Abstract
The use of DNA vaccines has become an attractive approach for generating antigen-specific cytotoxic CD8+ T lymphocytes (CTLs), which can mediate protective antitumor immunity. The potency of DNA vaccines encoding weakly immunogenic tumor-associated antigens (TAAs) can be improved by using an adjuvant injected together with checkpoint antibodies. In the current study, we evaluated whether the therapeutic effects of a DNA vaccine encoding human papilloma virus type 16 (HPV-16) E7 can be enhanced by combined application of an immune checkpoint blockade directed against the programmed death-1 (PD-1) pathway and secondary lymphoid tissue chemokine (SLC) also known as CCL21 adjuvant, in a mouse cervical cancer model. The therapeutic effects of the DNA vaccine in combination with CCL21 adjuvant plus PD-1 blockade was evaluated using a tumor growth curve. To further investigate the mechanism underlying the antitumor response, cytolytic and lymphocyte proliferation responses in splenocytes were measured using non-radioactive cytotoxicity and MTT assays, respectively. Vascular endothelial growth factor (VEGF) and IL-10 expression in the tumor and the levels of IFN-γ and IL-4 in supernatants of spleno-lymphocyte cultures were measured using ELISA. The immune efficacy was evaluated by in vivo tumor regression assay. The results showed that vaccination with a DNA vaccine in combination with the CCL21 adjuvant plus PD-1 blockade greatly enhanced cytotoxic T lymphocyte production and lymphocyte proliferation rates and greatly inhibited tumor progression. Moreover, the vaccine in combination with adjuvant and blockade significantly reduced intratumoral VEGF, IL-10 and splenic IL-4 but induced the expression of splenic IFN-γ. This formulation could be an effective candidate for a vaccine against cervical cancers and merits further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human papilloma viruses (HPVs) have been associated with the etiology of cervical cancer, the second most common form of cancer among young women [5].
There is growing evidence that the immune system is able to induce destructive responses against tumors that can be improved using several approaches [47]. Effective cancer immunotherapies must envision the stimulation of cell-mediated immunity as well as control negative immunological checkpoints that may interrupt effector T-cell responses [50].
Recent studies have significant implications for the design of therapeutic vaccines against HPV. DNA vaccines have been developed as an efficient therapeutic for HPV that shows promise as a potential treatment for HPV-associated cancers [23]. The viral oncogenes E6 and E7 are constitutively expressed by infected tumor cells and are responsible for the maintenance of the oncogenic transformation of HPV [16, 51]. Therefore, E6 and E7 are regarded as the preferred antigenic targets for immunotherapy of HPV-induced lesions and tumors.
However, naked DNA vaccines have the disadvantages of low immunogenicity, limited specificity for antigen-presenting cells (APCs) and restricted ability to spread between cells in vivo. These factors limit the potency of HPV DNA vaccines. To address these challenges, several strategies have been used to improve DNA vaccine potency and tumor-associated antigen (TAA) immunogenicity [38]. A number of studies have shown that co-administration of DNA vaccines with cytokines and chemokine adjuvants enhances immune responses to weak tumor-associated antigens and induce antitumor immunity [2, 28]. Chemokines are a unique family of soluble molecules that play a key role in the migration and recruitment of dendritic cells (DCs) [31]. Activation of tumor-associated CD8+ cytotoxic T lymphocytes (CTLs) often requires effective priming by DCs to boost vaccine-induced immune responses [21]. Induction and maintenance of CTL responses are considered key elements for regression of lesions in HPV-associated cancers [36].
The chemokine CCL21 has shown immunotherapeutic potential for anti-cancer vaccination approaches. CCL-21 can recruit both Th1 lymphocytes and antigen-stimulated dendritic cells into secondary lymphoid organs, resulting in T cell activation [15, 34]. Based on these properties, the hypothesis of the present study was that incorporating recombinant CCL21 into a DNA vaccine would increase tumor-specific immune responses.
Programmed cell death protein 1 (PD-1) is an immunosuppressive receptor on T cells that is expressed following T-cell activation [18]. In the tumor microenvironment, PD-L1 can be upregulated in tumor cells and tumor-infiltrating CD8+ T cells [14]. Furthermore, low cytotoxicity of T cells may be related to a high expression level of PD-1 [3, 44]. Therefore, targeting PD-1 with blocking antibodies enhances the efficiency of the tumor-specific CD8 T cell response in the tumor microenvironment, which results in tumor regression in experimental models.
The present study aimed to determine whether blocking of PD-1 in combination with adjuvanted DNA vaccine with CCL21 could induce synergistic anti-tumor effects and enhance E7-specific immune responses. The results showed that the simultaneous stimulation of different immune targets by a combination of treatments could produce a more effective antitumor immune response.
Materials and methods
Animals
Six- to eight-week-old female C57BL/6 mice were purchased from the Pasteur Institute (Karaj, Iran) and kept in the animal facility of Golestan University of Medical Sciences. All animals were housed in a specific-pathogen-free facility in microisolator cages with a 6- to18-hour light/dark cycle. All experiments were approved by the local animal ethics council of Golestan Ethics Committee of Golestan University of Medical Sciences (ethics number: et- 157495) and were performed in accordance with the national experimental guidelines.
Cell lines
The murine lung cancer cell line TC1 was derived from mouse lung epithelial cells immortalized with HPV-16 E6 and E7 and transformed with the c-Ha-ras oncogene (23).
The cell line was maintained in Roswell Park Memorial Institute (RPMI 1640) medium (GibcoBRL, Gaithersburg, MD, USA) supplemented with 10 % fetal calf serum (Invitrogen), 2.5 mM 2-mecaptoethanol (Invitrogen), 0.5 mM sodium pyruvate (Sigma), 2 mM L-glutamate (Invitrogen), and 10 IU of penicillin- streptomycin (Sigma) per ml.
For EL4 (a murine T-cell lymphoma of haplotype H-2b that was established in tissue culture from a lymphoma induced in a C57BL/6 mouse using 9,10-dimethyl-1,2-benzanthracene) cell culture, cells were seeded in 96-well tissue culture plates and grown in phenol-red-free RPMI containing 2 % FBS and 500 U of penicillin per ml for 3 days to confluency.
DNA vaccine, adjuvant and antibody
The recombinant eukaryotic expression vector pcDNA3.1-E7 containing human papilloma virus type 16 E7 (HPV-16 E7) was prepared as described previously [13]. It was propagated in E. coli DH5α and confirmed to contain the E7 cDNA sequence by agarose gel electrophoresis and DNA sequence analysis. Chinese hamster ovary (CHO) cells were transfected with the pcDNA3.1- E7, and the expression of E7 in the transfected cells was verified by western blot.
Large-scale production of endotoxin-free pcDNA3.1-E7DNA vaccine and pcDNA3.1 as control plasmid were prepared for in vivo immunization studies using an EndoFree® Plasmid Maxi Kit (QIAGEN, Hilden, Germany) and dissolved in endotoxin-free Tris-EDTA (Sigma, St. Louis, MO).
Recombinant murine CCL21 was obtained from R&D Systems (Minneapolis, MN) and was delivered in 50 μl of sterile PBS with 0.05 % normal mouse serum (Sigma). Control PBS injections also included 0.05 % normal mouse serum.
