Abstract
Increasing evidence confirms the involvement of virus infection and miRNA, such as miR-146a, in neuroinflammation-associated epilepsy. In the present study, we investigated the upregulation of miR-146a with RT-qPCR and in situ hybridization methods in a mice infection model of Japanese encephalitis virus (JEV) and in vitro. Subsequently we investigated the involvement of miR-146a in modulating JEV-induced neuroinflammation. It was demonstrated that JEV infection promoted miR-146a production in BALB/c mice brain and in cultured mouse microglial C8-B4 cells, along with pro-inflammatory cytokines, such as IL-1β, IL-6, TNF-α, IFN-β and IFN-α. We also found that miR-146a exerted negative regulatory effects upon IL-1β, IL-6, TNF-α, IFN-β and IFN-α in C8-B4 cells. Accordingly, miR-146a downregulation with a miR-146a inhibitor promoted the upregulation of IL-1β, IL-6, TNF-α, IFN-β and IFN-α, whereas miR-146a upregulation with miR-146a mimics reduced the upregulation of these cytokines. Moreover, miR-146a exerted no regulation upon JEV growth in C8-B4 cells. In conclusion, JEV infection upregulated miR-146a and pro-inflammatory cytokine production, in mice brain and in cultured C8-B4 cells. Furthermore, miR-146a negatively regulated the production of JEV-induced pro-inflammatory cytokines, in virus growth independent fashion, identifying miR-146a as a negative feedback regulator in JEV-induced neuroinflammation, and possibly in epilepsy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Experimental and clinical data have demonstrated that virus infection [1] and related inflammation [2–4] in the brain is mechanistically associated with epilepsy. Japanese encephalitis virus (JEV) infection has also been linked to the pathophysiological or pathological changes of epilepsy [1, 5]. Complicated and sustained inflammation has been found in surgical epilepsy specimens, including activation of microglia/macrophages and astrocytes [6] and induction of pro-epileptogenic inflammatory cytokines [4, 7].
Neuro-tropic virus infection not only results in an acute encephalomyelitis, but can also lead to secondary neuroinflammatory disorders [8, 9]. Infection with neuro-tropic viruses, such as West Nile Virus (WNV) [10], Influenza Virus [11] or JEV [1, 5] can cause cognitive dysfunction, seizure disorders (epilepsy), and other neurologic disorders [12]. In particular, JEV induces neuronal cell apoptotic death and the release of pro-inflammatory cytokines, which then promote subsequent apoptotic death of both infected and uninfected neurons [13], implying a crucial role for activated microglial cells and astrocytes in the pathogenesis of Japanese encephalitis (JE). In another mouse model, it has been shown that tumor necrosis factor alpha (TNF-α) is a key factor that mediates immunopathology in the central nervous system (CNS) during JE [14].
Accumulating evidence supports a critical regulatory role for microRNA-mediated post-transcriptional gene regulation in the pathogenesis of different neurodegenerative and neuroinflammatory disorders [15–17]. Moreover, there is a marked deregulation of brain miRNAs following prolonged seizures (status epilepticus), both in patients and in animal models [18–20]. These deregulated miRNAs are involved in pathways related to inflammation, stress signaling and neuronal excitation [19]. In particular, miRNA-146a has been shown to be upregulated in both experimental epilepsy models and human temporal lobe epilepsy (TLE) [21, 22]. There was also a strong miRNA-146a upregulation in astrocytes in a rat model of TLE after status epilepticus [23, 24], suggesting a key role for miRNA-146a in governing astrocyte activation and more broadly in epilepsy. However, little is known about the function of deregulated miR-146a in this process.
In the present study, we investigated upregulation of miR-146a in a mice infection model of JEV and in mouse microglial C8-B4 cells, which were infected with JEV. Furthermore, we investigated the involvement of miR-146a in JEV infection-induced upregulation of pro-inflammatory cytokines, such as IL-1β, IL-6, TNF-α and IFN-β, in C8-B4 cells. The present study indicates a regulatory role for miR-146a in JEV infection and probably in JEV infection-associated epilepsy.
