Abstract
A novel avian influenza A(H7N9) virus has emerged to infect humans in eastern China since 2013. An effective vaccine is needed because of the high mortality despite antiviral treatment and intensive care. We sought to develop an effective vaccine for A(H7N9) virus. The HA2 subunit was chosen as the vaccine antigen because it is highly conserved among the human A(H7N9) virus strains. Moreover, in silico analysis predicted two immunogenic regions within the HA2 subunit that may contain potential human B-cell epitopes. The HA2 fragment was readily expressed in Escherichia coli. In BALB/c mice, intraperitoneal immunization with two doses of HA2 with imiquimod (2-dose-imiquimod) elicited the highest geometric mean titer (GMT) of anti-HA2 IgG (12699), which was greater than that of two doses of HA2 without imiquimod (2-dose-no-adjuvant) (6350), one dose of HA2 with imiquimod (1-dose-imiquimod) (2000) and one dose of HA2 without imiquimod (1-dose-no-adjuvant) (794). The titer of anti-HA2 IgG was significantly higher in the 1-dose-imiquimod group than the 1-dose-no-adjuvant group. Although both hemagglutination inhibition titers and microneutralization titers were below 10, serum from immunized mice showed neutralizing activity in a fluorescent focus microneutralization assay. In a viral challenge experiment, the 2-dose-imiquimod group had the best survival rate (100 %), followed by the 2-dose-no-adjuvant group (90 %), the 1-dose-imiquimod group (70 %) and the 1-dose-no-adjuvant group (40 %). The 2-dose-imiquimod group also had significantly lower mean pulmonary viral loads than the 1-dose-imiquimod, 1-dose-no-adjuvant and non-immunized groups. This recombinant A(H7N9)-HA2 vaccine should be investigated as a complement to egg- or cell-based live attenuated or subunit influenza vaccines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The 2013 avian influenza A(H7N9) virus is among the most virulent and most common avian-origin influenza viruses affecting humans [5, 11, 30]. Although neuraminidase inhibitors may improve survival, they are not effective if started late during the course of illness [36]. Therefore, the prevention of A(H7N9) virus infection with effective human vaccines is important, especially for individuals with frequent contact with poultry or visits to live-poultry markets [11, 35].
Currently available human influenza virus vaccines are composed of either live attenuated virus, inactivated whole viral particles, or split virions [6]. These traditional vaccines, which elicit antibodies mainly targeting the globular head of the hemagglutinin, achieve low seroconversion rates in the vaccinees with chronic illness, even against antigenically similar strains [15]. Their protection against antigenically drifted strains may even be lower. Since the amino acid sequence of hemagglutinin from different human A(H7N9) strains can differ by as much as 1.6 % [12], a broadly reactive vaccine using a highly conserved immunogenic target is desirable. In recent years, studies have shown that vaccines composed of only the conserved HA2 region or the stem region of hemagglutinin offered good protection against viruses of different hemagglutinin subtypes in animal models [1, 17, 20, 22, 25–28]. Monoclonal antibodies against the hemagglutinin stem region could also protect mice challenged with different but related subtypes of influenza viruses [7–9]. This protection is speculated to be related to the inhibition of fusion between the virus and the host cell [1, 14]. In this study, we investigated whether an HA2 protein expressed in Escherichia coli could protect mice infected with A(H7N9) virus. Furthermore, we used the Toll-like receptor 7 (TLR7) agonist imiquimod as the adjuvant because previous studies showed that imiquimod can accelerate and augment the vaccine antibody response in mice and humans [16, 39].
Materials and methods
Bioinformatic analysis of the hemagglutinin HA2 subunit
Bioinformatic analysis of the HA2 subunit was performed as described previously [3]. The hemagglutinin sequences of the human A(H7N9) virus analyzed in this study were downloaded from the NCBI GenBank database, except for those of A/Anhui/1/2013 (Anhui/1) and A/Shanghai/1/2013, which was downloaded from GISAID. The strains that were compared in this study include A/Fujian/1/2013 (AGK82158), A/Hangzhou/1/2013 (AGI60301), A/Hangzhou/2/2013 (AGK84857), A/Hangzhou/3/2013 (AGK84860), A/Nanjing/1/2013 (AGJ73503), A/Shanghai/02/2013 (AGL44438), A/Shanghai/4664T/2013 (AGI60292), A/Zhejiang/DTID-ZJU01/2013 (AGJ51953), A/Zhejiang/HZ1/2013 (AGM16242), A/Nanchang/1/2013 AGO28204, A/Wuxi/1/2013 (AGN69474), A/Wuxi/2/2013 (AGN69462), Anhui/1 (EPI439507) and A/Shanghai/1/2013 (EPI439486). The immunogenic regions containing potential human B-cell epitopes were predicted using Epitopia. The transmembrane domain preceding the cytoplasmic tail was predicted using TMHMM version 2.0. Heptad repeat regions within the HA2 domains were predicted using MARCOIL with a threshold of 50 %.
Virus strain
The A(H7N9) virus strain Anhui/1 used in the in vitro and in vivo studies was obtained from the China Center for Disease Control and Prevention [12]. Anhui/1 was propagated in ten-day-old specific-pathogen-free (SPF) chicken embryos at 37 °C for 48 hours as described previously [4]. Allantoic fluid was titrated in Madin-Darby canine kidney (MDCK) cells for determination of the 50 % tissue culture infectious dose (TCID50). Aliquots of virus stock were stored at −80 °C until use. All experiments with Anhui/1 were conducted in a biosafety level 3 laboratory.