Rat anti-mouse PD-1 mAb (clone RMP1-14) and rat IgG2a isotype control were purchased from BioXcell.
Tumor treatment assay
Twelve groups of C57BL/6 mice (n = 10) were challenged by subcutaneous (s.c.) injection in the right flank with 6 × 105 TC-1 (ATCC) cells constitutively expressing wild-type HPV16E6E7 in 100 µl PBS. After one week, all mice were vaccinated with different injection formulations (Table 1).
Tumor-bearing mice were treated three times at 7-day intervals with intratumoral injections of 90 μg of plasmid encoding HPV-16 E7 (DNA vaccine).
In the adjuvanted vaccine groups, DNA vaccine encoding HPV-16 E7 (90 μg) in combination with 2 μg of recombinant murine CCL21 was administered intratumorally (i.t.) to mice on the same day as the DNA vaccine group (DNA vaccine/CCL21). Control mice were injected with empty plasmid in combination with CCL21 (empty plasmid/CCL21).
For the PD1 blockade groups, mice were vaccinated three times with plasmid encoding HPV-16 E7 and injected intraperitoneally (i.p.) with 200 μg of anti-PD1 mAb (DNA vaccine/anti-PD-1) (or isotype control mAb) (DNA vaccine/isotype control) at the same time as DNA immunization. Control mice were injected with empty plasmid and anti-PD1 mAb under the same protocol (empty plasmid/anti-PD-1).
In the combination treatment group, TC-1 mice were vaccinated three times at 7-day intervals with intratumoral injections of plasmid encoding HPV-16 E7 in combination with 2 μg of recombinant murine CCL21 and treated with intraperitoneal anti-PD1 mAb at the same times (DNA vaccine/anti-PD-1/CCL21). Control mice were injected with empty plasmid in combination with anti-PD-1 and CCL21 (empty plasmid/anti-PD-1/CCL21).
As controls, groups of mice were immunized with pcDNA3.1, anti-PD1 alone, CCL21 alone and PBS alone under the same protocol.
The vaccine volume was adjusted to 150 μL/mouse with PBS. All mice were immunized three times with the same dosage of the corresponding injection formulation at one-week intervals.
The schedule for tumor challenge, combination treatment, and immune analysis is illustrated in Figure 1.
Tumor growth was measured every other day using a caliper according to Carlsson’s formula [39] and plotted as a function of time to generate in vivo growth curves. The tumor volume in mm3 was calculated using the formula volume = (width)2 × length/2. Tumor growth was monitored for at least 42 days, and the immune response was analyzed one week after the last immunization. Statistical analysis was performed using Student’s t-test. The recorded data represent the mean ± standard deviation (S.D.) of three measurements.
Three mice per group were sacrificed one week after the third immunization, the spleens were removed aseptically, and cell proliferation, cytolytic activity and cytokine secretion were then assayed. Results are representative of three independent experiments.
Lymphocyte proliferation assay (LPA)
One week after the third immunization, spleens of three mice per group were removed in order to evaluate the cellular immune response. Splenocytes were isolated by sieving the dissected spleens through a 40-μm cell strainer, depleting red blood cells with NH4Cl lysis solution, and washing twice with RPMI 1640 medium. Cells were suspended at 2 × 105 cells/ml in RPMI-1640 supplemented with 10 % fetal calf serum, 1 % L-glutamine, 1 % HEPES, 0.1 % penicillin/streptomycin, and 25 mg of amphotericin B per ml.
Subsequently, lymphocytes were cultured in a 96-well plate and incubated at 37°C in 5 % CO2 in the presence of 1 µg of synthetic E749–57-specific H-2Db CTL epitope (specific antigen per ml) or in the absence of stimuli (medium only). Lymphoproliferation was determined by colorimetric MTT assay. MTT salt (3-(4,5-dimethyl tetrazolyl-2) 2,5 diphenyl) tetrazolyumbromide; Sigma Chemicals) is converted to purple formazan by the mitochondrial activity of living cells [39]. Seventy-two hours after stimulation, 30 µl of sterile MTT solution 5 mg/ml was added to each well and incubated for another 5 h for MTT reduction. DMSO (dimethyl sulfoxide) (100 µl) was then added to dissolve the formazan crystals that were produced. The optical density was measured using an automatic microplate reader at 540 nm wavelength, and the results were expressed as the stimulation index (SI) [28]. The SI was determined as the OD value of stimulated cells with E749–57 (Cs) minus the relative OD of unstimulated cells (Cu) divided by the relative OD value of unstimulated cells: SI = (Cs-Cu)/Cu
Cytotoxicity assay
C57BL/6 mice were immunized as described above. Seven days after the final immunization, spleens were collected from three mice per group, and a single-cell suspension of splenocytes was prepared from each spleen and used as effector cells. 4 × 104 EL4 cells in a volume of 100 μl (as a target cells) were cocultured with the effector cells (100 μl) at various effector/target (E/T) ratios (25:1, 50:1, 100:1) for 8 h in phenol-red-free RPMI 1640 containing 3 % FCS.
For preparation of target cells, EL4 cells were stimulated with a synthetic E7-specific CTL epitope at a concentration of 1 µg/ml (specific antigen) prior to a 4-h incubation.
After centrifugation, the coculture supernatant (50 μl per well) was transferred to 96-well flat-bottom plates (Nunc, Denmark), and lysis of target cells was determined by assaying lactate dehydrogenase (LDH) release using an LDH Cytotoxicity Detection Kit (Takara BIO INC, Shiga, Japan) according to the instructions provided by the manufacturer.
For all samples, including the controls, the assay was performed in triplicate. The LDH-mediated conversion of tetrazolium salt into a red formazan product was measured at 490 nm after incubation at room temperature for 30 min.
The spontaneous release of LDH by target cells or effector cells was assayed by incubation of target cells in the absence of effector cells and vice versa The maximum release of LDH was determined by incubation of the target cells in 1 % Triton X-100 in assay medium [12].
The percentage of specific cytolysis was calculated as follows:
[(experimental release - effector spontaneous release - target spontaneous release)/(target maximum release - target spontaneous release)] × 100.
Cytokine ELISA
Seven days after the last immunization, the mice were sacrificed, and their splenocytes were isolated.
Mononuclear cells from spleens of immunized mice at a concentration of 2 ×105 cells/well in a 96-well Costar plate (Nunc, Denmark) were incubated for 2 days in a total volume of 1.5 ml of RPMI-1640 supplemented with 10 % FCS, 1 % L-glutamine, 1 % HEPES, 0.1 % 2ME, and 0.1 % penicillin/streptomycin and pulsed with E7-specific H-2Db CTL epitope at a concentration of 1 µg/ml. IFN-γ and IL-4 secretion were evaluated 48 h after stimulation. Culture supernatant samples were collected at that time point and assayed for the presence of cytokines using commercially available sandwich-based ELISA kits (eBioscience, USA) according to the manufacturer’s instruction. The plates were read at 450 nm, and values were expressed as optical densities. Standard curves were created and used to calculate the cytokine level of each sample. All tests were performed in triplicate for each mouse.