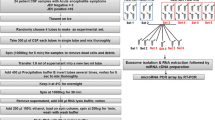
Materials and methods
JEV virus infection and brain sample preparation
The experiments involving infection of mice were approved by the ethics committee of First Affiliated Hospital to Science and Technology University of Henan (ST-M-2014031). Seven-week-old female BALB/c mice were infected via subcutaneous injection with JEV (vaccine strain SA14-14-2 (GenBank no. D90195)) stock or UV-inactivated JEV (105 PFU each), diluted in Dulbecco’s Modified Eagle’s Medium (DMEM; GIBCO, Rockville, MD, USA) supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA, USA). 1, 3, 5, 7 or 9 days post infection (D.P.I.), mice were sacrificed under anesthesia with isoflurane to collect infected brains. For miR-146a isolation, brain samples were homogenized in DMEM and centrifuged at 10,000 rpm for 30 min to remove cellular debris. The suspension was stored at -80 °C until further use. For in situ hybridization, the brain samples were immediately stored at -80 °C until further use.
Cell culture, infection with JEV and miR-146a manipulation
The C8-B4 cell line (a mouse microglia cell line) was purchased from American Type Culture Collection (ATCC) (Rockville, MD, USA) and cultured in DMEM (Thermo Scientific, Rockford, IL, USA) supplemented with 10% fetal bovine serum (FBS) (HyClone, Logan, UT, USA) at 37 °C, with 5% CO2. For JEV infection, C8-B4 cells were cultured in 6-well plates to a confluence of more than 85%. Post cell counting, JEV virus diluted with DMEM containing 2% FBS was inoculated onto the C8-B4 cell plate (100 μl per well) with a MOI (Multiplicity of Infection) of 1, 3 or 10. Cells were then incubated at 37 °C for 1 h, washed with warm phosphate-buffered saline (1× PBS, pH7.4) three times before fresh serum-free DMEM was added. Cells were then incubated at 37 °C for another 4, 8, 12 or 24 h, and the cell supernatants and cells were collected respectively, and stored at -80 °C for further analysis.
The miR-146a mimics, miR-146a inhibitor and scrambled RNA (as a control) were purchased from Sigma-Aldrich (St. Louis, MO, USA), and utilized to upregulate or downregulate miR-146a levels. 25 or 50 nM of miR-146a mimics, miR-146a inhibitor or scrambled RNA was transfected into C8-B4 cells using lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA).
Isolation of miRNA/mRNA and real-time quantitative PCR (RT-qPCR) analysis
mirVana miRNA Isolation Kit (Ambion, Austin, TX, USA) was utilized for the miRNA extraction according to the manufacturer’s guidance. The quantitative analysis of miR-146a levels was performed using the mirVana qRT-PCR miRNA Detection Kit (Ambion, Austin, TX, USA), with U6 small nuclear RNA as an internal control. The ∆∆Ct method was used for relative quantification [25]. Total cellular mRNA was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA). RT-qPCR analysis of IL-1β, IL-6, TNF-α, IFN-β or IFN-α mRNA levels was performed using the One Step SYBR PrimeScript RT-PCR Kit (Takara, Tokyo, Japan). All mRNA expression levels were normalized to β-actin, and the ∆∆Ct method was used for relative quantification [25].
In situ hybridization
In situ hybridization for miR-146a was performed using 5’ digoxigenin (DIG)-labeled antisense oligonucleotide probes (18 nt) containing Locked Nucleic Acid (LNA) and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide. The hybridizations were done on 6 mm sections of paraffin embedded materials, as described previously [26]. The hybridization signal was detected using a rabbit polyclonal Anti-DIG antibody and a horse radish peroxidase (HRP) labeled goat anti-rabbit polyclonal antibody (both from Abcam, Cambridge, UK) as a secondary antibody. Signal was detected with Diaminobenzidine (DAB) (Life Technologies, Grand Island, NY, USA).
Enzyme-linked immunosorbent assay (ELISA) for IL-1β, IL-6, TNF-α, or IFN-α/β
A sandwich enzyme-linked immunosorbent assay (ELISA) kit for IL-1β, IL-6, TNF-α, IFN-β or IFN-α (Excellbio, Shanghai, China) was used to detect the levels of each cytokine in mouse brain tissue or in the cell supernatant. Samples were serially diluted 1:10 and the kits were used according to the manufacturer’s guidance. A standard curve was generated using assay results from manufacturer-provided standard samples. Subsequently, for each sample cytokine levels were calculated according to this standard curve.
MTT assay for cellular viability
To examine the effect of transfection of scrambled RNA, miR-146a mimics or miR-146a inhibitor on the viability of C8-B4 cells, we performed a MTT (4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide) assay for the blank control, scrambled RNA-, miR-146a mimics- or miR-146a inhibitor-transfected cells. Briefly, 5 mg/ml of MTT buffer was added to transfected or non-transfected cells for an incubation period of 3 hours at 37 °C. 200 μl of DMSO was then added to stop the reaction before the cell viability was measured at a wavelength of 450 nm using a microplate reader (Bio-Rad, Hercules, CA, USA).
