Abstract
Millions of individuals around the world are afflicted with Parkinson’s disease (PD), a prevalent and incapacitating neurodegenerative disorder. Dr. Reichmann, a distinguished professor and neurologist, has made substantial advancements in the domain of PD research, encompassing both fundamental scientific investigations and practical applications. His research has illuminated the etiology and treatment of PD, as well as the function of energy metabolism and premotor symptoms. As a precursor to a number of neurotransmitters and neuromodulators that are implicated in the pathophysiology of PD, he has also investigated the application of tryptophan (Trp) derivatives in the disease. His principal findings and insights are summarized and synthesized in this narrative review article, which also emphasizes the challenges and implications for future PD research. This narrative review aims to identify and analyze the key contributions of Reichmann to the field of PD research, with the ultimate goal of informing future research directions in the domain. By examining Reichmann’s work, the study seeks to provide a comprehensive understanding of his major contributions and how they can be applied to advance the diagnosis and treatment of PD. This paper also explores the potential intersection of Reichmann’s findings with emerging avenues, such as the investigation of Trp and its metabolites, particularly kynurenines, which could lead to new insights and potential therapeutic strategies for managing neurodegenerative disorders like PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a long-term degenerative disorder characterized by a gradual decline in the number of neurons in the brain that produce dopamine (Kordower et al. 2013; Triarhou 2013; Meder et al. 2019). Dopamine plays a vital role in controlling movement, and a lack of it results in the distinct motor symptoms associated with PD, including tremors, rigidity, bradykinesia, and compromised balance and coordination (Kumar et al. 2022b; Mazzoni et al. 2012; Moustafa et al. 2016). In addition, PD can also affect non-motor functions, including mood, cognition, and sleep patterns (Poewe 2008; Burn et al. 2012; Herman et al. 2015; Maass and Reichmann 2013). While the exact etiology of PD remains uncertain, it is widely accepted that a combination of genetic and environmental factors plays a role in its pathogenesis (Tsuboi 2012; Polito et al. 2016; Fleming 2017). At present, there is no known remedy for PD; however, a range of medications and therapies exist to effectively alleviate symptoms and improve the overall well-being of individuals affected by this condition (Dong et al. 2016; Yuan et al. 2010; Kwok et al. 2016). Continuing research and progress in comprehending the disease provide optimism for potential future treatment alternatives and interventions (Valotto Neto et al. 2024; de Oliveira Zanuso et al. 2022; Buglio et al. 2022; Barbalho et al. 2022; Matias et al. 2021; Pagotto et al. 2024).
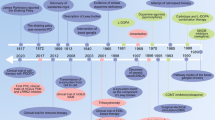
Prior to James Parkinson’s publication of his essay on shaking palsy in 1817, PD had been recognized and recorded by different cultures and medical professionals (Blonder 2018; Lees 2007). Ancient India referred to it as Kampavata and treated it with plants containing levodopa, a dopamine precursor (Ovallath and Deepa 2013; Bhattacharyya 2022). Mucuna pruiens, a tropical leguminous plant, has been used in Ayurvedic medicine for centuries (Verma et al. 2014). L-dopa accounts for approximately 5% of the phenolic compounds found in Mucuna seed velvet bean (Vadivel and Pugalenthi 2008; Foley 2003). In ancient Greece and Rome, Galen described it as shaking palsy and differentiated between resting and action tremors (Molina-Negro and Hardy 1975; Lanska 2009; Blattspieler 1946). In the 17th and 18th centuries, several European authors, such as Pápai Páriz, Sagar, Gaubius, Hunter, and Chomel, reported cases of PD and suggested various therapies (Blattspieler 1946; García Ruiz 2004; Arredondo-Blanco et al. 2018). However, it was James Parkinson who first identified PD as a distinct neurological disorder and provided a detailed description of its symptoms, in “An Essay on the Shaking Palsy” (Fig. 1) (Parkinson 1817, 2002; McDonald et al. 2018; Halliday et al. 2011).