Intratumoral cytokine assay
One week after the last immunization, the level of intratumoral IL-10 was determined using an ELISA Ready-Set-Go Kit (eBioscience, Inc. San Diego, CA). VEGF expression was also evaluated using a mouse VEGF ELISA kit (R&D Systems Inc., Minneapolis, MN, USA). For this, TC-1 tumors were harvested and tumor tissue extracts were prepared by mechanical homogenization and sonication of 100 mg of tumor in 500 μL of lysis buffer (50 mM Tris HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 0.1 % SDS, 1 % Triton X-100, 1 % sodium deoxycholate, and 1 mM phenylmethanesulfonyl fluoride [PMSF]). The samples were then homogenized and centrifuged at 14,000 rpm at 4°C for 30 min. The supernatants were used for cytokine measurement following the manufacturer’s instructions, and the data were expressed at pg/ml tumor tissue [39].
Statistical analysis
Lymphocyte proliferation, CTL and cytokine assay results were analyzed using a one-way ANOVA. Significant differences in tumor growth on given days were assessed by Student’s t-test. Differences were considered statistically significant when the P-value was less than 0.05. All tests were performed in triplicate, and all data are expressed as mean ± SD. To compare results between the different groups, a one-way ANOVA was used. The statistical software SPSS version 16.0 was utilized for statistical analysis.
Results
Tumor therapy
To test the hypothesis that PD-1 blockade and CCL21 adjuvant may synergize with DNA-based vaccine in a murine model of cervical cancer, the efficacy of blocking antibodies as a single agent was first evaluated. Tumor cells were subcutaneously injected into C57BL/6 mice on day 0. Ten days after tumor transplant, PD-1 blockade with or without DNA vaccine HPV-16 E7 adjuvanted with CCL21 adjuvant was initiated. The treatment was administered i.p. in tumor-bearing mice for a total of three doses. Tumor measurements recorded at one-week intervals are shown in Figure 2. Treatment with anti-PD-1 alone did not show a significant effect on tumor growth as compared with the isotype control IgG group (P = 0.53).
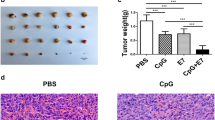
DNA vaccine in combination with CCL21 adjuvant plus PD-1 blockade inhibits the growth of established tumors in tumor cell (TC-1)-bearing mice. Tumor cells were injected s.c. into C57BL/6 mice on day 0. Ten days after tumor transplantation, PD-1 blockade with or without DNA vaccine HPV-16 E7 adjuvanted with CCL21 adjuvant was initiated. Tumor growth was monitored for at least 42 days by measuring diameters with calipers every other day. Line and scatter plot graphs illustrate quantitative evaluation of the tumor volume (mean ± standard deviation, n = 7) in each group. Statistical analysis was performed using Student’s t-test
The next step was to evaluate whether the DNA vaccine HPV-16 E7 adjuvanted with or without CCL21 adjuvant would induce an antigen-specific immune response that would reduce tumor growth in immunized mice. Compared with a relatively weak inhibitory effect on tumor growth when DNA vaccine alone was administrated (DNA vaccine), CCL21 adjuvant demonstrated a strong antitumor effect on the DNA vaccine (DNA vaccine/CCL21), mean ± SD: 371 ± 21 mm3 versus DNA vaccine, 656 ± 43 mm3; P < 0.001). In contrast, the use of CCL21 as an adjuvant and empty plasmid/CCL21 treatment had no antitumor effect (CCL21, mean ± SD, 809 ± 50 mm3 versus empty plasmid/CCL21, mean ± SD, 747 ± 29 mm3; P = 0.115).
Similarly, the tumor volume was also decreased 42 days post-tumor-challenge when the treatment with DNA vaccine and PD-1 blockade (DNA vaccine/anti-PD-1) was compared with empty plasmid/anti-PD-1 and DNA vaccine/ isotype control (DNA vaccine/anti-PD-1, mean ± SD, 281 ± 25 mm3 versus empty plasmid/anti-PD-1, mean ± SD, 699 ± 45 mm3; (P < 0.001), and DNA vaccine/anti-PD-1, mean ± SD, 281 ± 25 mm3 versus DNA vaccine/ isotype control, mean ± SD, 699 ± 30 mm3; (P < 0.001). Furthermore, the result showed that PD-1 blockade therapy combined with DNA vaccination significantly decreased tumor volume compared to DNA vaccine/ CCL21 treatment (DNA vaccine/anti-PD-1, mean ± SD, 281 ± 25 mm3 versus in DNA vaccine/ CCL21, mean ± SD, 371 ± 21 mm3; P < 0.01).
Tumor analysis of the combination treatment group showed that the systemic administration of anti-PD-1 antibody and intratumoral injection of CCL21 adjuvant reduced the tumor volume, and strongest tumor control in mice was observed when given in combination with DNA vaccine HPV-16 E7 compared to mice receiving DNA vaccine/ CCL21 and DNA vaccine/anti-PD-1 treatment, mean ± SD, 176 vs. 371 mm3; P < 0.001, and 176 vs. 281 mm3, respectively; P < 0.001 by Student’s t-test). In the combination treatment group, initial nonexponential tumor growth was observed for the first 3-4 weeks, followed by significant, durable growth inhibition of tumors. Tumors in the combination treatment group grew at a much slower rate than those in the DNA vaccine/anti-PD-1 group. At 4 weeks, the mean s.c. tumor volume for the combination treatment group was significantly lower than that of both the DNA vaccine/anti-PD-1 and empty plasmid/anti-PD-1/CCL21 groups (P < 0.001 by Student’s t-test). As expected, mice injected with pcDNA3.1 and PBS alone showed exponential tumor growth (Fig. 2). Thus, PD-1 checkpoint blockade plus vaccine has the potential to reduce tumor growth significantly, which could be maximized by boosting the antitumor immune response with adjuvant.
Lymphocyte proliferation assay
To study whether the administration of the PD-1 blockade potentiates cellular immune responses induced by DNA HPV-16 E7 adjuvanted with CCL21 in TC-1 tumor cell–bearing mice, an MTT assay detecting lymphocyte proliferation in response to E7 epitope stimulus was performed on splenocytes isolated from each group 1 week after the last immunization.
Mice in the group immunized with DNA vaccine/CCL21 showed significantly increased proliferation of spleen cells compared with the other two groups (DNA vaccine alone and CCL21 alone) on day 7 after the last immunization: DNA vaccine/CCL21, mean ± SD, 2.47 ± 0.21 vs. DNA vaccine, 1.35 ± 0.19 SI (P < 0.001), and DNA vaccine/CCL21, 2.47 ± 0.21 vs. CCL21, 0.52 ± 0.11 SI; P < 0.001, respectively.
For spleen cells from DNA vaccine/anti-PD-1-vaccinated mice, the treatment promoted significant proliferation compared to anti-PD-1 alone (DNA vaccine/anti-PD-1, mean ± SD, 2.68 ± 0.19 vs. anti-PD-1, 0.57 ± 0.1 SI (P < 0.001) but did not show a statistically significant proliferative effect over DNA vaccine/CCL21 (DNA vaccine/anti-PD-1, mean ± SD, 2.68 ± 0.19 vs. DNA vaccine/CCL21, 2.47 ± 0.21 SI, (P = 0.012)). Proliferation of the cells from combination treatment of mice was significantly higher than that induced in cells from the DNA vaccine/anti-PD-1 and DNA vaccine/CCL21 groups (DNA vaccine/anti-PD-1/CCL21, mean ± SD, 3.9 ± 0.14 SI; P < 0.001).