Viral growth curve and plaque forming assay
Growth curve analysis of JEV was performed by inoculation of C4-B8 cells at a MOI of 0.01 or 0.001 in 24-well plates. 85%-confluent C4-B8 cells were incubated with diluted JEV at 37 °C for one hour followed by three washes with PBS. Next, the cells were incubated with 1 ml of DMEM (with 2% FBS) per well at 37 °C for 0, 12, 24 or 48 hours. The supernatant samples were titrated by plaque forming assay on C4-B8 cells. The growth curves were determined in three independent experiments.
Statistical analysis
Graphpad Prism 6 statistical software was utilized for significance analysis. The data with normal distribution was expressed as mean ± SE; data without normal distribution was logarithmically transformed to normality for further analysis. ANOVA or t test was used for significance analysis with multiple variables or between two groups. P < 0.05 was considered statistically significant.
Results
miR-146a production is promoted by JEV infection in mice brain or in cultured mouse microglial C8-B4 cells
First, BALB/c mice were infected (105 PFU per mouse) with either JEV or UV-inactivated JEV. 1, 3, 5, 7 or 9 days post infection (D.P.I.) mice were sacrificed and brain samples were collected for miR-146a expression analysis using RT-qPCR or in situ hybridization (ISH). As shown in Figure 1A, JEV infection resulted in a significantly increased level of miR-146a in mice brain at 5 D.P.I., compared to the UV-inactivated JEV group (p < 0.05). miR-146a expression increased from 2 D.P.I onwards and reached a maximum at 5 D.P.I after which the expression decreased (p < 0.05 or p < 0.01, Figure 1B), whereas there was no significant time-dependent change in miR-146a expression in mice infected with the UV-inactivated JEV. Furthermore, we validated these results by ISH of miR-146a in the brains of JEV-infected mice. ISH results from brain sections of JEV-infected or UV-inactivated JEV-infected BALB/c mice demonstrated that miR-146a expression was strongly induced in the brain cortex area (Figure 1C), although there was a larger area of miR-146a positivity in JEV-infected brain samples than in the UV-inactivated JEV-infected controls. Thus, this strongly indicates that miR-146a expression is upregulated in BALB/c mice after JEV infection.
Japanese encephalitis virus infection promotes miR-146a expression in mice brains and in mouse microglial C8-B4 cells. BALB/c mice were infected with active or UV-inactivated JEV (105 PFU each). At 1, 3, 5, 7 or 9 days post infection (D.P.I.), mice were sacrificed for miR-146a analysis in brain samples, using RT-qPCR or in situ hybridization. The in vitro experiments were performed in mouse microglial C8-B4 cells with 1, 3 or 10 MOI for 0, 4, 8, 12 or 24 hours. A: miR-146a levels in active or UV-inactivated JEV-infected BALB/c mouse brain, 5 D.P.I.; B: Time-dependence of miR-146a upregulation in mice brain after JEV-infection; C and D: In situ hybridization of miR-146a in active (C) or UV-inactivated (D) JEV-infected mice brain samples. miR-146a expression is localized by arrows. E: RT-qPCR analysis of miR-146a expression in JEV-infected C8-B4 cells, 4, 8, 12 or 24 hours post inoculation (H.P.I.), with a multiplicity of infection (MOI) of 3; F: miR-146a levels in C8-B4 cells which were infected with 1, 3 or 10 MOI of active or UV-inactivated JEV, 8 H.P.I. * p < 0.05, or ** p < 0.01
To confirm the promotion of miR-146a expression by JEV infection, we evaluated miR-146a expression in cultured mouse C8-B4 cells which were infected with JEV. RT-qPCR analysis indicated that miR-146a expression increased markedly in JEV-infected C8-B4 cells, with a multiplicity of infection (MOI) of 3, from 4 hours post inoculation (H.P.I.) (p < 0.05), peaking at 12 H.P.I. (p < 0.001) (Figure 1E). This infection-induced expression pattern was repeatable and MOI-dependent (p < 0.01 or p < 0.001; Figure 1F). Taken together, we therefore confirmed the promotion of miR-146a expression by JEV infection in cultured C8-B4 cells.
