Abstract
Porcine parvovirus (PPV) infections can lead to significant losses to the swine industry by causing reproductive failure in pigs. Germacrone has been reported to efficiently suppress the replication of influenza virus. In this report, the antiviral activity of germacrone on PPV in swine testis (ST) cells was investigated. Here, we show for the first time that germacrone protects cells from PPV infection and suppresses the synthesis of viral mRNA and protein. Furthermore, we show that germacrone inhibits PPV replication at an early stage in a dose-dependent manner. These findings suggest that germacrone is a potential candidate for anti-PPV therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Porcine parvovirus (PPV) belongs to the family Parvoviridae, subfamily Parvovirinae, genus Protoparvovirus, species Ungulate protoparvovirus 1. The parvoviruses are small, non-enveloped, and single-stranded DNA viruses. The PPV mainly causes reproductive failure in sows, and is a significant contributor to porcine post-weaning multisystemic wasting syndrome (PMWS) [1, 7, 16]. PPV was first isolated from sick pigs in 1965 [13], and has caused significantly economic loss in the swine industry [2, 4, 6, 14, 16, 17]. Currently, vaccination is the primary measure to prevent PPV infection. However, the high cost of vaccination and biological safety issues such as recombination and mutation of viruses are problems that remain to be solved. On the other hand, PPV is still widespread in swine herds despite vaccination. Some studies have shown that although vaccination can relieve the clinical symptoms of PPV infection, the impairment caused by infection cannot be eliminated [8, 19]. PPV infection can also be restrained by antiviral drugs such as lithium chloride [3]. Therefore, antiviral therapies could be considered as an alternative strategy to control PPV infection.
Germacrone, which is extracted from Rhizoma Curcuma, is one of the main bio-active components in volatile oils (Fig. 1A) [20]. The compound exerts an anti-tumor effect in breast cancer by suppressing cell proliferation, metastasis and angiogenesis in vitro [11, 20, 21]. Recent studies have shown that germacrone can inhibit influenza viruses by interfering with the early stages of the viral life cycle in cells and can protect mice from lethal infection [10]. However, whether germacrone has an antiviral effect on other viruses has not been studied. In the present study, the antiviral activity of germacrone on PPV in swine testis (ST) cells was investigated. Germacrone suppressed the synthesis of viral mRNA and protein, and the inhibition of PPV replication occurred at an early stage in a dose-dependent manner.
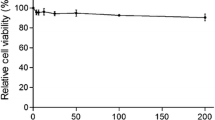
Structure and cytotoxic effect of germacrone. A. Structure of germacrone: (3E,7E)-3,7-dimethyl-10-propan-2-ylidenecyclodeca-3,7-dien-1-one. B. Cytotoxic effect of germacrone treatment on ST cells. Cells were treated with series of concentrations (0, 10, 50, 100, and 200 μM) of germacrone for 24 h. Relative cell viability was determined by CCK8 assay and normalized to the value of the untreated control (set as 100 %). Data are mean ± S.D. from three independent experiments
Materials and methods
Virus, cells and drug
PPV was isolated from a pig farm in Guangdong Province of China.
ST cells were obtained from the American Type Culture Collection (ATCC) and maintained in Dulbecco’s modified Eagle medium (DMEM) (Gibco, USA) containing 10 % fetal bovine serum (FBS) at 37 °C and 5 % CO2. Germacrone (Sichuan Weikeqi, China) was prepared in DMSO.
Cytotoxicity assay
Cells were cultured in 96-well plates in DMEM with 10 % FBS and incubated at 37 °C for 24 h. The cytotoxicity of germacrone was evaluated using a Cell Counting Kit-8 (CCK8; Donjindo, Japan) following the manufacturer’s instructions. Briefly, the cells in the 96-well culture plates were washed three times with PBS and then were incubated with 100 µl of serum-free DMEM containing germacrone at various concentrations (10, 20, 25, 30, 50, 100, 200, 250 μM) for 24 h. The optical density (OD) in each well was measured at a wavelength of 450 nm using a microplate reader (Bio-Rad). The relative cell viability rate was determined for each concentration as OD450 drug / OD450 control × 100. Concentrations under the 50 % cytostatic concentration (CC50) were defined as non-toxic.
Effect of drug addition at different stages of virus infection
Viral attachment stage
To evaluate whether germacrone could affect PPV attachment to cells, 1 × 104 ST cells were seeded per well in 24-well plates and infected with PPV (MOI = 0.1) and then treated with various non-toxic concentrations (0, 10, 50, 100, 150, and 200 μM) of germacrone for 1 h at 4 °C, a temperature that allowed viruses to bind to the surface of cells but not to replicate. DMSO alone was added to cells infected with PPV (MOI = 0.1) as a control. After removing the unbound virus by washing with PBS, cell lysates were prepared by three cycles of freezing and thawing, and the viral load in the supernatant was determined.