The proliferation was antigen-specific, since there was no expansion of splenocytes from C57BL/6 mice vaccinated with the control pcDNA3.1 plasmid and PBS in response to E7 antigen (Fig. 3). Thus, the combined therapeutic vaccine, anti-PD-1 and CCL21 significantly increased proliferation relative to monotherapy.
Proliferation of splenocytes collected from mice three weeks after immunization one week after stimulation for 3 days with 1 µg of E749–57-specific H-2Db CTL epitope (three per group) per ml measured by MTT assay to calculate the SI. Data are expressed as the mean ± SD absorbance values of stimulated cultures minus those of non-stimulated ones. *** indicates a statistically significant difference between the treated groups as determined by one-way ANOVA (P < 0.001) with control groups (PBS, pcDNA, CCL21 and PD-1). ### indicates a statistically significant difference between DNA vaccine/anti-PD-1/CCL21treatment and DNA vaccine/anti-PD-1 and DNA vaccine/CCL21 treatment (P < 0.001)
Cytotoxicity assay
To determine whether combining CCL21 adjuvant stimulation and PD-1 blockade with the DNA vaccine has the potential to enhance cytolytic activity against TC-1 tumors, cytotoxic T lymphocyte responses were measured based on lysis of syngeneic target EL4 lymphoma cells after one week of immunization.
The LDH release increased with the effector: target cell ratio up to the maximum ratio of 100:1; therefore, this ratio was used in the LDH analysis.
The results indicate a significant increase in cytotoxic activity against the target EL4 cells in the DNA vaccine plus CCL21 adjuvant group compared with the DNA vaccine alone: DNA vaccine/CCL21, mean ± SD, 42.2 ± 3.51 vs. DNA vaccine 29.4 ± 2.45, % cytotoxicity; P < 0.001).
Specific cytotoxic activity was significantly higher with DNA vaccine/anti-PD-1 treatment than with anti-PD-1 alone, empty plasmid/anti-PD-1 and DNA vaccine/ isotype control treatments (DNA vaccine/anti-PD-1, mean ± SD, 60.76 ± 4.11, anti- PD-1, mean ± SD, 17.2 ± 2.1, empty plasmid/anti-PD-1, mean ± SD, 19.3 ± 2.07; DNA vaccine/ isotype control, mean ± SD, 28.33 ± 1.3 % cytotoxicity; P < 0.001). Importantly, the E7-specific cytotoxicity was significantly higher in mice that received DNA vaccine/anti-PD-1 than in those treated with DNA vaccine plus CCL21 adjuvant (DNA vaccine/anti-PD-1, mean ± SD, 60.76 ± 4.11 vs. DNA vaccine/CCL21, mean ± SD, 42.2 ± 3.51 % cytotoxicity; P < 0.01).
The incorporation of systematic checkpoint blockade and local CCL21 adjuvant with the HPV16 E7 DNA vaccine demonstrated a synergistic effect on the specific CTL response, resulting in a significant difference in cytolytic activity compared to the other treatments (DNA vaccine/anti-PD-1/CCL21, mean ± SD, 80.1 ± 3.8 % cytotoxicity; P < 0.001). The control pcDNA3.1 plasmid and PBS induced no substantial increase in E7-specific cytotoxic activity (Fig. 4). These results indicate that the potency of vaccine-specific cytotoxic CD8+ T cells correlates with the therapeutic vaccine efficacy against established tumors.
C57BL/6 mice were immunized three times at one-week intervals, as described in Materials and methods. Seven days after the last immunization, CTL activity of the lymphocytes from immunized mice (three mice per group) was measured at a 100:1 E/T ratio using an LDH release assay kit. The graph shows the mean percent cytotoxicity of triplicate ± SD for a 100:1 splenocyte-to-EL4 (E:T) ratio and is representative of three independent experiments. *** indicates a statistically significant difference between the treated groups as determined by one-way ANOVA (P < 0.001) with control groups (PBS, pcDNA, CCL21 and PD-1). ### indicates a statistically significant difference between DNA vaccine/anti-PD-1/CCL21treatment and DNA vaccine/anti-PD-1 and DNA vaccine/CCL21 treatment (P < 0.001). ## indicates a statistically significant differences between DNA vaccine/anti-PD-1 treatment and DNA vaccine/CCL21 treatment (P < 0.01)
Cytokine ELISA
To determine whether the PD-1 blockade could enhance the antitumor immunity of the adjuvanted DNA-based vaccine, the IL-4/IFN-γ cytokine balance in restimulated spleen cells with E7-specific H-2Db CTL epitope was tested.
In spleen cells, higher IL-4 production was seen in DNA vaccine plus CCL21 adjuvant-treated mice compared with DNA-vaccinated mice (DNA vaccine/CCL21, mean ± SD, 231.1 ± 23.9 vs. DNA vaccine, mean ± SD, 132.1 ± 18.1 pg/ml; P < 0.001) (Fig. 5A), whereas this was not seen compared with DNA vaccine/anti-PD-1 mice (DNA vaccine/CCL21, mean ± SD, 231.1 ± 23.9 vs. DNA vaccine/anti-PD-1, mean ± SD, 223.4 ± 13.4 pg/ml; P = 0.07).
Quantitative ELISA analysis of IL-4 (A) and IFN-γ (B) secreted by lymphocytes upon re-stimulation with an E7-specific H-2Db CTL epitope. Splenocytes from mice that received DNA vaccine in conjunction with PD-1 blockade or CCL21 adjuvant were stimulated with E7 epitope or RPMI 1640 (negative control) for 48 h. Each bar represents the mean optical density values ± SD from three replicate wells from three individual mice. *** indicates a statistically significant difference between the treated groups (P < 0.001) compared with control groups (PBS, pcDNA, CCL21 and PD-1). ###, P < 0.001 for DNA vaccine/anti-PD-1/CCL21 combination group compared with the value for DNA vaccine/anti-PD-1 and DNA vaccine/CCL21 groups. ##, P < 0.01 for the DNA vaccine/anti-PD-1 group compared with the DNA vaccine/CCL21group
The level of IL-4 in E7-stimulated spleen cells was significantly elevated in mice treated with DNA vaccine/anti-PD-1 mice (DNA vaccine/anti-PD-1, mean ± SD, 223.4 ± 13.4 pg/ml; P < 0.001) compared to those in anti- PD-1 alone (mean ± SD: 71.1 ± 2.1 pg/ml), empty plasmid/anti-PD-1 (mean ± SD, 76.1 ± 17.7 pg/ml) and DNA vaccine/ isotype control (mean ± SD, 123.2 ± 3.7 pg/ml) treatments. It is noteworthy that, among all of the treatments, combination treatment induced the highest level of IL-4 secretion (DNA vaccine/anti-PD-1/CCL21, mean ± SD, 363.9 ± 25.3 pg/ml, P < 0.001) (Fig. 5A).
In spleen cells, higher IFN-γ production was seen in DNA vaccine plus CCL21 adjuvant mice compared with the DNA vaccine alone group (Fig. 5B) (DNA vaccine/CCL21, mean ± SD, 259.4 ± 23.9 pg/ml vs. DNA vaccine, 151.1 ± 10.2 pg/ml; P < 0.001).