Cloning, expression and purification of recombinant HA2 protein
Cloning, expression and purification of recombinant HA2 protein were done using a modification of our previous protocol [33]. Total nucleic acid was extracted from Anhui/1. RNA was reverse-transcribed to cDNA using a random hexamer strategy. A gene fragment encoding amino acids 1-192 of the HA2 protein (amino acid position 340-531 of the complete hemagglutinin protein) of Anhui/1 was amplified from cDNA by PCR with forward primer 5′-GGAATTCCATATGGGCCTATTTGGTGCTATAGCGGGTT-3′ and reverse primer 5′-ATAAGAATGCGGCCGCTTACCCGAAGCTAAACCAAAGTATCAC-3′ and cloned between the NdeI site and the NotI site of the pET-28b(+) vector (Novagen, Damstadt, Germany) with a six-His tag coding sequence at the N-terminus. The recombinant HA2 protein was expressed in E. coli and purified by Ni-nitrilotriacetic acid affinity chromatography (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. The purified protein was refolded by desalting from 8 M urea into phosphate-buffered saline (PBS) at a pH of 8.0 in refolding buffer consisting of 100 mM Tris base, 100 mM NaCl, 1 mM EDTA, 1 % glycine and 5 % glycerol. The purified HA2 protein was analyzed using sodium dodecyl sulfate polyacrylamide gel electrophoresis. The endotoxin level was measured using a Limulus Amebocyte Lysate Endotoxin Test (Endosafe, Charles River Laboratories, Charleston, USA).
Animals, vaccination schedule and immunogenicity
All animal-related experiments were performed according to standard operating procedures as described previously [38] and were approved by the Animal Ethics Committee (CULATR No. 2614-11). Six- to eight-week-old female BALB/c mice were obtained from the Laboratory Animal Unit of the University of Hong Kong. The animals were housed in SPF facilities with 12-hour light-dark cycles and standard pellet feed and water ad libitum. Groups of mice (n = 3 per group) were immunized by intraperitoneal injection of one dose of HA2 (25 μg in 100 μl) along with imiquimod––(InvivoGen, San Diego, CA; 50 μg in 100 μl) (1-dose-imiquimod), one dose of HA2 (25 μg in 100 μl) without imiquimod adjuvant (1-dose-no-adjuvant), two doses of HA2 with imiquimod given 14 days apart (2-dose-imiquimod), or two doses of HA2 without imiquimod given 14 days apart (2-dose-no-adjuvant). Mice in the non-immunized group were injected with the same volume of PBS. On day 21, after the first dose of vaccine, the mice were sacrificed and serum samples were collected for the determination of antibody titers.
Virus challenge study
Virus challenge experiments were carried out in biosafety level 3 animal facilities as described previously [39, 41]. Viral inoculation was performed under ketamine (100 mg/kg) and xylazine (10 mg/kg) anesthesia. Mice from each group were inoculated intranasally with 5 × 104 TCID50 of Anhui/1 21 days after the vaccination for the 1-dose-imiquimod group and 1-dose-no-adjuvant group, and 21 days after the first dose of vaccine for the 2-dose-imiquimod group and 2-dose-no-adjuvant group. Mice in the negative-control group were injected with the same volume of PBS. Nine to ten mice from each group were observed for survival in two separate experiments. Mice with weight loss of >25 % were considered to have reached the predefined humane endpoint and were euthanized. Another three mice from each group were sacrificed on day 4 postinfection to collect their lungs for viral load testing. The body weight, symptoms, and survival of the mice were monitored daily after virus inoculation. On day 21 postinfection, serum samples were collected from surviving mice.
Determination of viral load in homogenized lung tissue specimens
The viral loads in homogenized lung tissue specimens were quantified using a TCID50 assay as described previously [4].
Detection of anti-HA2 IgG in mouse serum samples
The enzyme immunoassay (EIA) was performed as we described previously, with modifications [29]. Ninety-six-well immunoplates (Costar, Corning, Tewksbury, USA) were coated overnight with 100 µl of HA2 protein at 1 µg/ml in 0.05 M carbonate buffer (pH 9.6) at 4 °C and then blocked using blocking reagent containing 2.5 g casein sodium salt, 1.21 g Tris base, 2 g gelatin, 20 g sucrose, 0.2 g Merthiolate and 5 ml Tween 20 per 1000 ml ddH2O (Sigma-Aldrich, Co., USA). After washing three times with PBS containing 0.05 % Tween 20 (PBS-T), 100 µl of serum sample in twofold serial dilutions in 0.1 % bovine serum albumin (BSA) was added to each well and incubated at 37 °C for 1 hour. After washing, 100 µl of goat anti-mouse IgG-horseradish peroxidase (dilution 1:1500) (Zymax, Invitrogen, USA) was added to each well as a secondary antibody for 30 min at 37 °C. The reaction was developed by adding 100 µl of diluted 3,3′,5,5′-tetramethylbenzidine single solution (Invitrogen, USA) for 10 min at room temperature and stopped with 100 µl of 0.3 N H2SO4. The optical density (OD) was read at 450 nm. All samples were tested in duplicate, and the mean absorbance was calculated. A Western blot was performed to confirm the specific binding between the mouse serum antibody and the HA2 protein.