JEV infection induces pro-inflammatory cytokines in mice brains
In order to uncover the pathogenesis of brain damage caused by JEV infection, we examined the induction of pro-inflammatory cytokines in the brains of BALB/c mouse infected with 105 PFU JEV. It was demonstrated that at 1 day post infection, there was no significant cytokine promotion in mouse brain (data not shown). As shown in Figure 2, from 3 D.P.I., pro-inflammatory cytokines, such as IL-1β (Figure 2A), IL-6 (Figure 2B), TNF-α (Figure 2C), IFN-β (Figure 2D) and IFN-α (Figure 2E) were significantly upregulated in the brains of mice infected with JEV (p < 0.05 or p < 0.01). This upregulation was sustained at 5 D.P.I. (p < 0.01 or p < 0.001; Figure 2A-E).
JEV infection induces pro-inflammatory cytokine expression in mice brains. BALB/c mice were infected with active or UV-inactivated JEV (105 PFU each), and subsequently sacrificed for analysis of pro-inflammatory cytokines in brain samples at 3 or 5 D.P.I.. Mice brain samples were homogenized and examined for IL-1β (A), IL-6 (B), TNF-α (C) or IFN-β (D) via enzyme-linked immunosorbent assay (ELISA). Each value is an average of three mouse brain samples, and is expressed as a mean fold change, compared to control samples. * p < 0.05, ** p < 0.01 or *** p < 0.001
To confirm the promotion of pro-inflammatory cytokine expression by JEV infection in vitro, we subsequently examined the expression level of four cytokines in JEV-infected C8-B4 cells (MOI of 3). Interestingly, increased expression of IL-1β (Figure 3A), IL-6 (Figure 3B), TNF-α (Figure 3C), IFN-β (Figure 3D) and IFN-α (Figure 3E) was also significant in JEV-infected C8-B4 cells (p < 0.05, p < 0.01 or p < 0.001), and was even more significant than in infected mouse brains. In particular, the expression of TNF-α in JEV-infected C8-B4 cells, when compared to control cells, was greater than 200 fold higher at 24 hours post inoculation (Figure 3C). Thus, the promotion of pro-inflammatory cytokines by JEV infection was confirmed in vivo and in vitro.
JEV infection induces pro-inflammatory cytokine expression in C8-B4 astrocytes. C8-B4 cells were infected with active or UV-inactivated JEV (3 MOI) for 12 or 24 hours and the supernatant collected for analysis of pro-inflammatory cytokines. The level of IL-1β (A), IL-6 (B), TNF-α (C) or IFN-β (D) was examined with enzyme-linked immunosorbent assay (ELISA) method. Each value represents the average of three independent experiments, ** p < 0.01 or *** p < 0.001
A miR-146a inhibitor reduced JEV-induced cytokine induction in cultured C8-B4 cells
To correlate increased miR-146a expression with the upregulation of pro-inflammatory cytokines in JEV infection, we utilized a miR-146a inhibitor to downregulate miR-146a levels in JEV-infected C8-B4 cells before re-evaluating cytokine promotion after JEV infection. Figure 4A demonstrates that transfection with 25 or 50 nM of miR-146a inhibitor significantly decreases the miR-146a level in C8-B4 cells, in contrast to transfection with a scrambled RNA control (p < 0.05 or p < 0.01); whereas transfection of a miR-146a mimic markedly promoted miR-146a levels at concentrations of both 25 or 50 nM (p < 0.001 respectively). This inhibitory/promotory regulation of miR-146a by the miR-146a inhibitor or mimic was evident at 8 or 12 hours post infection in JEV-infected C8-B4 cells (p < 0.05, p < 0.01 or p < 0.001, Figure 4B). To investigate the regulatory role of miR-146a in promotion of pro-inflammatory cytokines after JEV infection, we re-examined the levels of IL-1β, IL-6, TNF-α, IFN-β and IFN-α. When compared to the scrambled RNA group, transfection with 50 nM of a miR-146a mimic significantly reduced the level of IL-1β (Figure 4C), IL-6 (Figure 4D), TNF-α (Figure 4E), IFN-β (Figure 4F) and IFN-α (Figure 4G) in JEV-infected C8-B4 cells, at both 12 and 24 H.P.I. (p < 0.05 or p < 0.01).