Timeline of Parkinson’s disease. Parkinson’s disease (PD) has a long history of recognition and treatment in various cultures, predating James Parkinson’s seminal 1817 essay. Ancient Indian Ayurvedic medicine referred to PD as Kampavata and utilized plants like Mucuna pruriens, a tropical legume rich in levodopa, the primary drug used to treat the condition. Similarly, ancient Greek and Roman physicians, as well as European doctors in the 17th and 18th centuries, documented PD symptoms and trialed various therapies. However, it was James Parkinson who first identified PD as a distinct neurological disorder in 1817. French neurologist Jean-Martin Charcot improved its diagnosis, and Arvid Carlsson’s discovery of levodopa’s effectiveness revolutionized treatment. PD research continues to evolve further, building on the contributions of these pioneers
James Parkinson, an English physician, was the first to characterize PD in 1817, identifying symptoms like tremor, rigidity, and bradykinesia (Parkinson 1817, 2002; Schiller 1986; Bhattacharyya 2017). He named it paralysis agitans and suggested treatments like opium and bloodletting (Parkinson 2002). Jean-Martin Charcot, a French neurologist, improved the diagnosis of PD and named it after Parkinson (Charcot 1877; Lees 2007). He together with Leopold Ordenstein and then Wilhelm Erb utilized anticholinergic medications hyoscyamine and scopolamine, respectively to address certain symptoms (Goetz 2011; Schulz et al. 2011; Foley 2003). Arvid Carlsson, a Swedish pharmacologist, found that reserpinized hares’ brains with dopamine deficits exhibited PD-like symptoms. The conditions were alleviated by DL-dopa (Carlsson et al. 1957, 1958; Carlsson 1964). He used levodopa, the initial successful treatment for the condition, earning him the Nobel Prize in 2000 (Carlsson et al. 1957; Birkmayer and Hornykiewicz 1961; Carlsson 2001). George C. Cotzias, a Greek-American neurologist, was a trailblazer in utilizing high doses of levodopa and researching its impacts and adverse reactions (Cotzias et al. 1967, 1969; Cotzias 1971). Meanwhile, seminal clinical studies revealed that combining levodopa with the dopa decarboxylase inhibitor (DDI) had a levodopa-saving effect. The combination of levodopa and DDI has now become the gold standard for treating PD (Birkmayer and Mentasti 1967; Keller et al. 2011). Cotzias also experimented with other medications, such as dopamine receptor agonist apomorphine, for PD (Cotzias et al. 1970). Anders Björklund, a prominent Swedish neuroscientist, is a pioneer in the area of neural transplantation for PD. He and his colleagues implant fetal dopamine neurons into the brains of PD patients to enhance their symptoms and analyze their survival and incorporation (Lindvall et al. 1990). However, adverse reactions, including graft-induced dyskinesias, were observed, necessitating the withdrawal of this strategy and its ongoing investigation (Bjorklund and Kordower 2013). These individuals are prominent in the field of PD, but there are numerous others who have contributed significantly to the understanding and management of this condition. Ongoing research is being conducted on PD, with the development of new therapies aimed at improving the quality of life for individuals affected by the condition (Fig. 1).
A German neurologist Dr. Reichmann has made substantial contributions to the field of PD research, which is essential for enhancing our comprehension of this intricate neurodegenerative condition (Klingelhoefer and Reichmann 2017). His innovative research has provided valuable insights into different facets of the illness, such as its causes, development, and possible points of intervention for treatment (Reichmann 2011; Klingelhoefer and Reichmann 2015; Ossig and Reichmann 2013). His research has contributed to better understand alpha-synuclein (α-syn) protein aggregation in the development of PD, contributing to the understanding of the processes that lead to the degeneration of dopaminergic neurons (Riederer et al. 2019; Becker et al. 1995; Pan-Montojo et al. 2012). Moreover, investigations into the genetic and environmental factors linked to the disease have yielded valuable insights into its multifactorial nature (Klingelhoefer and Reichmann 2017; Balbona et al. 2022; Kidd 2000). By understanding his contributions to PD research, we can enhance our understanding of the disease and facilitate the progress of developing efficacious treatments, which could potentially enhance the quality of life for millions of individuals afflicted by this incapacitating condition.
Although Reichmann and other researchers have made significant research efforts, there is still a lack of knowledge in the field of PD that requires additional investigation. PD, a multifaceted neurological disorder, has been subject to thorough investigation over time, resulting in substantial advancements in our understanding of its etiology, manifestations, and progression (Kouli et al. 2018; Rodriguez-Oroz et al. 2009; Poewe and Mahlknecht 2009). Nevertheless, the specific mechanisms responsible for its onset and progression remain unclear. Although there are many treatments that show promise in managing symptoms, there is a need for the development of more effective and targeted therapies to slow or halt disease progression (Oertel and Schulz 2016; Ntetsika et al. 2021; Sardi et al. 2018). Continuing research is crucial to unravel the complexities of this condition and discover new therapeutic targets and personalized interventions for patients, ultimately improving their quality of life.