In spleen cells stimulated with E7, IFN-γ production was significantly lower in DNA vaccine plus CCL21 adjuvant-treated mice than in the DNA vaccine/anti-PD-1-treated mice (DNA vaccine/CCL21, mean ± SD, 259.4 ± 23.9 pg/ml vs. DNA vaccine/anti-PD-1, mean ± SD, 379.3 ± 19.4; P < 0.01). IFN-γ was significantly higher after DNA vaccine/anti-PD-1 treatment than after anti-PD-1 alone (mean ± SD, 80.1 ± 12.2 pg/ml), empty plasmid/anti-PD-1 (mean ± SD, 81.1 ± 10.7 pg/ml) and DNA vaccine/isotype control treatments (mean ± SD, 165.8 ± 25.7 pg/ml) (P < 0.001). In comparison with all treatments, the combination DNA vaccine/anti-PD-1/CCL21 group (mean ± SD, 671.6 ± 24.3 pg/ml; P < 0.001) showed the most elevated IFN-γ secretion.
As expected, a significant IL-4 and IFN-γ increase was observed with all vaccine formulations compared to the pcDNA3.1 plasmid, PBS, and CCL21 controls. The increase in IFN-γ expression was much higher than that of IL-4, which indicates a switch from a Th2- to a Th1-dominated profile.
Th1 dominance in DNA vaccine/anti-PD-1/CCL21 and DNA vaccine/anti-PD-1 mice was evident from the 4.5- and 2.5-fold higher IFN-γ secretion, respectively, in E7-pulsed splenocytesas compared to those in the DNA-vaccine-alone group.
These data show that combining CCL21 adjuvant stimulation and PD-1 blockade with DNA vaccine has the potential to induce a protective Th1 cytokine shift in the Th1/Th2 balance, and it regulates cytokine production.
Intratumoral cytokine assay
To analyze whether the observed effects on tumor growth regression of combining CCL21 adjuvant stimulation and PD-1 blockade with DNA vaccine treatment could be correlated with the inhibitory action of the tumor microenvironment, the concentrations of tumor-derived immunosuppressive factors were determined in tumor lysates by ELISA assay.
All treated groups demonstrated a significant decrease in IL-10 and VEGF expression compared to pcDNA3.1 plasmid (IL-10 mean ± SD, 425.4 ± 39.6 and VEGF mean ± SD, 345.4 ± 26.3 pg/ml), PBS (IL-10 mean ± SD, 447.1 ± 40.6 and VEGF mean ± SD, 351.3 ± 28.7 pg/ml), anti-PD-1 (IL-10 mean ± SD, 330.1 ± 33.2 and VEGF mean ± SD, 306.1 ± 19.5 pg/ml) and CCL21 controls (IL-10 mean ± SD, 359.7 ± 30.6 and VEGF mean ± SD, 319.9 ± 22.6 pg/ml) (P < 0.001).
Concentrations of IL-10 and VEGF cytokines in mice treated with DNA vaccine alone were significantly higher than those in DNA vaccine plus CCL21 adjuvant group (DNA vaccine, IL-10 mean ± SD, 270.8 ± 38.7 vs. DNA vaccine plus CCL21, 227.1 ± 28.3 pg/ml; P < 0.001), and DNA vaccine, VEGF mean ± SD, 268.8 ± 18 vs. DNA vaccine plus CCL21, 207.5 ± 13 pg/ml; P < 0.001), while, the levels in DNA vaccine plus anti-PD-1 were significantly lower than DNA vaccine plus CCL21 adjuvant: DNA vaccine/anti-PD-1, IL-10 mean ± SD, 173.5 ± 11.8 vs. DNA vaccine plus CCL21, 227.1 ± 28.3 pg/ml (P < 0.001) and DNA vaccine plus anti-PD-1, VEGF mean ± SD,140.5 ± 22.2 vs. DNA vaccine plus CCL21, 207.5 ±13 pg/ml; P < 0.001.
Both cytokines displayed a significant reduction in intratumoral IL-10 and VEGF in mice treated with DNA vaccine/anti-PD-1/CCL21 (IL-10 mean ± SD, 107.2 ± 9.1 pg/ml; P < 0.001 and VEGF mean ± SD, 93.1 ± 8.4 pg/ml; P < 0.001) compared to tumors treated with DNA vaccine plus CCL21 adjuvant or with DNA vaccine plus anti-PD-1 (Fig. 6 A and B). These data suggest that the addition of anti-PD-1 and CCL21 leads to a reduction in immunosuppressive IL-10 and VEGF levels within the tumor microenvironment when combined with the DNA vaccine, which might improve the natural anti-tumor immune response. The decrease in expression of these immunosuppressive cytokines is in line with the enhancement of the splenic immune response following blockade of PD-1-mediated inhibition of lymphocyte activation.
Assessment of intratumoral cytokine secretion after PD-1 blockade in combination with adjuvanted DNA vaccine with CCL21. The concentrations of tumor-derived immunosuppressive factors IL-10 (A) and VEGF (B) were determined in tumor lysates by ELISA assay. The results demonstrate that DNA vaccine in conjunction with PD-1 blockade or adjuvant significantly reduced the levels of IL-10 and VEGF expression. Each bar represents the mean ± SD of the immunosuppressive cytokines in the tumor microenvironment from three individual mice. *** indicates a statistically significant difference between the treated groups (P < 0.001) compared with control groups (PBS, pcDNA, CCL21 and PD-1). ###, P < 0.001 for DNA vaccine/anti-PD-1/CCL21 combination group compared with the DNA vaccine/anti-PD-1 and DNA vaccine/CCL21 groups. +++, P < 0.001 for DNA vaccine/anti-PD-1 group compared with the DNA vaccine/CCL21group
Discussion
Although the DNA-vaccine -based approach has the potential to offer safe, systemic, and detectable immune responses against tumor-specific antigens [1], protective long-lasting antitumoral immunity in cancer patients remains to be achieved.
A successful therapeutic effect and overcoming cancer immune evasion involves both the innate and adaptive arms of the immune system [49]. Therefore, various approaches have been attempted in order to improve DNA vaccine immunogenicity, including manipulation of the immune system via immunomodulatory antibodies against checkpoint inhibitors and addition of adjuvants [20, 27].
A number of clinical findings in parallel with preclinical results using animal models have shown that targeting T cell inhibitory factors including cytotoxic T lymphocyte antigen 4 (CTLA4) and programmed cell death protein 1 (PD1) and its ligand programmed death-1 ligand 1 (PD-L1) may represent an effective therapeutic approach for a variety of cancers [43].
PD-1 is an inhibitory receptor that negatively regulates T-cell activation and might be responsible for compromised tumor immunity [32]. Therefore, inhibition of this pathway using blocking monoclonal antibodies against PD-1 provides a basis for targeting this pathway for cancer therapy.
Another possible approach to promoting the tumor-specific CD8 T cell responses is the use of chemokine adjuvants. Chemokines are a family of secreted chemoattractant cytokines that mediate leukocyte migration and initiation of a specific immune response [8]. One such chemokine, CCL21 is a CC chemokine that is selective for the chemokine receptor CCR7 on mature DCs and distinct T-and B-cell subpopulations, and it is constitutively expressed in T-cell zones of both spleen and lymph nodes [19]. These observations emphasize the potential of CCL21 as adjuvant for developing tumor-specific immunity. Since PD-1 and its ligand PD-L1 play an important role in tumor immune escape by maintaining an immunosuppressive tumor microenvironment, and secondary lymphoid tissue chemokine (SLC, also known as CCL21) elicited a considerable recruitment of T lymphocytes and DCs inside the tumor microenvironment, it was hypothesized that application of the PD-1 inhibitors in combination with a DNA vaccine could reverse the tumor microenvironment and improve the endogenous antitumor immune responses mediated by CCL21-induced chemoattraction.