Hemagglutination inhibition (HI) assay
The HI assay was performed as we described previously, with modifications [2, 15, 37]. Nonspecific inhibitors in the serum were removed with receptor-destroying enzyme (RDE; 1:3 [Denka Seiken Co., Ltd., Tokyo, Japan]), and the sample was incubated overnight at 37 °C and heat inactivated at 56 °C for 30 min. Serial twofold dilutions of RDE-treated serum at a starting dilution of 1:10 were mixed with four hemagglutinin units of Anhui/1, followed by incubation at room temperature for 1 hour. Next, 0.5 % turkey erythrocytes were added to the serum-virus mixture, followed by further incubation at room temperature for 30 min.
Microneutralization (MN) assay
The MN assay was performed as we described previously [2, 29]. Serial dilutions of serum were mixed with 100 TCID50 of Anhui/1 for 2 hours at 37 °C and then added to MDCK cells. One hour after infection, the virus-serum mixture was removed, and serum-free minimal essential medium with 2 μg of L-1-tosylamide-2-phenylethyl chloromethyl ketone-treated trypsin (TPCK-trypsin; Sigma Immunochemical) per ml was added to each well. A cytopathic effect was observed three days after incubation at 37 °C. The highest serum dilution that protected at least 50 % of the cells from cytopathology was considered to be the MN titer. All samples were tested in duplicate.
Fluorescent focus microneutralization (FFMN) assay
The FFMN assay was performed as described previously, with modifications [2]. Briefly, serum samples diluted 1 to 10 were mixed with Anhui/1 at a multiplicity of infection of 1 (108.4 PFU/ml) at room temperature for 1 hour and then added to MDCK cells. After 1 hour of incubation at 37 °C, the serum-virus mixture was removed, and the cells were further incubated for 6 hours at 37 °C. The seeded cells were then fixed in chilled acetone and methanol (1:1) at −20 °C for 10 minutes and stained with monoclonal antibody against influenza A nucleoprotein at 37 °C. This was followed by the addition of goat anti-mouse fluorescein-labeled conjugate (Millipore, California) and further incubation at 37 °C for 45 min. The percentage of positive cells was determined by visual examination using a fluorescence microscope. 4′,6-diamidino-2-phenylindole staining was performed to quantify the MDCK cells in each well.
Influenza-virus-induced hemolysis assay
Virus-cell membrane fusion was assessed by the influenza-virus-induced hemolysis assay as described previously [19]. Briefly, serially diluted sera from vaccinated or unvaccinated mice were mixed with A(H7N9) virus with a PR8 backbone at room temperature for 30 min. The serum-virus mixture was then added to 50 μl of 2 % human erythrocytes and incubated at 4 °C for 20 min. Next, 200 μl of sodium citrate at a pH of 5.2 was added to the serum-virus-erythrocyte mixture at 37 °C for 90 min. After incubation, the mixture was centrifuged at 800g for 5 min. The heme of lysed erythrocyte in the supernatant was quantified by measuring its absorbance at 405 nm using a VICTOR™ X3 Multilabel Plate Reader (PerkinElmer, Massachusetts, USA). The hemolysis titer is expressed as % hemolysis of control as given by the following formula: ([A405 (experimental) − A405 (no virus added)]/[A405 (H7N9 virus only) − A405 (no virus added)] × 100 %). The experiment was performed in duplicate.
Statistical analysis
Mouse survival rates were analyzed by the Kaplan-Meier method and log-rank test using GraphPad Prism 6.0. The anti-HA2 IgG titer was defined as the titer at which the absorbance (optical density) was nearest to 1.0. HI and MN titers <10 were arbitrarily assigned a value of 5, while anti-HA2 titers <1000 were assigned a value of 500. Log-transformed geometric mean titers (GMT) were compared using the Mann-Whitney U test [29]. Body weights and pulmonary viral loads were analyzed by Student’s t-test. A P-value of <0.05 was considered statistically significant.
Results
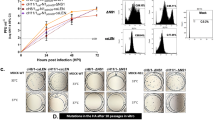
The HA2 amino acid sequence (position 340-560 of hemagglutinin) of Anhui/1 was 100 % identical to that of other human A(H7N9) viruses, except for A/Shanghai/01/2013 and A/Wuxi/1/2013 (Table 1). The amino acid sequence of HA2 was much less variable than that of the HA1 subunit. Two immunogenic regions, which may contain potential human B-cell epitopes, were predicted by Epitopia software at HA2 amino acid position 26-40 (amino acid position 365-379 of hemagglutinin) and at HA2 amino acid position 155-183 (amino acid position 494-522 of hemagglutinin) (Fig. 1A). A heptad repeat region at HA2 amino acid position 34-79 overlaps with the immunogenic region at position 26-40.