A miR-146a inhibitor reduced the induction of pro-inflammatory cytokines by JEV infection in cultured C8-B4 cells. A: miR-146a inhibitor down-regulated miR-146a levels in C8-B4 cells, when compared to the scrambled RNA and miR-146a mimics; B: A miR-146a inhibitor (50 nM) reduced miR-146a expression after JEV infection (3 MOI) in C8-B4 cells at 8 or 12 H.P.I.; C-F: A miR-146a inhibitor (50 nM) reduced JEV infection-mediated induction of IL-1β (C), IL-6 (D), TNF-α (E) or IFN-β (F) in C8-B4 cells at 8 or 12 H.P.I.; C8-B4 cells were transfected with miR-146a inhibitor or scrambled RNA and then infected with JEV(3 MOI) for 8 or 12 hours. The supernatant in each group was then collected and examined for IL-1β, IL-6, TNF-α or IFN-β using an ELISA kit. Each result was independently performed in triplicate, and was expressed as mean fold change, relative to control samples. * p < 0.05, ** p < 0.01 or *** p < 0.001
In addition, we re-evaluated proinflammatory cytokine induction in JEV-infected C8-B4 cells, post transfection with a miR-146a inhibitor. As indicated in Figure 5A, there was a higher level of IL-1β in miR-146a inhibitor-transfected C8-B4 cells, than in scrambled RNA-transfected C8-B4 control cells (p < 0.05 or p < 0.01). Furthermore miR-146a inhibitor transfection also promoted JEV-induced IL-6 (Figure 5B), TNF-α (Figure 5C), IFN-β (Figure 5D) and IFN-α (Figure 5E) expression (p < 0.05, p < 0.01 or p < 0.001). Taken together, this indicates a negative regulatory role for miR-146a in the promotion of pro-inflammatory cytokines after JEV infection in C8-B4 cells.
Transfection of miR-146a mimics promoted pro-inflammatory cytokine expression in cultured C8-B4 cells. C8-B4 cells were transfected with 50 nM of a miR-146a mimic or scrambled RNA for 12 or 24 hours. The supernatant in each group was then collected and examined for IL-1β (A), IL-6 (B), TNF-α (C) or IFN-β (D) expression using an ELISA kit. H.P.I.: Hours post transfection; All results were independently performed in triplicate. * p < 0.05, ** p < 0.01 or ns: no significance
miR-146a-mediated cytokine reduction is virus-independent
To investigate whether miR-146a-mediated cytokine reduction was dependent on JEV infection, we then correlated miR-146a expression and JEV growth. Figure 6A demonstrates that transfection of scrambled RNA, miR-146a mimics or miR-146a inhibitor slightly reduced the viability of C8-B4 cells, but not significantly. Plaque forming assays demonstrated that there was no significant difference in the number of JEV-induced plaques in normal C8-B4 cells (blank) when compared to C8-B4 cells transfected with scrambled RNA-, miR-146a mimics- or miR-146a inhibitor (Figure 6B and 6C). In addition growth curve analysis of JEV showed that viral replication was not significantly different in these four types of C8-B4 cells, with a MOI of 0.01 or 0.001(Figure 6D and 6E). Thus, we concluded that miR-146a-mediated cytokine reduction was virus-independent.
miR-146a exerted no regulatory effect on JEV growth in cultured C8-B4 cells. A: An MTT assay for the viability of blank or miR-146-regulated C8-B4 cells (transfected with 50 nM scrambled, miR-146a mimics or miR-146a inhibitor for 24 hours); B and C: A plaque forming assay for JEV virus in scrambled-, miR-146a mimic- or miR-146a inhibitor-transfected C8-B4 cells; D and E: Growth curve analysis of JEV in the scrambled-, miR-146a mimic- or miR-146a inhibitor-transfected C8-B4 cells with a MOI of 0.01 (D) or 0.001 (E). All results were independently performed in triplicate. ns: no significance
Discussion
Japanese encephalitis (JE) is characterized by the neuronal destruction/dysfunction caused by neuroinflammation [27]. JEV infection induces neuronal apoptotic cell death and the release of pro-inflammatory cytokines, which then promote subsequent apoptotic death of both infected and uninfected neurons [13]. The virus-mediated killing and cytokine-mediated cytotoxicity have been reported to cause neuronal death [28]. In particular, microglia, as a mononuclear phagocytic population in the CNS parenchyma, represent an important component of the innate immune response against invading pathogens [28], and is widely considered to play a key role in the pathogenesis of JE and the immune response against JEV infection [28–32]. Recently, the inflammatory responses and consequences of astrocyte activation after JEV infection have also been examined. It was demonstrated that pro-inflammatory mediators as TNF-α and IL-1β were also promoted by JEV infection in astrocytes or in microglial cells [33–36]. However, the activation mechanism after JEV infection is not fully understood.
