB2401-2015 is a recombinant of genotype I and II strains.
Phylogenetic analysis based on nucleotide sequences of the gB (A), gC (B), gD (C), gE (D), and TK (E) genes of the 36 PRV isolates obtained in this study. The phylogenetic tree was generated by the neighbor-joining method with 1000 bootstrap replicates, using MEGA 11.0 software. The black triangles represent the 36 PRV isolates.
Analysis of the gB, gC, gD, gE, and TK genes
Sequence analysis of the major PRV genes gB, gC, gD, gE, and TK showed that they had a maximum nucleotide sequence divergence of 1.8, 4.8, 1.6, 2.6, and 12.6%, respectively, when compared to reference strains from other countries and 1.8, 4.2, 0.7, 0.9, and 12.3% divergence, respectively, when compared to the prevalent strains in China before 2012. After 2012, the maximum nucleotide sequence divergence for these genes was 1.7, 4.8, 0.7, 0.7, and 12.4%, respectively. The maximum amino acid sequence divergence in gB, gC, gD, gE, and TK was 5.6, 8.3, 1.2, 2.1, and 23.6%, respectively, for the Chinese isolates, and 5.6, 8.3, 3.5, 4.8, and 23.9%, respectively, when compared to isolates from other countries. Further sequence analysis showed that the maximum amino acid sequence divergence was 3.2, 6.9, 1.0, 2.1, and 23.2%, respectively, when compared to variant PRV strains and 3.4, 7.3, 0.7, 1.0, and 23.2%, respectively, when compared to the classical PRV strains (Table 5). Notably, the TK gene was found to be highly conserved among the different strains, except for a deletion in the TK gene of the GXGG2480-2016 isolate. The deduced amino acid sequences of the gB, gC, gD, gE, and TK proteins of the 36 PRV isolates were aligned with those of reference strains, as shown in Figure 3. In the gB protein, there were three contiguous amino acid deletions (75SPG77) in all of the isolates except for GXLB2401-2015 when compared to the Bartha-Hungary, Kaplan-Hungary, and NIA-3-Japan isolates (Fig. 3A), and except for the GXLB2401-2015 isolate, which showed a high level of sequence similarity to those of foreign strains, all of the isolates had a 7-amino-acid insertion (63AAASTPA69) in the gC protein, as observed in genotype II PRV strains (Fig. 3B). When compared to the Chinese variant PRV strains, amino acid sequence alignments of the gD protein also revealed a two-amino-acid deletion (280RP281) in 21 of the isolates (Fig. 3C). An amino acid sequence alignment of gE protein sequences showed that the isolates from China had a single amino acid insertion at position 48 (D) when compared to the strains from other countries. Five isolates were similar to the classical PRV strains in that each had an amino acid deletion at position 497 (D) (Fig. 3D). The only amino acid sequence difference between the TK proteins of the Chinese isolates and those of the genotype I PRV strains was a single amino acid substitution at position 215 (V-T) (Fig. 3E).
Pathogenicity of the PRV strains in mice
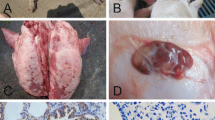
Pathogenicity assays were performed in order to evaluate the virulence of the PRV strains isolated in this study. SPF BALB/c mice were inoculated subcutaneously in the groin area with different doses of the PRV isolates GXBB1776-2013, GXNN2020-2014, and GXGG1901-2013 and the vaccine strain Bartha-K61, as well as DMEM as a control. The clinical signs of each group were monitored daily for 14 days. The results showed that the mice developed typical PRV symptoms such as pruritus, convulsions, and decreased appetite within 48-54 h after inoculation with the PRV variant strains GXBB1776-2013 and GXNN2020-2014 and the classical strain GXGG1901-2013 isolated in this study, and these mice died 8-12 h thereafter. The LD50 values for these isolates were between 104.17 and 104.63, whereas mice inoculated with the vaccine strain Bartha-K61 showed clinical symptoms only at the high dose of 107 TCID50 at 72 h postinfection and the mice died at 84-96 h postinfection. However, no further morbidity or mortality was observed 7 days after infection. The LD50 of Bartha-K61 was determined to be 105.78 (Table 6). Necropsy of the dead mice showed that death was caused by hemorrhaging and congestion in their brain tissues (Fig. 4B). The control group injected with the same volume of DMEM did not show any clinical signs during the observation period. Overall, our results suggest that, compared to the classical PRV strain, the variant strains isolated in this study had a higher pathogenicity in mice.