Viral entry stage
To determine whether germacrone could affect entry of PPV into cells, 1 × 104 ST cells were seeded per well in 24-well plates and infected with PPV (MOI = 0.1) at 4 °C for 1 h. After removing the unbound virus by washing with PBS, cells were treated with various non-toxic concentrations (0, 10, 50, 100, 150, and 200 μM) of germacrone at 37 °C for 1 h. DMSO alone was added to cells infected with PPV (MOI = 0.1) as a control. Subsequently, the drug was removed by washing three times with PBS, and the cells were grown in fresh medium for another 24 h. Cell lysates were prepared by three cycles of freezing and thawing, and the viral load in the supernatant was determined.
Viral replication assay
To determine whether germacrone could affect the replication of PPV in cells, 1 × 104 ST cells were seeded per well in 24-well plates and infected with PPV (MOI = 0.1) at 37 °C for 1 h to allow virus entry. After removing the unbound virus by washing with PBS, cells were treated with various non-toxic concentrations (0, 10, 50, 100, 150, and 200 μM) of germacrone at 37 °C for 24 h. DMSO alone was added to cells infected with PPV (MOI = 0.1) as a control. Subsequently, cell lysates were prepared by three cycles of freezing and thawing, and the viral load was measured.
Effect of the time of drug addition on virus replication
ST cells (1 × 104 per well) were seeded in 24-well plates and infected with PPV (MOI = 0.1) at 37 °C for 1 h. They were then washed three times with PBS and grown in fresh medium (set as 0 h). A non-toxic concentration (200 μM) of germacrone was added to the cells at 0, 1, 3, 6, 9, 12, 14, 18, and 24 h postinfection (hpi), which included the most drug-sensitive phase of virus replication. Cell lysates were prepared by three cycles of freezing and thawing, and the viral load was determined at 24 hpi.
Virus titration
ST cells (1 × 104 per well) were seeded in 96-well plates and infected 24 h later. Cell lysates from the preceding experiments were serially diluted tenfold in DMEM and cultivated on ST cells in five replicates. After incubation at 37 °C for 72 h, the 50 % tissue culture infectious dose (TCID50) was calculated by the method of Reed and Muench [15].
Real-time PCR
Total RNA was extracted using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. cDNA was obtained using a RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, USA). The NS1 gene of PPV was used as the target for real-time quantitative PCR, and the primers used for amplification are shown in Table 1. The real-time quantitative PCR was performed using a 7500 Real-Time PCR System (Applied Biosystems, USA) with a SYBR Select Master Mix Kit (Applied Biosystems, USA) according to the manufacturer’s instructions. The relative mRNA expression levels were calculated using the 2- △△CT method with normalization of the internal control GAPDH [12]. The mean mRNA level of the mock-treated control was set as 1.00.
Indirect immunofluorescence assay (IFA)
After washing with PBS, the cells of the PPV replication stage assay that had been treated with various nontoxic concentrations of germacrone after allowing virus entry were fixed with 4 % paraformaldehyde in PBS for 15 min and were then permeabilized with 0.2 % Triton X-100 in PBS for 10 min. After washing, the cells were incubated with rabbit anti-PPV antibody (1:200) for 1 h, followed by washing with PBS and secondary antibody staining using rhodamine-conjugated goat anti-rabbit IgG (1:500) (Zhongshan, China), and fluorescence was observed under a Leica DMI4000 B confocal microscope (Leica, Wetzlar, Germany).
Statistical analysis
All experiments were performed in triplicate, and the data are presented as mean ± standard deviation (SD). A Student’s t-test was performed to compare sets of data. A two-tailed p-value < 0.05 was considered statistically significant.
Results
Determination of nontoxic concentrations of germacrone
Cytotoxicity assays were performed according the instructions of the manufacturer of CCK8. The relative cell viability was above 95 % at all concentrations tested, and no difference in cell morphology was observed when compared to the mock-treated cells (data not shown), even at the highest concentration tested (Fig. 1B). Therefore, germacrone did not show detectable toxicity on ST cells, and the concentrations of 0-200 μM germacrone were used in the subsequent antiviral assays.
Germacrone has no effect on PPV attachment and entry
Viral attachment and entry assays were performed to evaluate the effect of germacrone on PPV attachment to and entry into cells. For both the viral attachment and entry assays, the PPV titers of cells treated with 10 μM, 50 μM, 100 μM, and 200 μM of germacrone and mock-treated cells were all approximately 6 (Fig. 2A and B). The negligible differences in viral titers between drug-treated and mock-treated cells in viral attachment and entry assays indicated that, under non-toxic concentrations, germacrone had no effect on PPV attachment to or entry into the ST cells.