In the present study, we demonstrate that antitumor vaccination with a DNA vaccine encoding a tumor-associated antigen E7 from human papillomavirus-16 can be significantly enhanced by combining vaccination with PD-1 blocking and CCL21 stimulation. Thus, the combination of CCL21-adjuvanted vaccine plus PD-1 blockade conferred robust antitumor effects and synergistically suppressed tumor growth in tumor cell (TC-1)-bearing mice.
In addition to driving the proliferation of tumor-specific CD4 T cells and the cytolytic activity of CD8 T lymphocytes, this combination promotes cellular immunity through the production of splenic IFN-γ and downregulation of tumor-derived immunosuppressive factors VEGF and IL-10.
We have previously developed a DNA vaccine encoding the HPV-16 E7 gene that generates modest antitumor effects [12, 45]. We have also demonstrated that the administration of adjuvants such as Toll-like receptor agonists facilitates the induction of tumor-specific CD8+ T cells and enhances the efficacy of this DNA vaccine in a murine TC-1 tumor model [11, 39].
In 2000, the first studies using intratumoral CCL21 as a monotherapy in lung carcinoma tumor models revealed enhanced cytolytic capacity, suggesting generation of systemic immune responses [42].
Since CCL21 is potent inducer of T cell proliferation, it has been demonstrated that mice injected with Her2/neu DNA vaccine plus CCL21 had substantially improved antitumor immune responses [30]. Physiologically, SLC or CCL21 serves to recruit both T lymphocytes and mature DCs and regulate DC homeostasis and function. In vivo studies have also shown that CCL21 is involved in effective T cell priming [46].
The results of the present study showed that mice immunized with an adjuvanted DNA vaccine with CCL21 induced an antitumor immune response, although to a lesser degree than that observed with DNA vaccine in combination with blockade of the inhibitory PD-1 pathway.
Increased expression of the immune modulatory molecule PD-L1 and immunosuppressive molecules in the tumor microenvironment produces unfavorable conditions for T-cell expansion stimulated by adjuvanted DNA vaccine treatment.
The importance of the PD-1/PD-L1 pathway in cancer immunity has been extensively studied, showing that therapeutic approaches targeting PD-1 and PD-L1 can reverse the suppressive state of the tumor microenvironment and thereby enhance antitumor immunity [37].
As a monotherapy, consistent with reported data [4], PD-L1 blockade did not induce any significant tumor protection in the subcutaneous tumor model. Enhanced anti-tumor responses are therefore expected if this blockade is combined with an immunotherapy such as a DNA vaccine.
Curran et al. have shown that blockade of PD-1 combined with tumor vaccine significantly improved survival rates and synergistically increased the ratio of effector T cells to myeloid-derived suppressor cells (MDSCs) within B16 melanomas [6]. A 2013 study indicated that PD-1/PD-L1 blockade enhanced tumor regression by increasing effector T cell activity, thereby diminishing regulatory T cell (Treg) suppression [9].
Similar findings have been reported by another group of investigators, who found that PD-1 blockade shows synergistic effects with several tumor vaccines to improve tumor-specific T cell responses and induce tumor regression in animal models [9, 24, 26].
In agreement with the findings of the present study, it has been reported recently that the antitumor activity of a DNA vaccine encoding TAAs could be improved when combined with antibodies blocking the PD-1 pathway [33]. Consequently, these studies, along with ours, demonstrate that blocking of PD-1 inhibitory signals by a monoclonal antibody might overcome the failure of current therapeutic vaccination approaches by potentiating anti-tumor immunity in mice [48].
Although the results of the present study have shown that a DNA vaccine in conjunction with PD-1 blockade or adjuvant is more effective than the vaccine alone, further improvement of the vaccine is still required to regulate the magnitude of the antitumor response.
Recent animal studies have suggested that combination approaches involving several immunotherapeutic agents may boost the therapeutic efficacy of cancer vaccines [7, 10]. These results were confirmed in the present study, demonstrating that i.t. injection of adjuvanted DNA vaccine with CCL21 combined with i.p. administration of anti-PD1 mAb has the greatest effect on regressing tumors in most of the tumor-bearing mice and most efficiently boosted epitope-specific CD8+ T cells.
The synergic effect of the PD-1 blockade in the combination therapy could be due to interactions with DCs, which express PD-L1 and PDL2.The PD-1 blockade could promote DC function and restore the effector function of T cells [29, 40].
Because the tumor cells can activate PD-L1 expression via multiple oncogenic signaling pathways [17, 22], it is also likely that PD-1 blockade improves the cytolytic activity of CD8 T lymphocytes, increasing antitumor immunity and perhaps promoting lymphocyte proliferation, activation and cytokine production in the tumor microenvironment.
It has been hypothesized that the DNA-vaccine-specific antitumor CD8 T-cell and lymphocyte proliferative responses induced against a plasmid encoding the tumor-associated antigen E7 from human papillomavirus-16 are in general ineffective and that the administration of CCL21 and anti-PD-1 mAb expands these responses or induces other antitumor T-cell responses that provide greater tumor control.
The ability of PD-1 blockade or CCL21 stimulation in conjunction with vaccine to attenuate the production of tumor-derived VEGF and IL-10 is highly desirable. Our findings indicate that DNA vaccine combined with adjuvant and PD-1 blockade most efficiently reduced angiogenic mediators (VEGF and IL-10).
Growth, progression, and metastasis of tumors are angiogenesis-dependent processes. VEGF is a main stimulant of angiogenesis and has an essential role in promoting progressive tumor growth mediated by tumor angiogenesis [41].
A recent study showed that VEGF can interfere with antitumor immunity through the generation of a local immunosuppressive tumor microenvironment [35]. IL-10 is also essential for regulatory T cells to mediate immune tolerance and functions as a suppressor cytokine that impairs antigen presentation [25]. In agreement with the current study, these studies highlight that reduction of VEGF and IL-10 expression could facilitate antitumor immunity, providing synergistic benefits when combined with immunotherapy.
Consistent with these results, in a recent study, Cappuccini et al. found that the combination of vaccine with PD-1 blocking antibody significantly improved survival of experimental animals, suggesting that the observed synergistic effect could be taking place at the tumor microenvironment, but not in the periphery [4]. This intratumoral evidence provides further new insights into the mechanisms underlying the antitumor synergistic effect of PD-1 blockade and CCL21 adjuvant in combination with DNA vaccine.
Our results suggest that the mechanisms underlying the synergistic effects of vaccine could possibly be due to the capacity of vaccine to increase the frequency of CD8+ tumor-infiltrating lymphocytes (TILs), which could be activated by CTLA-4 and PD-1 blockade.