Pathogenicity of the PRV strains isolated in this study. Six-week-old SPF BALB/c mice were infected with 105 TCID50 of a PRV strain (GXBB1776-2013, GXGG1901-2013, GXNN2020-2014, or Bartha-K61) or inoculated with the same volume of DMEM as a negative control. The clinical symptoms and survival rates of each group were observed and recorded daily for 14 days. (A) The survival curves of each group infected with a different PRV strain. (B) Mice infected with PRV strains isolated in this study showed pruritus (a, b), and cerebral hemorrhage and edema were observed after dissection (c, d). The brain tissues in the negative control group were found to be normal (e).
Discussion
Currently, PR is widely prevalent worldwide, severely restricting the development of the pig industry. Since 2011, PRV has re-emerged on several pig farms in China where the animals had been vaccinated with a conventional PRV vaccine, and the virus has rapidly spread to most Chinese provinces and cities, triggering an epidemic of PR in China and causing huge economic losses to the large-scale farming industry [21]. Studies have shown that the pathogen that caused the resurgence of PRV outbreaks on Chinese pig farms was a new mutant strain of PRV, and it had significantly enhanced virulence and pathogenicity when compared to the classical PRV strain. This resulted in an inability of the PRV vaccines that were in current use to provide complete protection [22, 23]. Therefore, the present situation is that PR remains widely prevalent and China is at risk of outbreaks from this virus.
To understand the epidemiological characteristics of PRV in Guangxi province, China, 819 tissue samples were collected in this region from 2013 to 2019 and tested for the presence of the virus. The positivity rate was 25.27% (231/819), suggesting that PRV infection is common on swine farms in Guangxi province. A recent statistical survey of PRV prevalence in several regions of China found that the overall seropositivity rate for PRV gE in China from 2011 to 2020 was 29.87% (76,553/256,326) [24]. However, some variations in the prevalence of PRV were observed in different regions of China. Yao et al. and others reported that the gE gene seropositivity rates in Yunnan, Hebei, and Hunan provinces were 31.37, 46.27, and 23.55%, respectively [25,26,27]. These results suggest that vaccination with the Bartha-K61 vaccine may not provide full protection against the PRV strains that are currently prevalent in China, as has been reported previously. However, some studies have reported that vaccination with Bartha-K61 provided effective protection only against the Chinese variant [28,29,30,31]. We speculate that the discrepancy between these results may be related to inappropriate immunization procedures, poor rearing environments, or coinfections with other farm pathogens in the field infection studies, and this needs to be investigated further. Currently, due to the ongoing African swine fever epidemic in China, the measures taken to prevent and control disease on swine farms have continued to improve. In addition, the biosecurity systems in Chinese farms have gradually improved, and these measures have contributed to a significant decrease in infections with other pathogens. It is for this reason that the rate of PRV infection on Chinese swine farms has also decreased significantly in recent years. Nevertheless, the eradication of the virus from these farms is still an urgent priority for China's pig farming industry.
To further investigate the genetic evolution and biological characteristics of PRV in Guangxi province, 36 wild-type PRV strains were isolated from the positive tissue samples obtained. These isolates could be stably passaged in Vero cells, in which they exhibited obvious CPE and reached high titers, indicating that they grew well in Vero cells. Phylogenetic analysis based on the gB, gC, gD, and gE genes showed that 30 of these PRV isolates formed a relatively independent clade and were closely related to PRV variants isolated in China since 2012, while five of them were related to classical PRV strains. These results suggest that variant PRV strains are the dominant epidemic strains in Guangxi province. This is consistent with previous studies. For example, in 2019, Zhai et al. [32] detected PRV variants in pigs that had been inoculated with the Bartha-K61 strain in eastern China, and the isolates showed unique molecular characteristics similar to those of variant PRV strains. In 2018, Sun et al. [33] found that the currently prevalent Chinese PRV isolates showed characteristic variations in the gB, gC, and gE proteins that were significantly different from those found in isolates from other countries and earlier Chinese isolates. These findings confirmed that PRV variants have become prevalent in China. Outbreaks of PR on many Bartha-K61-immunized Chinese pig farms have caused significant economic losses to the pig industry.