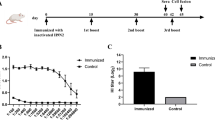
Expression of the HA2 protein in E. coli and its immunogenicity as a vaccine. (A) Bioinformatics analysis of the HA2 protein of human A(H7N9) virus. Immunogenic regions of at least 10 residues in length, predicted using Epitopia, are indicated by a black line. The numbering in the figure represents the amino acid position within the HA2 subunit. (B) Sodium dodecyl sulfate polyacrylamide gel electrophoresis of the purified HA2 protein, showing the expression of HA2 protein at the expected size of 24.2 kDa (arrow). The gel was stained with Coomasie blue. M, protein molecular mass marker; N, supernatant of the positive transformant before induction; I, supernatant of the positive transformant after induction; P, purified HA2 protein. (C) Determination of serum antibody using western blot analysis. Serum samples were collected on day 21 after the first dose of vaccine. One serum sample from each group, diluted 1:10,000, was randomly chosen for the western blot analysis. Lane 1, non-immunized; lane 2, 1-dose-no-adjuvant; lane 3, 1-dose-imiquimod; lane 4, 2-dose-no-adjuvant; lane 5, 2-dose-imiquimod. (D) FFMN assay, showing inhibition of fluorescent foci in serum samples collected from immunized mice. Panel a, influenza-virus-infected MDCK cells without mouse serum; panel b, no inhibition of fluorescent foci with a serum sample obtained from a non-immunized/uninfected mouse; panel c, >90 % inhibition of fluorescent foci with a serum sample obtained from a mouse in the 2-dose-no-adjuvant group 21 days after the first dose of vaccine; panel d, 100 % inhibition of fluorescent foci with a serum sample collected from mice on day 21 postinfection; top panel, the infected cells stained with FITC-conjugated antibody against the influenza A nucleoprotein; bottom panel, cells stained with 4′,6-diamidino-2-phenylindole
The HA2 subunit amino acid position 1-192 (amino acid position 340-531 of hemagglutinin protein) was cloned into a pET-28b(+) vector and introduced into E. coli by transformation. The HA2 subunit was successfully expressed and purified with an expected molecular mass of 24.2 kDa (Fig. 1B). The endotoxin level was 38.7 IU/dose of vaccine, which is less than the recommended upper limit of 100 IU/dose for human influenza vaccine [32]. Using the purified HA2 protein, we developed an enzyme immunoassay (EIA) to detect anti-HA2 IgG. The performance of the EIA was evaluated with a convalescent serum sample from a mouse that survived infection with 5 × 104 TCID50 of Anhui/1 (positive control), and 33 serum samples collected from uninfected/non-immunized mice (negative control). When diluted 1000-fold, the OD was 1.5 for convalescent serum samples from the infected mouse, while the OD was <0.12 for all serum samples from uninfected/non-immunized mice.
Next, we determined the immunogenicity of the vaccine. Groups of mice were immunized by intraperitoneal injection of one dose of HA2 (25 μg in 100 μl) along with imiquimod––(InvivoGen, San Diego, CA; 50 μg in 100 μl) (1-dose-imiquimod), one dose of HA2 (25 μg in 100 μl) without imiquimod adjuvant (1-dose-no-adjuvant), two doses of HA2 with imiquimod given 14 days apart (2-dose-imiquimod), or two doses of HA2 without imiquimod given 14 days apart (2-dose-no-adjuvant). Mice in the non-immunized group were injected with the same volume of PBS. Antibody titers (anti-HA2 IgG, HI and MN titers) were measured at 21 days after the first dose of vaccine for three mice per group. The 1-dose-imiquimod group had a significantly higher anti-HA2 IgG titer than the 1-dose-no-adjuvant group (GMT, 2000 vs 794; P = 0.034), but there was no significant difference between the 2-dose-imiquimod group and the 2-dose-no-adjuvant group (GMT, 12699 vs 6350; P = 0.637). Both the 2-dose-imiquimod group and the 2-dose-no-adjuvant group had significantly higher anti-HA2 IgG titers than the 1-dose-imquimod group (P = 0.037 and 0.034, respectively). Western blot confirmed that antibodies from the immunized mice could react specifically with the HA2 protein (Fig. 1C). The HI and MN titers were below 10 for all immunized and non-immunized mice. Since the MN titers were below 10 for immunized mice, we performed the FFMN assay to determine whether a low level of neutralizing activity was present. Serum samples collected from immunized mice showed inhibition of fluorescent foci in the FFMN assay (Fig. 1D). Both the 2-dose-imiquimod group and the 2-dose-no-adjuvant group showed >90% inhibition of fluorescent foci, while both the 1-dose-imiquimod and the 1-dose-no-adjuvant group showed only 25-40 % inhibition (data not shown) (Fig. 1D, panel b and c). Complete inhibition of fluorescent foci was observed in serum collected from mice surviving the Anhui/1 infection (Fig. 1D, panel d). To assess whether the anti-HA2 antibody induced by the HA2 vaccine can inhibit fusion of the virus and the host cell membrane, a red blood cell hemolysis assay was used. Postvaccination sera from two mice in the 2-dose-no-adjuvant group and two mice in the non-immunized group were compared. The mean inhibition of hemolysis was 93.1 % (standard error of mean [SEM], 7.21 %) for the 2-dose-no-adjuvant group, compared to 47.6 % (SEM, 1.24 %) for the non-immunized group.