Reichmann’s invaluable contributions to research on PD have greatly enhanced our comprehension of this intricate neurodegenerative disorder. PD has been linked to oxidative stress, reduced antioxidant capacity, excitotoxicity, mitochondrial dysfunction, proteasomal dysfunction, apoptosis, lysosomal dysfunction, and impaired autophagy. These factors contribute to α-synuclein fibril formation, neuroinflammation, and cell death. Understanding these mechanisms can inform the development of targeted therapies to slow or prevent disease progression (Cohen 1983; Heikkila et al. 1984; Riederer et al. 2021). His groundbreaking research on the impact of oxidative stress in PD has uncovered the harmful consequences of free radicals on dopaminergic neurons, suggesting that oxidative stress could be a viable target for therapy (Reichmann et al. 2012b; Gille et al. 2004; Dexter and Jenner 2013; Jászberényi et al. 2024). Furthermore, his investigation into inflammation and specifically, neuroinflammation has yielded vital understandings regarding the progression of diseases and possible approaches to intervention (Holzer et al. 2017; Reichmann et al. 2002; Nagatsu and Sawada 2005; Nagatsu et al. 2000). His work not only enhanced our comprehension of PD but also expanded the potential for future investigation. Given these discoveries, upcoming research should focus on investigating innovative therapeutic approaches that specifically address oxidative stress and inflammation (Pajares et al. 2020; Colombo et al. 2020; Jiang et al. 2016; Tanaka and Vécsei 2021; Fornari Laurindo et al. 2023; de Souza et al. 2020). Additionally, it is important to explore potential biomarkers that can be used for the early detection and continuous monitoring of disease advancement (Berg 2008; Molochnikov et al. 2012; Lotankar et al. 2017; Tanaka and Vécsei 2020; Höglinger et al. 2024; Höglinger and Lang 2024). In order to make progress in the field, potential areas for future research could include studying the molecular pathways involved in the development of PD, identifying early biomarkers for accurate diagnosis, and developing targeted interventions to slow down or stop the progression of the disease (Tanaka et al. 2023b Török et al. 2020).
Moreover, Reichmann’s research has made a substantial contribution to presenting potential strategies for improved diagnosis and treatment. The outcomes of his research have facilitated the development of improved diagnostic methods, enabling the earlier and more precise identification of PD (Bhidayasiri and Reichmann 2013; Ossig and Reichmann 2015a). Timely diagnosis enables the implementation of suitable treatments and interventions to effectively control symptoms and decelerate the progression of the disease (Pahwa and Lyons 2010; Bloem et al. 2019). Furthermore, his research has also investigated novel therapeutic methods, such as neuroprotective strategies and deep brain stimulation, that show potential for enhancing the quality of life for individuals with PD (Polanski et al. 2010; Keller et al. 2020; Sarkar et al. 2016; McKinnon et al. 2019; Tanaka and Vécsei 2021). Thus, Reichmann’s impact on PD research is multifaceted and far-reaching. This narrative review article aims to provide a short but clear overview of his significant contributions. By highlighting key areas such as motor and non-motor symptoms, health-related quality of life, energy metabolism, and etiopathogenesis, readers gain insight into the breadth of Reichmann’s work. Additionally, this review attempts to identify research gaps and sets the stage for discussing potential future avenues. These avenues may include novel biomarkers, innovative therapies, and personalized medicine approaches. Ultimately, the article underscores the patient-centric goal: improving diagnosis and treatment outcomes for those affected by this debilitating condition.
The progress in understanding the etiology and management of Parkinson’s disease
Prior to the pioneering research conducted by Reichmann, PD was primarily recognized as a neurodegenerative disorder characterized by the loss of dopamine-producing neurons in the brain, resulting in classical Parkinsonian motor symptoms such as tremors, rigidity, and bradykinesia (Goetz 2011; Reichmann and Jost 2023a). The etiology of the disease is associated with a complex interplay of genetic and environmental factors (Pang et al. 2019; Allam et al. 2005; Bogers et al. 2023). Traditional treatment strategies have focused on alleviating symptoms through dopamine replacement therapy, including levodopa, and other medications that target motor impairments as well as non-motor symptoms, such as depression and sleep disturbances.