The nucleotide and amino acid sequence comparisons of the gB, gC, gD, gE, and TK genes of the 35 isolates showed that the sequences of these genes were 96.4%-100% identical, with the exception of strain GXGG2480-2016, which had a 69-amino-acid deletion in the TK gene. Multiple sequence comparisons with reference strains showed that 30 of the PRV variants had characteristic variant sites in the amino acid sequences of the gB, gC, gD, and gE proteins. Similar amino acid changes have been reported in previous studies [26, 27, 32]. We speculate that some of these amino acid changes affect neutralization epitopes or adhesion properties of the virus. This would, in turn, affect the protective efficacy of conventional vaccines and could also affect the virulence of the currently prevalent PRV strains in China.
Interestingly, the gB, gC, and TK genes of the GXLB2401-2015 isolate were found to be most closely related to the foreign strains, while the gD and gE genes were most closely related to Chinese PRV variant strains. This indicated that some recombination events might have occurred in the GXLB2401-2015 strain. Our laboratory previously provided evidence that this strain is a natural recombinant between a PRV variant strain and the Bartha-K61 vaccine strain [34]. Natural recombination can play an important role in viral evolution. Several studies have recently reported natural recombination between different PRV genotypes as well as between vaccine and wild-type strains. Huang et al. [22] reported that the FJ62 variant, which they isolated, may have been a natural recombination of a wild boar PRV genotype I strain and a Chinese domestic pig genotype II PRV strain. In addition, Bo et al. [18] demonstrated that the JSY13 strain is a natural recombinant between the genotype I Bartha-K61 vaccine strain and a genotype II PRV variant strain. These reports suggest that PRV recombination events are not coincidental, and we speculate that the recombination phenomenon reported here might have resulted from the immune pressure associated with the PRV vaccine. The widespread use of live attenuated vaccines in China might have increased the likelihood of recombination events between the vaccine and field strains. Therefore, it is necessary to continuously monitor the genetic evolution of PRV in China as well as in other countries where such vaccines are commonly used.
Pathogenicity assays in mice showed that the PRV strains isolated in this study caused significant clinical signs, such as anorexia, mental fatigue, and convulsions in the infected animals. Brain tissues obtained from infected mice were found to be congested, and the LD50 of these isolated strains ranged from 104.17 to 104.63. The LD50 of classical strains of PRV is significantly higher than that of variant strains, indicating that the wild-type strains isolated in this study are more pathogenic in mice.
Conclusions
In summary, 819 tissue samples were collected from Guangxi province of China, and 25.27% of these were found to be infected with PRV. We isolated 36 wild-type PRV strains, all of which were able to multiply to high titers in cultured Vero cells. Most of the isolated virulent strains belonged to a group of mutant strains and showed a strong pathogenicity in mice. Our results will provide a reference for our future understanding of the epidemiological and genetic evolutionary patterns of PRV in China.