A viral challenge study was performed to assess the protective efficacy of one or two doses of vaccine with or without imiquimod (n = 9-10 per group). All mice in the 2-dose-imquimod group and 90 % of mice in the 2-dose-no-adjuvant group survived (P = 0.34) (Fig. 2A). The survival was significantly better in the 2-dose-imiquimod group (P = 0.0002) and in the 2-dose-no-adjuvant group (P = 0.0006) than in the PBS control group. The 1-dose-imiquimod group had a higher survival rate than the 1-dose-no-adjuvant group, but the difference was not statistically significant (70 % vs 40 %, P = 0.17). However, the 1-dose-imiquimod group had a significantly higher survival rate than the PBS control group (70 % vs 10 %, P = 0.02), while there was no significant difference between the survival of 1-dose-no-adjuvant group and the PBS control group (40 % vs 10 %, P = 0.40). When comparing between the 2-dose and 1-dose regimens, only the survival of 2-dose-imiquimod group was significantly better than that of the 1-dose-no-adjuvant group (P = 0.007). The body weight started to stabilize on day 6 postinfection for the immunized mice but continued to drop for the non-immunized mice (Fig. 2B). After day 6 postinfection, the body weight was regained more quickly in the 2-dose-imiquimod and 2-dose-no-adjuvant group than the 1-dose-imiquimod group and the 1-dose-no-adjuvant group. The mean pulmonary viral loads of the 2-dose-imiquimod group at day 4 postinfection were 4.25 log10 TCID50/ml, which was significantly lower than that of the 1-dose-imiquimod group, the 1-dose-no-adjuvant group and the PBS control group, but was not significantly different from the 2-dose-no-adjuvant group (Fig. 2C).
Viral challenge study. (A) Mortality and (B) body weight of mice challenged with influenza virus subtype A(H7N9). Mice were inoculated intranasally with 20 μl of virus and observed for 14 days for mortality. Data are for 9-10 mice per group. Error bars indicate standard error of the mean. *P < 0.05 and **P < 0.01 when compared with the PBS group. (C) Pulmonary viral titer at day 4 postinfection. Data are for three mice per group. Error bars indicate standard error of the mean. *P < 0.05; **P < 0.01
Discussion
Avian influenza A(H7N9) virus has affected >450 patients with about 30 % mortality. Notably, this number is about ten times the total number of cases of human A(H5N1) infection in the last 10 years in China [34]. Since the key risk factor for influenza A(H7N9) or A(H5N1) virus infection is exposure to poultry, as over half of all cases had direct or indirect contact with poultry, this difference in incidence strongly suggests that the A(H7N9) virus has a much higher poultry-to-human transmissibility than the A(H5N1) virus. The most effective way to control human infection is to prevent poultry infection. However, the A(H7N9) virus does not cause deaths or severe disease in poultry [40]. This poses great difficulty in locating the poultry source for control by depopulation. Moreover, viral genome sequence analysis of A(H7N9) virus has revealed many genetic signatures of mammalian adaptation in the hemagglutinin H7 and the polymerase complex PB2 and PA genes [5, 31]. Transmission studies have suggested some degree of droplet-borne transmission between ferrets, which alerts to the possibility of A(H7N9) having a higher potential to evolve into a pandemic agent [24, 40]. Moreover, in a seroprevalence study, the general population was found to lack protective HI antibody titer against A(H7N9) virus [35]. Since an A(H7N9) vaccine for poultry is not yet available, the importance of a human A(H7N9) vaccine for poultry workers and the general population in the anticipation of a pandemic cannot be over-emphasized.
In this study, we have successfully expressed the HA2 subunit of the hemagglutinin from A(H7N9) in E. coli. In silico analysis showed that this HA2 protein contains two immunogenic regions. One of the immunogenic regions at position 26–40 of HA2 overlaps with the heptad repeat region at position 34–79, and antibodies against this region may affect the conformational change in HA2, thereby preventing fusion between the virus and the host-cell membrane. The HA2 protein, with or without imiquimod, was highly immunogenic in mice, eliciting high titers of anti-HA2 IgG, and improved the survival of mice with A(H7N9) virus infection. The survival rate correlates with the titer of anti-HA2 IgG. The HA2 subunit of the hemagglutinin was chosen because this is a conserved region amongst the newly emerged A(H7N9) viruses. Immunity elicited by the HA2 region should be effective against all A(H7N9) viruses.
Despite the high titers of anti-HA2 IgG, the HI titers were low in immunized mice. This is consistent with a previous study that showed that monoclonal antibody against the hemagglutinin stalk region did not have HI activity [28]. The lack of HI activity is not unexpected, as the antibody elicited by the HA2 protein binds to the hemagglutinin at a site distant from the receptor-binding site. Instead, antibody elicited by the HA2 vaccine should inhibit virus membrane fusion, although a recent study has shown that anti-HA2 antibody could enhance fusion activity [19]. Furthermore, the MN titers were also <10, as reported in a previous study [1]. However, the FFMN assay showed that immunization with our HA2 vaccine induced antibodies with neutralizing activity in the serum, suggesting the presence of low-level neutralizing activities that could not be detected by MN assay. The MN assay depends on the inhibition of viral replication, while the FFMN assay depends only on viral entry and nucleoprotein expression and is not affected by the later steps in the viral life cycle. Since the inhibition in the FFMN is not 100 % even for the 2-dose-imiquimod group, some cells become infected and are therefore able to produce sufficient numbers of virus particles to cause cytopathic effects. It is important to note that even low titers of neutralizing antibody can provide significant protection in mouse models [1, 27]. One possible reason for the ability of the HA2 vaccine to protect mice from lethal challenge is that the Fc portion of the anti-HA2 antibody could interact with Fc receptors for IgG and protect mice via antibody-dependent cellular cytotoxicity [7]. This is similar to what has been observed with vaccination based on the ectodomain of matrix protein 2 (M2e). M2e vaccination protects mice from lethal infection. Anti-M2e does not have neutralizing activity but acts via an Fc-dependent mechanism [10, 23].