The viral load in ST cells treated with different concentrations of germacrone at different stages of the viral life cycle. A. viral attachment stage. B. viral entry stage. C. viral replication stage. D. Viral load in ST cells treated with drug during the viral replication stage, determined by real-time PCR. (*, P < 0.05; **, P < 0.01; ***, P < 0.001). E. Viral load in ST cells treated with germacrone during the viral replication stage, determined by IFA
Germacrone inhibits PPV replication
Viral replication assays were performed to evaluate the effect of germacrone on the replication of PPV in ST cells. The mean viral titers obtained with mock-treated and 10 μM, 50 μM, 100 μM, and 200 μM drug-treated samples were 5.87, 5.51, 4.4, 2.8, 2.27, and 2.39, respectively, which indicates that the PPV viral titer decreased in a dose-dependent manner in ST cells when treated with germacrone (Fig. 2C). To confirm the inhibitory effect of germacrone, a real-time PCR targeting the NS1 gene of PPV (Fig. 2D) and an IFA for detection of PPV protein were performed. Untreated ST cells produced stronger fluorescent signals at 48 hpi than did drug-treated cells. The fluorescence signals declined when cells were treated with 50 μM, 100 μM, or 200 μM germacrone. No fluorescence signals were detected in untreated mock-infected cells (Fig. 2E).
Germacrone inhibits PPV replication at an early stage
To determine the stages of PPV replication that was affected by germacrone, a non-toxic concentration of 200 μM germacrone was added to cells at different times postinfection. The virus titer was determined, and real-time PCR and IFA were performed at 24 h postinfection. In contrast to the control, PPV replication was significantly repressed when germacrone was added at 0, 1, 3, 6, and 9 hpi, but no further reductions were seen at 12, 14, 18, and 24 hpi (Fig. 3A, B, and C), indicating that the inhibitory effect of germacrone on PPV replication occurs mainly at an early stage.
Time course of PPV replication in ST cells with germacrone treatment: A. Viral titers. B. Real-time PCR. C. IFA. ST cells were infected with PPV (MOI = 0.1) followed by treatment with 200 μM germacrone at the indicated time (hpi). Virus titers were determined at 24 hpi. “-” indicates that cells were not treated with germacrone. Values represent the mean ± standard deviation for three independent experiments. The asterisks indicate significant differences between mock-treated and germacrone-treated cells (***, P < 0.001)
Discussion
Rhizoma Curcuma is widely used as a traditional Chinese medicine for treatment of tumor and inflammation [9]. Germacrone, which is a key component of the essential oils extracted from Rhizoma Curcuma has been reported to exert a wide range of biological activities, including anti-depressant, anti-inflammatory, antiulcer, antifeedant, antibacterial, antifungal, antitumor, antitussive, vasodilator, choleretic and hepatoprotector effects [5, 18]. Recently, germacrone has shown antiviral activity against influenza virus replication in a dose-dependent manner [10]. PPV is the leading cause of reproductive failure in sows, and antiviral therapies may be an alternative strategy to control PPV infection.
Our study is the first to show that germacrone inhibits the replication of PPV as well as the synthesis of viral RNA and protein. Treatment with germacrone concentrations up to 200 μM showed no significant toxicity to ST cells. Incubation of ST cells with PPV at 37 °C for 1 allows virus entry, whereas incubation for 24 h allows replication of PPV in cells. We demonstrated that germacrone had no effect on PPV attachment or entry into ST cells, which demonstrates that germacrone affects neither the interaction between PPV and the cell receptor nor the cell-entry process of PPV. To further determine which stage of PPV replication is inhibited by germacrone, cells were exposed to germacrone at several time points in the viral life cycle. We found the inhibitory effect occurred primarily at an early stages. Real-time PCR and IFA results clearly confirmed that germacrone inhibited PPV replication in a dose-dependent manner.
In conclusion, the inhibition of PPV replication in ST cells by germacrone was dose-dependent. Additionally, germacrone targets the early phase of PPV replication. These results indicate that germacrone is a potential candidate anti-PPV drug with low toxicity. Further studies will be required to explore the mechanism of the antiviral effect of germacrone on PPV infection in vivo.