In the present study, we examined the role of miR-146a in the promotion of pro-inflammatory cytokines in mouse brain, and separately in C8-B4 microglial cells in vitro, post JEV infection. Results demonstrated that JEV infection induced a significant upregulation of miR-146a levels in BALB/c mice brains, in a time-dependent fashion, observed by both RT-qPCR and ISH. The miR-146a upregulation was also confirmed in cultured mouse microglial C8-B4 cells after JEV infection. The expression of the two key microRNA processing enzymes Drosha and Dicer was also significantly upregulated in JEV-infected cultured C8-B4 cells, at both the mRNA and protein levels. The present study then confirmed the role of the upregulated pro-inflammatory cytokines in brain damage caused by JEV infection. Results demonstrated that pro-inflammatory cytokines such as IL-1β, IL-6, TNF-α and IFN-β were also significantly upregulated in the brains of BALB/c mouse infected with JEV. The induction of pro-inflammatory cytokines by JEV infection was also confirmed in vitro, since these four cytokines were also significantly upregulated in JEV-infected C8-B4 cells. Moreover, the present study associated miR-146a with the upregulation in pro-inflammatory cytokines by JEV infection; since a miR-146a inhibitor reduced JEV-promoted pro-inflammatory cytokine induction in cultured microglial C8-B4 cells. In addition, our results demonstrated that miR-146a-mediated negative regulation of cytokines was virus growth-independent, since miR-146a upregulation or downregulation exerted no effect on virus growth in C8-B4 cells.
miR-146a has been shown to increase in response to virus infection or viral products, such as human T-cell leukemia virus type I (HTLV-1) [37], human immunodeficiency virus (HIV) [38] and Kaposi’s sarcoma-associated herpesvirus (KSHV) [39]. miR-146a induction leads to an impaired antiviral state against Varicella-zoster virus (VZV) infection, via targeting TNFR-associated factor 6 (TRAF6), and IL-1R-associated kinase (IRAK) 1 and 2 [40, 41]. We speculated that miR-146a induction might also be a strategy for JEV to antagonize the innate immune system against JEV infection. However, the possible mechanism underlying such a response remains unclear.
Conclusion
In summary, the present study confirmed the upregulation of miR-146a in JEV-infected microglial cells, and that this miR-146a promotion contributed to the upregulation of pro-inflammatory cytokines, such as IL-1β, IL-6, TNF-α, IFN-β and IFN-α. It implies a regulatory role for miR-146a in JEV infection and probably in JEV infection-associated epilepsy.
References
Che W, Dong Y, Quan HB (2015) RANKL inhibits cell proliferation by regulating MALAT1 expression in a human osteoblastic cell line hFOB 1.19. Cell Mol Biol (Noisy-le-grand) 61:7–14
Vezzani A, Ravizza T, Balosso S, Aronica E (2008) Glia as a source of cytokines: implications for neuronal excitability and survival. Epilepsia 49(Suppl 2):24–32
Hirvonen J, Kreisl WC, Fujita M, Dustin I, Khan O, Appel S, Zhang Y, Morse C, Pike VW, Innis RB, Theodore WH (2012) Increased in vivo expression of an inflammatory marker in temporal lobe epilepsy. J Nucl Med 53:234–240
Aronica E, Crino PB (2011) Inflammation in epilepsy: clinical observations. Epilepsia 52(Suppl 3):26–32
Swarup V, Ghosh J, Duseja R, Ghosh S, Basu A (2007) Japanese encephalitis virus infection decrease endogenous IL-10 production: correlation with microglial activation and neuronal death. Neurosci Lett 420:144–149
Blumcke I, Vinters HV, Armstrong D, Aronica E, Thom M, Spreafico R (2009) Malformations of cortical development and epilepsies: neuropathological findings with emphasis on focal cortical dysplasia. Epileptic Disord 11:181–193
Maroso M, Balosso S, Ravizza T, Liu J, Bianchi ME, Vezzani A (2011) Interleukin-1 type 1 receptor/Toll-like receptor signalling in epilepsy: the importance of IL-1beta and high-mobility group box 1. J Intern Med 270:319–326