Imiquimod is a TLR7 agonist and has been used successfully as an adjuvant for influenza vaccine, eliciting higher levels of serum IgG and neutralizing antibody titers in mice [39]. In that study, imiquimod alone without vaccine did not improve survival, suggesting that the benefit of imiquimod relies on the vaccine. In a double-blind randomized controlled trial, we have also shown that topical imiquimod can accelerate, enhance and prolong the immunogenicity of intradermal influenza vaccination in individuals with comorbidities [16]. TLR7 is a pathogen-recognition receptor that can sense viral genomic RNA. Stimulation of TLR7 triggers the signaling pathway, leading to an increase in inflammatory cytokines and type 1 interferons responsible for the antiviral response [18]. In our study, imiquimod boosted the anti-HA2 titer in both the 1-dose and 2-dose vaccine groups, although it was only statistically significant for the 1-dose groups. The higher anti-HA2 titers in the groups with imiquimod also correlated with better survival in the 1-dose-imiquimod group when compared to the 1-dose-no-adjuvant group. Therefore, imiquimod can effectively improve the efficacy of the one-dose vaccine.
In the virus challenge study, there was an initial mean weight loss of 20 % in the immunized groups, although the weight loss was significantly less than that of non-immunized mice. Initial weight loss in vaccine studies for mice is commonly observed [22], and is related to the relatively high viral inoculum necessary for lethal infection. This is unlike the scenario in humans, where the inoculation dose would be expected to be much lower. Although the intraperitoneal route is not used for vaccination of humans, it is commonly used in mouse studies [13, 21]. We did not use the intramuscular route, because the large volume of vaccine and adjuvant causes leakage.
Conclusions
The 2013 A(H7N9) virus has caused widespread infection in China, and the number of infections increased rapidly during the winter season of 2013/2014. It is likely that A(H7N9) virus will continue to cause poultry-to-human epidemics. Our study showed that HA2-based vaccine together with imiquimod can improve survival of mice with A(H7N9) infection and should be further investigated for clinical trials in humans.
References
Bommakanti G, Citron MP, Hepler RW, Callahan C, Heidecker GJ, Najar TA, Lu X, Joyce JG, Shiver JW, Casimiro DR, ter Meulen J, Liang X, Varadarajan R (2010) Design of an HA2-based Escherichia coli expressed influenza immunogen that protects mice from pathogenic challenge. Proc Natl Acad Sci USA 107:13701–13706
Chan KH, To KK, Hung IF, Zhang AJ, Chan JF, Cheng VC, Tse H, Che XY, Chen H, Yuen KY (2011) Differences in antibody responses of individuals with natural infection and those vaccinated against pandemic H1N1 2009 influenza. Clin Vaccine Immunol 18:867–873
Chan KH, Chan JF, Tse H, Chen H, Lau CC, Cai JP, Tsang AK, Xiao X, To KK, Lau SK, Woo PC, Zheng BJ, Wang M, Yuen KY (2013) Cross-reactive antibodies in convalescent SARS patients’ sera against the emerging novel human coronavirus EMC (2012) by both immunofluorescent and neutralizing antibody tests. J Infect 67:130–140
Chan KH, To KK, Chan JF, Li CP, Chen H, Yuen KY (2013) Analytical sensitivity of seven point-of-care influenza virus detection tests and two molecular tests for detection of avian origin H7N9 and swine origin H3N2 variant influenza A viruses. J Clin Microbiol 51:3160–3161
Chen Y, Liang W, Yang S, Wu N, Gao H, Sheng J, Yao H, Wo J, Fang Q, Cui D, Li Y, Yao X, Zhang Y, Wu H, Zheng S, Diao H, Xia S, Zhang Y, Chan KH, Tsoi HW, Teng JL, Song W, Wang P, Lau SY, Zheng M, Chan JF, To KK, Chen H, Li L, Yuen KY (2013) Human infections with the emerging avian influenza A H7N9 virus from wet market poultry: clinical analysis and characterisation of viral genome. Lancet 381:1916–1925
Cheng VC, To KK, Tse H, Hung IF, Yuen KY (2012) Two years after pandemic influenza A/2009/H1N1: what have we learned? Clin Microbiol Rev 25:223–263
DiLillo DJ, Tan GS, Palese P, Ravetch JV (2014) Broadly neutralizing hemagglutinin stalk-specific antibodies require FcgammaR interactions for protection against influenza virus in vivo. Nat Med 20:143–151
Ekiert DC, Bhabha G, Elsliger MA, Friesen RH, Jongeneelen M, Throsby M, Goudsmit J, Wilson IA (2009) Antibody recognition of a highly conserved influenza virus epitope. Science 324:246–251