Conclusions
Collectively, the current study shows that a tumor-targeted vaccine that was adjuvanted with an immunostimulatory agent could optimally combine with anti-PD-L1 antibody to promote antitumor immunity by modulating the tumor microenvironment and the adaptive immune responses. To our knowledge, this work represents the first report of strong synergy between chemokine adjuvant in conjunction with PD-1 blockade in DNA cancer vaccine preparations.
In summary, the current study illustrates the importance of combining therapeutic approaches such as immunotherapy together with immune checkpoint inhibition and adjuvant for efficient treatment of tumors through lymphocytic proliferation and cytolytic activity of splenocytes as well as switching the immune balance to the host protective Th1 response in vivo.
Abbreviations
- HPV:
-
Human papilloma virus
- APC:
-
Antigen-presenting cell
- CTL:
-
Cytolytic CD8+T lymphocyte
- DC:
-
Dendritic cells
- IFN- γ:
-
Interferon γ
- IL-4:
-
Interleukin 4
- VEGF:
-
Vascular endothelial growth factor
- IL-10:
-
Interleukin 10
- LDH:
-
Lactate dehydrogenase
- MTT:
-
3[4,5-Dimethylthiazol-2-ll]-2,5-diphenyltetrazolium bromide, thiazolyl-blue
- DMSO:
-
Dimethyl sulfoxide
- OD:
-
Optical density
- FBS:
-
Fetal bovine serum
- RPMI:
-
1640 Roswell Park Memorial Institute (name of the medium)
- Th:
-
T helper
- PD-1:
-
Programmed death-1
- SLC:
-
Secondary lymphoid tissue chemokine
- TAA:
-
Tumor-associated antigen
- TIL:
-
Tumor-infiltrating lymphocyte
References
Bahrami AA, Ghaemi A, Tabarraei A, Sajadian A, Gorji A, Soleimanjahi H (2014) DNA vaccine encoding HPV-16 E7 with mutation in L-Y-C-Y-E pRb-binding motif induces potent anti-tumor responses in mice. J Virol Methods 206:12–18
Bobanga ID, Petrosiute A, Huang AY (2013) Chemokines as cancer vaccine adjuvants. Vaccines 1:444–462
Bos R, Marquardt KL, Cheung J, Sherman LA (2012) Functional differences between low-and high-affinity CD8+ T cells in the tumor environment. Oncoimmunology 1:1239–1247
Cappuccini F, Stribbling S, Pollock E, Hill AV, Redchenko I (2016) Immunogenicity and efficacy of the novel cancer vaccine based on simian adenovirus and MVA vectors alone and in combination with PD-1 mAb in a mouse model of prostate cancer. Cancer immunology, immunotherapy : CII
Chen AA, Gheit T, Franceschi S, Tommasino M, Clifford GM (2015) Human Papillomavirus 18 Genetic Variation and Cervical Cancer Risk Worldwide. J Virol 89:10680–10687
Curran MA, Montalvo W, Yagita H, Allison JP (2010) PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proceed Nat Acad Sci USA 107:4275–4280
Drake C (2012) Combination immunotherapy approaches. Ann Oncol 23:viii41–viii46
Dubinett SM, Lee JM, Sharma S, Mule JJ (2010) Chemokines: can effector cells be redirected to the site of the tumor? Cancer journal (Sudbury, Mass) 16:325-335
Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G (2013) Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res 73:3591–3603
Friedberg JW, Kim H, McCauley M, Hessel EM, Sims P, Fisher DC, Nadler LM, Coffman RL, Freedman AS (2005) Combination immunotherapy with a CpG oligonucleotide (1018 ISS) and rituximab in patients with non-Hodgkin lymphoma: increased interferon-α/β–inducible gene expression, without significant toxicity. Blood 105:489–495
Gableh F, Saeidi M, Hemati S, Hamdi K, Soleimanjahi H, Gorji A, Ghaemi A (2016) Combination of the toll like receptor agonist and alpha-Galactosylceramide as an efficient adjuvant for cancer vaccine. J Biomed Sci 23:16
Ghaemi A, Soleimanjahi H, Bamdad T, Soudi S, Arefeian E, Hashemi SM, Ebtekar M (2007) Induction of humoral and cellular immunity against latent HSV-1 infections by DNA immunization in BALB/c mice. Comp Immunol Microbiol Infect Dis 30:197–210
Ghaemi A, Soleimanjahi H, Gill P, Hassan ZM, Razeghi S, Fazeli M, Razavinikoo SM (2011) Protection of mice by a lambda-based therapeutic vaccine against cancer associated with human papillomavirus type 16. Intervirology 54:105–112
He J, Hu Y, Hu M, Li B (2015) Development of PD-1/PD-L1 pathway in tumor immune microenvironment and treatment for non-small cell lung cancer. Sci Rep 5
Hong C, Lee HJ, Kim HJ, Lee JJ (2014) The Lymphoid Chemokine CCL21 Enhances the Cytotoxic T Lymphocyte-Inducing Functions of Dendritic Cells. Scand J Immunol 79:173–180
Huang Z, Peng S, Knoff J, Lee SY, Yang B, Wu TC, Hung CF (2015) Combination of proteasome and HDAC inhibitor enhances HPV16 E7-specific CD8+ T cell immune response and antitumor effects in a preclinical cervical cancer model. J Biomed Sci 22
Ji M, Liu Y, Li Q, Li X-D, Zhao W-Q, Zhang H, Zhang X, Jiang J-T, Wu C-P (2015) PD-1/PD-L1 pathway in non-small-cell lung cancer and its relation with EGFR mutation. Journal of translational medicine 13:1
Jin H-T, Ahmed R, Okazaki T (2010) Role of PD-1 in regulating T-cell immunity. Negative Co-Receptors and Ligands. Springer, pp 17–37
Kirk CJ, Hartigan-O’Connor D, Nickoloff BJ, Chamberlain JS, Giedlin M, Aukerman L, Mule JJ (2001) T cell-dependent antitumor immunity mediated by secondary lymphoid tissue chemokine: augmentation of dendritic cell-based immunotherapy. Cancer Res 61:2062–2070
Kyi C, Postow MA (2014) Checkpoint blocking antibodies in cancer immunotherapy. FEBS Lett 588:368–376
Lin K, Roosinovich E, Ma B, Hung CF, Wu TC (2010) Therapeutic HPV DNA vaccines. Immunol Res 47:86–112
Lu L, Xu X, Zhang B, Zhang R, Ji H, Wang X (2014) Combined PD-1 blockade and GITR triggering induce a potent antitumor immunity in murine cancer models and synergizes with chemotherapeutic drugs. J Trans Med 12:36
Ma B, Xu Y, Hung C-F, Wu T-C (2010) HPV and therapeutic vaccines: where are we in 2010? Curr Cancer Therapy Rev 6:81–103
McGray AJ, Bernard D, Hallett R, Kelly R, Jha M, Gregory C, Bassett JD, Hassell JA, Pare G, Wan Y, Bramson JL (2012) Combined vaccination and immunostimulatory antibodies provides durable cure of murine melanoma and induces transcriptional changes associated with positive outcome in human melanoma patients. Oncoimmunology 1:419–431
Mittal SK, Roche PA (2015) Suppression of antigen presentation by IL-10. Curr Opin Immunol 34:22–27
Mkrtichyan M, Najjar YG, Raulfs EC, Abdalla MY, Samara R, Rotem-Yehudar R, Cook L, Khleif SN (2011) Anti-PD-1 synergizes with cyclophosphamide to induce potent anti-tumor vaccine effects through novel mechanisms. Euro J Immunol 41:2977–2986