Hosking MP, Lane TE (2014) ELR(+) chemokine signaling in host defense and disease in a viral model of central nervous system disease. Front Cell Neurosci 8:165
Venkatesan A, Benavides DR (2015) Autoimmune encephalitis and its relation to infection. Curr Neurol Neurosci Rep 15:529
Hart JJ, Tillman G, Kraut MA, Chiang HS, Strain JF, Li Y, Agrawal AG, Jester P, Gnann JJ, Whitley RJ (2014) West Nile virus neuroinvasive disease: neurological manifestations and prospective longitudinal outcomes. BMC Infect Dis 14:248
van Riel D, Leijten LM, Verdijk RM, GeurtsvanKessel C, van der Vries E, van Rossum AM, Osterhaus AD, Kuiken T (2014) Evidence for influenza virus CNS invasion along the olfactory route in an immunocompromised infant. J Infect Dis 210:419–423
Centers for Disease Control and Prevention (2013) Influenza vaccination practices of physicians and caregivers of children with neurologic and neurodevelopmental conditions—United States, 2011–12 influenza season. MMWR Morb Mortal Wkly Rep 62:744–746
Myint KS, Kipar A, Jarman RG, Gibbons RV, Perng GC, Flanagan B, Mongkolsirichaikul D, Van Gessel Y, Solomon T (2014) Neuropathogenesis of Japanese encephalitis in a primate model. PLoS Negl Trop Dis 8:e2980
Ye J, Jiang R, Cui M, Zhu B, Sun L, Wang Y, Zohaib A, Dong Q, Ruan X, Song Y, He W, Chen H, Cao S (2014) Etanercept reduces neuroinflammation and lethality in mouse model of Japanese encephalitis. J Infect Dis 210:875–889
Sethi P, Lukiw WJ (2009) Micro-RNA abundance and stability in human brain: specific alterations in Alzheimer’s disease temporal lobe neocortex. Neurosci Lett 459:100–104
Lopez-Ramirez MA, Wu D, Pryce G, Simpson JE, Reijerkerk A, King-Robson J, Kay O, de Vries HE, Hirst MC, Sharrack B, Baker D, Male DK, Michael GJ, Romero IA (2014) MicroRNA-155 negatively affects blood-brain barrier function during neuroinflammation. FASEB J 28:2551–2565
Hill JM, Zhao Y, Clement C, Neumann DM, Lukiw WJ (2009) HSV-1 infection of human brain cells induces miRNA-146a and Alzheimer-type inflammatory signaling. Neuroreport 20:1500–1505
Dogini DB, Avansini SH, Vieira AS, Lopes-Cendes I (2013) MicroRNA regulation and dysregulation in epilepsy. Front Cell Neurosci 7:172
Jimenez-Mateos EM, Henshall DC (2013) Epilepsy and microRNA. Neuroscience 238:218–229
Gorter JA, Iyer A, White I, Colzi A, van Vliet EA, Sisodiya S, Aronica E (2014) Hippocampal subregion-specific microRNA expression during epileptogenesis in experimental temporal lobe epilepsy. Neurobiol Dis 62:508–520
Aronica E, Fluiter K, Iyer A, Zurolo E, Vreijling J, van Vliet EA, Baayen JC, Gorter JA (2010) Expression pattern of miR-146a, an inflammation-associated microRNA, in experimental and human temporal lobe epilepsy. Eur J Neurosci 31:1100–1107
Omran A, Peng J, Zhang C, Xiang QL, Xue J, Gan N, Kong H, Yin F (2012) Interleukin-1beta and microRNA-146a in an immature rat model and children with mesial temporal lobe epilepsy. Epilepsia 53:1215–1224
Gorter JA, van Vliet EA, Aronica E, Breit T, Rauwerda H, Lopes DSF, Wadman WJ (2006) Potential new antiepileptogenic targets indicated by microarray analysis in a rat model for temporal lobe epilepsy. J Neurosci 26:11083–11110
Aronica E, Gorter JA (2007) Gene expression profile in temporal lobe epilepsy. Neuroscientist 13:100–108
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108
Budde BS, Namavar Y, Barth PG, Poll-The BT, Nurnberg G, Becker C, van Ruissen F, Weterman MA, Fluiter K, Te BE, Aronica E, van der Knaap MS, Hohne W, Toliat MR, Crow YJ, Steinling M, Voit T, Roelenso F, Brussel W, Brockmann K, Kyllerman M, Boltshauser E, Hammersen G, Willemsen M, Basel-Vanagaite L, Krageloh-Mann I, de Vries LS, Sztriha L, Muntoni F, Ferrie CD, Battini R, Hennekam RC, Grillo E, Beemer FA, Stoets LM, Wollnik B, Nurnberg P, Baas F (2008) tRNA splicing endonuclease mutations cause pontocerebellar hypoplasia. Nat Genet 40:1113–1118