Ekiert DC, Friesen RH, Bhabha G, Kwaks T, Jongeneelen M, Yu W, Ophorst C, Cox F, Korse HJ, Brandenburg B, Vogels R, Brakenhoff JP, Kompier R, Koldijk MH, Cornelissen LA, Poon LL, Peiris M, Koudstaal W, Wilson IA, Goudsmit J (2011) A highly conserved neutralizing epitope on group 2 influenza A viruses. Science 333:843–850
El Bakkouri K, Descamps F, De Filette M, Smet A, Festjens E, Birkett A, Van Rooijen N, Verbeek S, Fiers W, Saelens X (2011) Universal vaccine based on ectodomain of matrix protein 2 of influenza A: Fc receptors and alveolar macrophages mediate protection. J Immunol 186:1022–1031
Gao HN, Lu HZ, Cao B, Du B, Shang H, Gan JH, Lu SH, Yang YD, Fang Q, Shen YZ, Xi XM, Gu Q, Zhou XM, Qu HP, Yan Z, Li FM, Zhao W, Gao ZC, Wang GF, Ruan LX, Wang WH, Ye J, Cao HF, Li XW, Zhang WH, Fang XC, He J, Liang WF, Xie J, Zeng M, Wu XZ, Li J, Xia Q, Jin ZC, Chen Q, Tang C, Zhang ZY, Hou BM, Feng ZX, Sheng JF, Zhong NS, Li LJ (2013) Clinical findings in 111 cases of influenza A (H7N9) virus infection. N Engl J Med 368:2277–2285
Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, Chen J, Jie Z, Qiu H, Xu K, Xu X, Lu H, Zhu W, Gao Z, Xiang N, Shen Y, He Z, Gu Y, Zhang Z, Yang Y, Zhao X, Zhou L, Li X, Zou S, Zhang Y, Li X, Yang L, Guo J, Dong J, Li Q, Dong L, Zhu Y, Bai T, Wang S, Hao P, Yang W, Zhang Y, Han J, Yu H, Li D, Gao GF, Wu G, Wang Y, Yuan Z, Shu Y (2013) Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med 368:1888–1897
Gocnik M, Fislova T, Mucha V, Sladkova T, Russ G, Kostolansky F, Vareckova E (2008) Antibodies induced by the HA2 glycopolypeptide of influenza virus haemagglutinin improve recovery from influenza A virus infection. J Gen Virol 89:958–967
Gomez Lorenzo MM, Fenton MJ (2013) Immunobiology of influenza vaccines. Chest 143:502–510
Hung IF, Levin Y, To KK, Chan KH, Zhang AJ, Li P, Li C, Xu T, Wong TY, Yuen KY (2012) Dose sparing intradermal trivalent influenza (2010/2011) vaccination overcomes reduced immunogenicity of the 2009 H1N1 strain. Vaccine 30:6427–6435
Hung IF, Zhang AJ, To KK, Chan JF, Li C, Zhu HS, Li P, Li C, Chan TC, Cheng VC, Chan KH, Yuen KY (2014) Immunogenicity of intradermal trivalent influenza vaccine with topical Imiquimod, a double blind randomized controlled trial. Clin Infect Dis. doi:10.1093/cid/ciu582
Janulikova J, Stanekova Z, Mucha V, Kostolansky F, Vareckova E (2012) Two distinct regions of HA2 glycopolypeptide of influenza virus hemagglutinin elicit cross-protective immunity against influenza. Acta Virol 56:169–176
Kawai T, Akira S (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11:373–384
Khurana S, Loving CL, Manischewitz J, King LR, Gauger PC, Henningson J, Vincent AL, Golding H (2013) Vaccine-induced anti-HA2 antibodies promote virus fusion and enhance influenza virus respiratory disease. Sci Transl Med 5:200ra114
Mallajosyula VV, Citron M, Ferrara F, Lu X, Callahan C, Heidecker GJ, Sarma SP, Flynn JA, Temperton NJ, Liang X, Varadarajan R (2014) Influenza hemagglutinin stem-fragment immunogen elicits broadly neutralizing antibodies and confers heterologous protection. Proc Natl Acad Sci USA 111:E2514–E2523
Manicassamy B, Medina RA, Hai R, Tsibane T, Stertz S, Nistal-Villan E, Palese P, Basler CF, Garcia-Sastre A (2010) Protection of mice against lethal challenge with 2009 H1N1 influenza A virus by 1918-like and classical swine H1N1 based vaccines. PLoS Pathog 6:e1000745
Margine I, Krammer F, Hai R, Heaton NS, Tan GS, Andrews SA, Runstadler JA, Wilson PC, Albrecht RA, Garcia-Sastre A, Palese P (2013) Hemagglutinin stalk-based universal vaccine constructs protect against group 2 influenza A viruses. J Virol 87:10435–10446
Neirynck S, Deroo T, Saelens X, Vanlandschoot P, Jou WM, Fiers W (1999) A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat Med 5:1157–1163
Richard M, Schrauwen EJ, de Graaf M, Bestebroer TM, Spronken MI, van Boheemen S, de Meulder D, Lexmond P, Linster M, Herfst S, Smith DJ, van den Brand JM, Burke DF, Kuiken T, Rimmelzwaan GF, Osterhaus AD, Fouchier RA (2013) Limited airborne transmission of H7N9 influenza A virus between ferrets. Nature 501:560–563
Schneemann A, Speir JA, Tan GS, Khayat R, Ekiert DC, Matsuoka Y, Wilson IA (2012) A virus-like particle that elicits cross-reactive antibodies to the conserved stem of influenza virus hemagglutinin. J Virol 86:11686–11697
Stanekova Z, Adkins I, Kosova M, Janulikova J, Sebo P, Vareckova E (2013) Heterosubtypic protection against influenza A induced by adenylate cyclase toxoids delivering conserved HA2 subunit of hemagglutinin. Antiviral Res 97:24–35
Steel J, Lowen AC, Wang TT, Yondola M, Gao Q, Haye K, Garcia-Sastre A, Palese P (2010) Influenza virus vaccine based on the conserved hemagglutinin stalk domain. mBio. doi:10.1128/mBio.00018-10