References
Muller T, Hahn EC, Tottewitz F, Kramer M, Klupp BG, Mettenleiter TC, Freuling C (2011) Pseudorabies virus in wild swine: a global perspective. Arch Virol 156:1691–1705
Klupp BG, Hengartner CJ, Mettenleiter TC, Enquist LW (2004) Complete, annotated sequence of the pseudorabies virus genome. J Virol 78:424–440
Pomeranz LE, Reynolds AE, Hengartner CJ (2005) Molecular biology of pseudorabies virus: impact on neurovirology and veterinary medicine. Microbiol Mol Biol Rev 69:462–500
Laval K, Enquist LW (2020) The neuropathic itch caused by pseudorabies virus. Pathogens 9
Mettenleiter TC (2000) Aujeszky’s disease (pseudorabies) virus: the virus and molecular pathogenesis–state of the art, June 1999. Vet Res 31:99–115
Zheng HH, Fu PF, Chen HY, Wang ZY (2022) Pseudorabies virus: from pathogenesis to prevention strategies. Viruses 14
Yang H, Han H, Wang H, Cui Y, Liu H, Ding S (2019) A case of human viral encephalitis caused by pseudorabies virus infection in China. Front Neurol 10:534
Liu Q, Wang X, Xie C, Ding S, Yang H, Guo S, Li J, Qin L, Ban F, Wang D, Wang C, Feng L, Ma H, Wu B, Zhang L, Dong C, Xing L, Zhang J, Chen H, Yan R, Wang X, Li W (2021) A novel human acute encephalitis caused by pseudorabies virus variant strain. Clin Infect Dis 73:e3690–e3700
Freuling CM, Müller TF, Mettenleiter TC (2017) Vaccines against pseudorabies virus (PrV). Vet Microbiol 206:3–9
Zhou M, Wu X, Jiang D, Sui C, Chen L, Cong X, Xin X, Wang G, Li Y, Tian F, Chen Z, Zhang H, Qi J, Wang Z, Wu J, Shan H, Du Y (2019) Characterization of a moderately pathogenic pseudorabies virus variant isolated in China, 2014. Infect Genet Evol 68:161–171
Hu D, Zhang Z, Lv L, Xiao Y, Qu Y, Ma H, Niu Y, Wang G, Liu S (2015) Outbreak of variant pseudorabies virus in Bartha-K61-vaccinated piglets in central Shandong Province, China. J Vet Diagn Investig 27:600–605
Gu Z, Hou C, Sun H, Yang W, Dong J, Bai J, Jiang P (2015) Emergence of highly virulent pseudorabies virus in southern China. Can J Vet Res 79:221–228
An TQ, Peng JM, Tian ZJ, Zhao HY, Li N, Liu YM, Chen JZ, Leng CL, Sun Y, Chang D, Tong GZ (2013) Pseudorabies virus variant in Bartha-K61-vaccinated pigs, China, 2012. Emerg Infect Dis 19:1749–1755
Luo Y, Li N, Cong X, Wang CH, Du M, Li L, Zhao B, Yuan J, Liu DD, Li S, Li Y, Sun Y, Qiu HJ (2014) Pathogenicity and genomic characterization of a pseudorabies virus variant isolated from Bartha-K61-vaccinated swine population in China. Vet Microbiol 174:107–115
Ishikawa K, Tsutsui M, Taguchi K, Saitoh A, Muramatsu M (1996) Sequence variation of the gC gene among pseudorabies virus strains. Vet Microbiol 49:267–272
Ye C, Zhang QZ, Tian ZJ, Zheng H, Zhao K, Liu F, Guo JC, Tong W, Jiang CG, Wang SJ, Shi M, Chang XB, Jiang YF, Peng JM, Zhou YJ, Tang YD, Sun MX, Cai XH, An TQ, Tong GZ (2015) Genomic characterization of emergent pseudorabies virus in China reveals marked sequence divergence: Evidence for the existence of two major genotypes. Virology 483:32–43
He W, Auclert LZ, Zhai X, Wong G, Zhang C, Zhu H, Xing G, Wang S, He W, Li K, Wang L, Han GZ, Veit M, Zhou J, Su S (2019) Interspecies transmission, genetic diversity, and evolutionary dynamics of pseudorabies virus. J Infect Dis 219:1705–1715
Bo Z, Miao Y, Xi R, Gao X, Miao D, Chen H, Jung YS, Qian Y, Dai J (2021) Emergence of a novel pathogenic recombinant virus from Bartha vaccine and variant pseudorabies virus in China. Transbound Emerg Dis 68:1454–1464
Sun Y, Luo Y, Wang CH, Yuan J, Li N, Song K, Qiu HJ (2016) Control of swine pseudorabies in China: opportunities and limitations. Vet Microbiol 183:119–124
Reed LJ, Muench H (1938) A simple method of estimating fifty per cent endpoints12. Am J Epidemiol 27:493–497