Chen CJ, Ou YC, Lin SY, Raung SL, Liao SL, Lai CY, Chen SY, Chen JH (2010) Glial activation involvement in neuronal death by Japanese encephalitis virus infection. J Gen Virol 91:1028–1037
Jiang R, Ye J, Zhu B, Song Y, Chen H, Cao S (2014) Roles of TLR3 and RIG-I in mediating the inflammatory response in mouse microglia following Japanese encephalitis virus infection. J Immunol Res 2014:787023
Olson JK, Miller SD (2004) Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol 173:3916–3924
Pareek S, Roy S, Kumari B, Jain P, Banerjee A, Vrati S (2014) MiR-155 induction in microglial cells suppresses Japanese encephalitis virus replication and negatively modulates innate immune responses. J Neuroinflamm 11:97
Thongtan T, Thepparit C, Smith DR (2012) The involvement of microglial cells in Japanese encephalitis infections. Clin Dev Immunol 2012:890586
Thongtan T, Wikan N, Wintachai P, Rattanarungsan C, Srisomsap C, Cheepsunthorn P, Smith DR (2012) Characterization of putative Japanese encephalitis virus receptor molecules on microglial cells. J Med Virol 84:615–623
Chen CJ, Ou YC, Chang CY, Pan HC, Liao SL, Raung SL, Chen SY (2011) TNF-alpha and IL-1beta mediate Japanese encephalitis virus-induced RANTES gene expression in astrocytes. Neurochem Int 58:234–242
Mishra MK, Kumawat KL, Basu A (2008) Japanese encephalitis virus differentially modulates the induction of multiple pro-inflammatory mediators in human astrocytoma and astroglioma cell-lines. Cell Biol Int 32:1506–1513
Mishra MK, Kumawat KL, Basu A (2008) Japanese encephalitis virus differentially modulates the induction of multiple pro-inflammatory mediators in human astrocytoma and astroglioma cell-lines. Cell Biol Int 32:1506–1513
Das S, Mishra MK, Ghosh J, Basu A (2008) Japanese Encephalitis Virus infection induces IL-18 and IL-1beta in microglia and astrocytes: correlation with in vitro cytokine responsiveness of glial cells and subsequent neuronal death. J Neuroimmunol 195:60–72
Tomita M, Tanaka Y, Mori N (2012) MicroRNA miR-146a is induced by HTLV-1 tax and increases the growth of HTLV-1-infected T-cells. Int J Cancer 130:2300–2309
Rom S, Rom I, Passiatore G, Pacifici M, Radhakrishnan S, Del VL, Pina-Oviedo S, Khalili K, Eletto D, Peruzzi F (2010) CCL8/MCP-2 is a target for mir-146a in HIV-1-infected human microglial cells. FASEB J 24:2292–2300
Punj V, Matta H, Schamus S, Tamewitz A, Anyang B, Chaudhary PM (2010) Kaposi’s sarcoma-associated herpesvirus-encoded viral FLICE inhibitory protein (vFLIP) K13 suppresses CXCR4 expression by upregulating miR-146a. Oncogene 29:1835–1844
Taganov KD, Boldin MP, Chang KJ, Baltimore D (2006) NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA 103:12481–12486
Hou J, Wang P, Lin L, Liu X, Ma F, An H, Wang Z, Cao X (2009) MicroRNA-146a feedback inhibits RIG-I-dependent Type I IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J Immunol 183:2150–2158
Acknowledgements
The present study was supported by the grant from First Affiliated Hospital to Science and Technology University of Henan (ST-2014-017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The protocol of current study was approved by the Ethics Committee of the First Affiliated Hospital to Science and Technology University of Henan. Animal experiments were performed according to the Guide for the Care and Use of Laboratory Animals of China.
Conflict of interest
Authors declare no conflict of interests regarding the publication of this article.
Additional information
M. Deng and G. Du contributed equally to this work.
Rights and permissions
About this article
Cite this article
Deng, M., Du, G., Zhao, J. et al. miR-146a negatively regulates the induction of proinflammatory cytokines in response to Japanese encephalitis virus infection in microglial cells. Arch Virol 162, 1495–1505 (2017). https://doi.org/10.1007/s00705-017-3226-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-017-3226-3