Tan GS, Krammer F, Eggink D, Kongchanagul A, Moran TM, Palese P (2012) A pan-H1 anti-hemagglutinin monoclonal antibody with potent broad-spectrum efficacy in vivo. J Virol 86:6179–6188
To KK, Zhang AJ, Hung IF, Xu T, Ip WC, Wong RT, Ng JC, Chan JF, Chan KH, Yuen KY (2012) High titer and avidity of nonneutralizing antibodies against influenza vaccine antigen are associated with severe influenza. Clin Vaccine Immunol 19:1012–1018
To KK, Chan JF, Chen H, Li L, Yuen KY (2013) The emergence of influenza A H7N9 in human beings 16 years after influenza A H5N1: a tale of two cities. Lancet Infect Dis 13:809–821
To KK, Song W, Lau SY, Que TL, Lung DC, Hung IF, Chen H, Yuen KY (2014) Unique reassortant of influenza A(H7N9) virus associated with severe disease emerging in Hong Kong. J Infect 69:60–68
Wong SS, Yuen KY (2005) Influenza vaccination: options and issues. Hong Kong Med J 11:381–390
Woo PC, Lau SK, Tsoi HW, Chen ZW, Wong BH, Zhang L, Chan JK, Wong LP, He W, Ma C, Chan KH, Ho DD, Yuen KY (2005) SARS coronavirus spike polypeptide DNA vaccine priming with recombinant spike polypeptide from Escherichia coli as booster induces high titer of neutralizing antibody against SARS coronavirus. Vaccine 23:4959–4968
World Health Organization Influenza at the human-animal interface. Summary and assessment as of 4 July 2013. Available at http://www.who.int/influenza/human_animal_interface/Influenza_Summary_IRA_HA_interface_03July13.pdf. Accessed on 15 Aug 2013
Yang S, Chen Y, Cui D, Yao H, Lou J, Huo Z, Xie G, Yu F, Zheng S, Yang Y, Zhu Y, Lu X, Liu X, Lau SY, Chan JF, To KK, Yuen KY, Chen H, Li L (2014) Avian-origin influenza A(H7N9) infection in influenza A(H7N9)-affected areas of China: a serological study. J Infect Dis 209:265–269
Yu L, Wang Z, Chen Y, Ding W, Jia H, Chan JF, To KK, Chen H, Yang Y, Liang W, Zheng S, Yao H, Yang S, Cao H, Dai X, Zhao H, Li J, Bao Q, Chen P, Hou X, Li L, Yuen KY (2013) Clinical, virological, and histopathological manifestations of fatal human infections by avian influenza A(H7N9) virus. Clin Infect Dis 57:1449–1457
Zhang AJ, To KK, Tse H, Chan KH, Guo KY, Li C, Hung IF, Chan JF, Chen H, Tam S, Yuen KY (2011) High incidence of severe influenza among individuals over 50 years of age. Clin Vaccine Immunol 18:1918–1924
Zhang AJ, To KK, Li C, Lau CC, Poon VK, Chan CC, Zheng BJ, Hung IF, Lam KS, Xu A, Yuen KY (2013) Leptin mediates the pathogenesis of severe 2009 pandemic influenza A(H1N1) infection associated with cytokine dysregulation in mice with diet-induced obesity. J Infect Dis 207:1270–1280
Zhang AJ, Li C, To KK, Zhu HS, Lee AC, Li CG, Chan JF, Hung IF, Yuen KY (2014) Toll-like receptor 7 agonist Imiquimod in combination with influenza vaccine expedites and augments humoral immune responses against influenza A(H1N1)pdm09 virus infection in BALB/c mice. Clin Vaccine Immunol 21:570–579
Zhang Q, Shi J, Deng G, Guo J, Zeng X, He X, Kong H, Gu C, Li X, Liu J, Wang G, Chen Y, Liu L, Liang L, Li Y, Fan J, Wang J, Li W, Guan L, Li Q, Yang H, Chen P, Jiang L, Guan Y, Xin X, Jiang Y, Tian G, Wang X, Qiao C, Li C, Bu Z, Chen H (2013) H7N9 influenza viruses are transmissible in ferrets by respiratory droplet. Science 341:410–414
Zheng BJ, Chan KW, Lin YP, Zhao GY, Chan C, Zhang HJ, Chen HL, Wong SS, Lau SK, Woo PC, Chan KH, Jin DY, Yuen KY (2008) Delayed antiviral plus immunomodulator treatment still reduces mortality in mice infected by high inoculum of influenza A/H5N1 virus. Proc Natl Acad Sci USA 105:8091–8096
Acknowledgments
We are grateful to Dr. Desmond Yap and Dr. Loretta Yuk-Yee Chan for performing the endotoxin assay. This work was supported in part by the Providence Foundation Ltd., in memory of the late Lui Hac Minh, and Mr. Larry Chi-Kin Yung. We thank the China Center for Disease Control and Prevention for providing the A/Anhui/1/2013 (H7N9) isolate. We acknowledge Yuelong Shu for his written permission to use his sequence data deposited in the Global Initiative on Sharing Avian Influenza Data (GISAID).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
K. K. W. To and A. J. X. Zhang contributed equally to this wok.
Rights and permissions
About this article
Cite this article
To, K.K.W., Zhang, A.J.X., Chan, A.S.F. et al. Recombinant influenza A virus hemagglutinin HA2 subunit protects mice against influenza A(H7N9) virus infection. Arch Virol 160, 777–786 (2015). https://doi.org/10.1007/s00705-014-2314-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-014-2314-x