Müller D (2015) Antibody fusions with immunomodulatory proteins for cancer therapy. Pharmacol Ther 154:57–66
Naderi M, Saeedi A, Moradi A, Kleshadi M, Zolfaghari MR, Gorji A, Ghaemi A (2013) Interleukin-12 as a genetic adjuvant enhances hepatitis C virus NS3 DNA vaccine immunogenicity. Virologica Sinica 28:167–173
Nagato T, Lee YR, Harabuchi Y, Celis E (2014) Combinatorial immunotherapy of polyinosinic-polycytidylic acid and blockade of programmed death-ligand 1 induce effective CD8 T-cell responses against established tumors. Clin Cancer Res Off J Am Assoc Cancer Res 20:1223–1234
Nguyen-Hoai T, Baldenhofer G, Sayed Ahmed MS, Pham-Duc M, Vu MD, Lipp M, Dorken B, Pezzutto A, Westermann J (2012) CCL21 (SLC) improves tumor protection by a DNA vaccine in a Her2/neu mouse tumor model. Cancer Gene Therapy 19:69–76
Nguyen-Hoai T, Pham-Duc M, Gries M, Dörken B, Pezzutto A, Westermann J (2016) CCL4 as an adjuvant for DNA vaccination in a Her2/neu mouse tumor model. Cancer Gene Therapy
Pedoeem A, Azoulay-Alfaguter I, Strazza M, Silverman GJ, Mor A (2014) Programmed death-1 pathway in cancer and autoimmunity. Clin Immunol 153:145–152
Rekoske BT, Smith HA, Olson BM, Maricque BB, McNeel DG (2015) PD-1 or PD-L1 Blockade Restores Antitumor Efficacy Following SSX2 Epitope-Modified DNA Vaccine Immunization. Cancer Immunol Res 3:946–955
Riedl K, Baratelli F, Batra RK, Yang S, Luo J, Escuadro B, Figlin R, Strieter R, Sharma S, Dubinett S (2003) Overexpression of CCL-21/secondary lymphoid tissue chemokine in human dendritic cells augments chemotactic activities for lymphocytes and antigen presenting cells. Mol Cancer 2:1
Roland CL, Dineen SP, Lynn KD, Sullivan LA, Dellinger MT, Sadegh L, Sullivan JP, Shames DS, Brekken RA (2009) Inhibition of vascular endothelial growth factor reduces angiogenesis and modulates immune cell infiltration of orthotopic breast cancer xenografts. Mol Cancer Ther 8:1761–1771
Rosales R, Rosales C (2014) Immune therapy for human papillomaviruses-related cancers. World J Clin Oncol 5:1002
Rozali EN, Hato SV, Robinson BW, Lake RA, Lesterhuis WJ (2012) Programmed death ligand 2 in cancer-induced immune suppression. Clin Dev Immunol 2012:656340
Saade F, Petrovsky N (2012) Technologies for enhanced efficacy of DNA vaccines. Expert Rev Vaccines 11:189–209
Sajadian A, Tabarraei A, Soleimanjahi H, Fotouhi F, Gorji A, Ghaemi A (2014) Comparing the effect of Toll-like receptor agonist adjuvants on the efficiency of a DNA vaccine. Arch Virol 159:1951–1960
Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC (2010) Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med 207:2187–2194
Schmitz V, Vilanueva H, Raskopf E, Hilbert T, Barajas M, Dzienisowicz C, Gorschlüter M, Strehl J, Rabe C, Sauerbruch T (2006) Increased VEGF levels induced by anti-VEGF treatment are independent of tumor burden in colorectal carcinomas in mice. Gene Therapy 13:1198–1205
Sharma S, Stolina M, Luo J, Strieter RM, Burdick M, Zhu LX, Batra RK, Dubinett SM (2000) Secondary lymphoid tissue chemokine mediates T cell-dependent antitumor responses in vivo. J Immunol (Baltimore, Md : 1950) 164:4558–4563
Sharon E, Streicher H, Goncalves P, Chen HX (2014) Immune checkpoints in cancer clinical trials. Chinese Journal of Cancer 33:434–444
Shi L, Chen S, Yang L, Li Y (2013) The role of PD-1 and PD-L1 in T-cell immune suppression in patients with hematological malignancies. J Hematol Oncol 6:74
Tahamtan A, Ghaemi A, Gorji A, Kalhor HR, Sajadian A, Tabarraei A, Moradi A, Atyabi F, Kelishadi M (2014) Antitumor effect of therapeutic HPV DNA vaccines with chitosan-based nanodelivery systems. J Biomed Sci 21:69
Tolba KA, Bowers WJ, Muller J, Housekneckt V, Giuliano RE, Federoff HJ, Rosenblatt JD (2002) Herpes simplex virus (HSV) amplicon-mediated codelivery of secondary lymphoid tissue chemokine and CD40L results in augmented antitumor activity. Cancer Res 62:6545–6551
Vanneman M, Dranoff G (2012) Combining immunotherapy and targeted therapies in cancer treatment. Nature Rev Cancer 12:237–251
Vezys V, Penaloza-MacMaster P, Barber DL, Ha SJ, Konieczny B, Freeman GJ, Mittler RS, Ahmed R (2011) 4-1BB signaling synergizes with programmed death ligand 1 blockade to augment CD8 T cell responses during chronic viral infection. J Immunol (Baltimore, Md : 1950) 187:1634–1642
Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, Lichtor T, Decker WK, Whelan RL, Kumara HS (2015) Immune evasion in cancer: Mechanistic basis and therapeutic strategies. In: Seminars in Cancer Biology. Elsevier, pp S185–S198
Waldmann TA (2006) Effective cancer therapy through immunomodulation. Ann Rev Med 57:65–81
Yang MC, Yang A, Qiu J, Yang B, He L, Tsai YC, Jeang J, Wu TC, Hung CF (2016) Buccal injection of synthetic HPV long peptide vaccine induces local and systemic antigen-specific CD8+ T-cell immune responses and antitumor effects without adjuvant. Cell Biosci 6
Acknowledgments
The authors would like to acknowledge Institute Pasteur of Iran and Golestan Medical University for the financial support. This project was extracted from a MSC thesis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by Institute Pasteur of Iran and Research Deputy at Golestan Medical University through Grant Project Number 157495.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The animal protocol used in this study was approved by the local animal ethics council of Golestan Ethics Committee of Golestan University of Medical Sciences (ethics number: et-157495). All experimental procedures involving mice were performed in accordance with the national experimental guidelines.
Additional information
The original version of this article was revised: “Unfortunately, the fourth author name “Mahdieh Mondanizadeh” was incorrectly published in this original version and the same is corrected here”.
An erratum to this article is available at http://dx.doi.org/10.1007/s00705-016-3160-9.
Rights and permissions
About this article
Cite this article
Moeini, S., Saeidi, M., Fotouhi, F. et al. Synergistic effect of programmed cell death protein 1 blockade and secondary lymphoid tissue chemokine in the induction of anti-tumor immunity by a therapeutic cancer vaccine. Arch Virol 162, 333–346 (2017). https://doi.org/10.1007/s00705-016-3091-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-016-3091-5