Ye C, Guo JC, Gao JC, Wang TY, Zhao K, Chang XB, Wang Q, Peng JM, Tian ZJ, Cai XH, Tong GZ, An TQ (2016) Genomic analyses reveal that partial sequence of an earlier pseudorabies virus in China is originated from a Bartha-vaccine-like strain. Virology 491:56–63
Huang J, Zhu L, Zhao J, Yin X, Feng Y, Wang X, Sun X, Zhou Y, Xu Z (2020) Genetic evolution analysis of novel recombinant pseudorabies virus strain in Sichuan, China. Transbound Emerg Dis 67:1428–1432
Fan J, Zeng X, Zhang G, Wu Q, Niu J, Sun B, Xie Q, Ma J (2016) Molecular characterization and phylogenetic analysis of pseudorabies virus variants isolated from Guangdong province of southern China during 2013–2014. J Vet Sci 17:369–375
Tan L, Yao J, Yang Y, Luo W, Yuan X, Yang L, Wang A (2021) Current status and challenge of pseudorabies virus infection in China. Virol Sin 36:588–607
Zhang C, Cui H, Zhang W, Meng L, Chen L, Wang Z, Zhao K, Chen Z, Qiao S, Liu J, Guo Z, Dong S (2022) Epidemiological investigation of porcine pseudorabies virus in Hebei Province, China, 2017–2018. Front Vet Sci 9:930871
Lin Y, Tan L, Wang C, He S, Fang L, Wang Z, Zhong Y, Zhang K, Liu D, Yang Q, Wang A (2021) Serological investigation and genetic characteristics of pseudorabies virus in Hunan Province of China From 2016 to 2020. Front Vet Sci 8:762326
Yao J, Li J, Gao L, He Y, Xie J, Zhu P, Zhang Y, Zhang X, Duan L, Yang S, Song C, Shu X (2022) Epidemiological investigation and genetic analysis of pseudorabies virus in Yunnan Province of China from 2017 to 2021. Viruses 14
Papageorgiou KV, Michailidou M, Grivas I, Petridou E, Stamelou E, Efraimidis K, Chen L, Drew TW, Kritas SK (2022) Bartha-K61 vaccine protects nursery pigs against challenge with novel european and asian strains of suid herpesvirus 1. Vet Res 53:47
Zhou J, Li S, Wang X, Zou M, Gao S (2017) Bartha-k61 vaccine protects growing pigs against challenge with an emerging variant pseudorabies virus. Vaccine 35:1161–1166
Wang J, Cui X, Wang X, Wang W, Gao S, Liu X, Kai Y, Chen C (2020) Efficacy of the Bartha-K61 vaccine and a gE(-)/gI(-)/TK(-) prototype vaccine against variant porcine pseudorabies virus (vPRV) in piglets with sublethal challenge of vPRV. Res Vet Sci 128:16–23
Ren Q, Li L, Pan H, Wang X, Gao Q, Huan C, Wang J, Zhang W, Jiang L, Gao S, Kai Y, Chen C (2022) Same dosages of rPRV/XJ5-gI(-)/gE(-)/TK(-) prototype vaccine or Bartha-K61 vaccine similarly protects growing pigs against lethal challenge of emerging vPRV/XJ-5 strain. Front Vet Sci 9:896689
Zhai X, Zhao W, Li K, Zhang C, Wang C, Su S, Zhou J, Lei J, Xing G, Sun H, Shi Z, Gu J (2019) Genome characteristics and evolution of pseudorabies virus strains in Eastern China from 2017 to 2019. Virol Sin 34:601–609
Sun Y, Liang W, Liu Q, Zhao T, Zhu H, Hua L, Peng Z, Tang X, Stratton CW, Zhou D, Tian Y, Chen H, Wu B (2018) Epidemiological and genetic characteristics of swine pseudorabies virus in mainland China between 2012 and 2017. PeerJ 6:e5785
Qin Y, Qin S, Huang X, Xu L, Ouyang K, Chen Y, Wei Z, Huang W (2023) Isolation and identification of two novel pseudorabies viruses with natural recombination or TK gene deletion in China. Vet Microbiol 280:109703
Acknowledgements
This research was funded by the National Natural Science Foundation (Grant no. 32260875). The authors would like to thank Dr. Dev Sooranna, Imperial College, London, for editing the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Additional information
Handling Editor: Ana Cristina Bratanich.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, X., Qin, S., Wang, X. et al. Molecular epidemiological and genetic characterization of pseudorabies virus in Guangxi, China. Arch Virol 168, 285 (2023). https://doi.org/10.1007/s00705-023-05907-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00705-023-05907-2