References
Allan GM, Kennedy S, McNeilly F, Foster JC, Ellis JA, Krakowka SJ, Meehan BM, Adair BM (1999) Experimental reproduction of severe wasting disease by co-infection of pigs with porcine circovirus and porcine parvovirus. J Comp Pathol 121:1–11
Cartwright SF, Lucas M, Huck RA (1969) A small haemagglutinating porcine DNA virus. I. Isolation and properties. J Comp Pathol 79:371–377
Chen Y, Yan H, Zheng H, Shi Y, Sun L, Wang C, Sun J (2015) Antiviral effect of lithium chloride on cell infection by porcine parvovirus. Arch Virol 160:1015–1020
Cheung AK, Wu G, Wang D, Bayles DO, Lager KM, Vincent AL (2010) Identification and molecular cloning of a novel porcine parvovirus. Arch Virol 155:801–806
Cho W, Nam JW, Kang HJ, Windono T, Seo EK, Lee KT (2009) Zedoarondiol isolated from the rhizoma of Curcuma heyneana is involved in the inhibition of iNOS, COX-2 and pro-inflammatory cytokines via the downregulation of NF-kappa B pathway in LPS-stimulated murine macrophages. Int Immunopharmacol 9:1049–1057
Cui J, Wang X, Ren Y, Cui S, Li G, Ren X (2012) Genome sequence of Chinese porcine parvovirus strain PPV2010. J Virol 86:2379
Ellis JA, Bratanich A, Clark EG, Allan G, Meehan B, Haines DM, Harding J, West KH, Krakowka S, Konoby C, Hassard L, Martin K, McNeilly F (2000) Coinfection by porcine circoviruses and porcine parvovirus in pigs with naturally acquired postweaning multisystemic wasting syndrome. J Vet Diagn Invest 12:21–27
Jozwik A, Manteufel J, Selbitz HJ, Truyen U (2009) Vaccination against porcine parvovirus protects against disease, but does not prevent infection and virus shedding after challenge infection with a heterologous virus strain. J Gen Virol 90:2437–2441
Li Y, Wo JM, Liu Q, Li X, Martin RC (2009) Chemoprotective effects of Curcuma aromatica on esophageal carcinogenesis. Ann Surg Oncol 16:515–523
Liao QJ, Qian ZX, Liu R, An LW, Chen XL (2013) Germacrone inhibits early stages of influenza virus infection. Antivir Res 100:578–588
Liu YY, Wang W, Fang B, Ma FY, Zheng Q, Deng PY, Zhao SS, Chen MJ, Yang GX, He GY (2013) Anti-tumor effect of germacrone on human hepatoma cell lines through inducing G2/M cell cycle arrest and promoting apoptosis. Eur J Pharmacol 698:95–102
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408
Mahnel H, Mayr A, Bibrack B (1966) Multiplication of swine fever virus with cytopathogenic effect in piglet testis culture. Zentralblatt fur Veterinarmedizin Reihe B (J Vet Med Series B) 13:250–259
Mengeling WL (1972) Porcine parvovirus: properties and prevalence of a strain isolated in the United States. Am J Vet Res 33:2239–2248
Reed LJ, Muench H (1938) A simple method of estimating fifty per cent endpoints. Am J Epidemiol 27:493–497
van Leengoed LA, Vos J, Gruys E, Rondhuis P, Brand A (1983) Porcine Parvovirus infection: review and diagnosis in a sow herd with reproductive failure. Vet Q 5:131–141
Wu R, Wen Y, Huang X, Wen X, Yan Q, Huang Y, Ma X, Cao S (2014) First complete genomic characterization of a porcine parvovirus 5 isolate from China. Arch Virol 159:1533–1536
Xiao X, Zhao Y, Yuan H, Xia W, Zhao J, Wang X (2002) Study on the effect of Rhizoma Curcuma Longa on gastrin receptor. Zhong Yao Cai (J Chin Med Mater) 25:184–185
Zeeuw EJ, Leinecker N, Herwig V, Selbitz HJ, Truyen U (2007) Study of the virulence and cross-neutralization capability of recent porcine parvovirus field isolates and vaccine viruses in experimentally infected pregnant gilts. J Gen Virol 88:420–427
Zhong ZF, Chen XP, Zhou KY, Wu T, Cui LA, Wang YT (2010) Rhizoma curcuma exerts synthetic effect of anti-breast cancer through suppressing cell proliferation, metastasis and angiogenesis in vitro. Int J Mol Med 26:S39–S139
Zhong ZF, Chen XP, Tan W, Xu ZT, Zhou KY, Wu T, Cui L, Wang YT (2011) Germacrone inhibits the proliferation of breast cancer cell lines by inducing cell cycle arrest and promoting apoptosis. Eur J Pharmacol 667:50–55
Author information
Authors and Affiliations
Corresponding authors
Additional information
Y. Chen, Y. Dong and Y. Jiao are co-first authors.
Rights and permissions
About this article
Cite this article
Chen, Y., Dong, Y., Jiao, Y. et al. In vitro antiviral activity of germacrone against porcine parvovirus. Arch Virol 160, 1415–1420 (2015). https://doi.org/10.1007/s00705-015-2393-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-015-2393-3