Dr. Reichmann’s contributions to Parkinson’s disease research and treatment
Reichmann’s extensive research on energy metabolism, premotor symptoms, and the etiopathogenesis and treatment of PD has significantly advanced our understanding of this condition (Reichmann and Janetzky 2000; Reichmann 2016, 2017; Klingelhoefer and Reichmann 2015; Storch et al. 2013). His groundbreaking contributions have played a crucial role in enhancing our knowledge of PD and its associated disorders, including neuromuscular diseases and neurosarcoidosis (Reichmann et al. 1993a, 1995). Furthermore, his research delved into the treatment methods for gastrointestinal dysfunction in PD, offering valuable insights into the development of novel therapeutic strategies and future research directions (Klingelhoefer and Reichmann 2015; Reichmann 2021).
Beyond the traditional focus on motor impairments and dopamine replacement therapy, emerging research underscores the significance of cognitive and social functioning alterations in PD. These changes are believed to stem from disrupted activity within cortical and subcortical brain structures (Battaglia et al. 2023c, d, 2024d; Battaglia and Thayer 2022). Consequently, there is a growing imperative to develop holistic management strategies for PD that encompass not only motor symptoms but also address the cognitive and social challenges faced by individuals living with the disease (Candini et al. 2021; Di Gregorio et al. 2024; Tortora et al. 2023; Di Gregorio and Battaglia 2023). Additionally, rehabilitation programs, including speech therapy, occupational therapy, and physical therapy, are recommended as complementary treatments to help manage the functional limitations associated with the disease (Weiner and Singer 1989; Riklan and Cooper 1961; Hunt 1988). Continued efforts have been made to discover novel therapeutic approaches, with a growing interest in investigating the role of gastrointestinal dysfunction and autonomic disorders (Camilleri 1990; Goetz et al. 1986).
The function of energy metabolism
Energy metabolism, which involves the conversion of food and oxygen into energy and waste products within cells, is a fundamental process necessary for proper functioning of the brain and body (McKenna et al. 2012; Wagner et al. 2011; Laurindo et al. 2023a, 2024; Bosso et al. 2023). PD disrupts motor and coordination functions as well as other aspects, arising from the loss of dopamine-producing neurons in the substantia nigra, which is responsible for regulating motor control activity in other brain regions (Mazzoni et al. 2012; Ingvarsson et al. 1997; Zgaljardic et al. 2003; Castrioto et al. 2016). Prior to Reichmann’s research, we had knowledge of impaired energy metabolism in PD; however, the underlying mechanisms remain unclear (Cohen 1983; Heikkila et al. 1984).
Reichmann’s extensive research has primarily concentrated on three crucial aspects related to PD. Firstly, his investigations into energy metabolism have provided valuable insights into the underlying mechanisms of this neurodegenerative disorder (Reichmann and Janetzky 2000; Reichmann 2016, 2017; Klingelhoefer and Reichmann 2015). By studying the cellular processes involved in energy production and utilization within the brain, he has been able to identify key mitochondrial metabolic dysfunctions that contribute to the development and progression of PD. His findings have shed light on how alterations in energy metabolism impact neuronal function and survival, thus paving the way for potential therapeutic interventions targeting these pathways.
Previous hypotheses and findings
The following are some of the hypotheses and findings that had been proposed or uncovered prior to Reichmann’s research. Patients diagnosed with PD exhibit lower levels of glucose and oxygen consumption in the brain, particularly in the striatum, which is the primary target of dopamine neurons (Kuhl et al. 1984; Wolfson et al. 1985). This suggests that brain cells are less active and more efficient in their energy utilization. Additionally, individuals with PD have lower levels of the primary energy currency of the cell, adenosine triphosphate (ATP), and higher levels of anaerobic glycolysis byproduct lactate (Reichmann and Janetzky 2000; Gille et al. 2004; Emamzadeh and Surguchov 2018). These findings indicate that the cells are relying more on the less efficient anaerobic pathway to produce energy and that there exists an imbalance between energy supply and demand.
Meanwhile, patients with PD exhibit elevated levels of oxidative stress, which is the harm caused by reactive oxygen species (ROS) on cellular components, such as DNA, proteins, and lipids (Weng et al. 2018; Dorszewska et al. 2021; Chang and Chen 2020; Laurindo et al. 2023a; Nunes et al. 2023; Reichmann and Riederer 1989; Schapira et al. 1989; Mizuno et al. 1989). ROS are ordinarily generated during cellular respiration, but they can also be produced by environmental toxins, inflammation, or mitochondrial dysfunction (Franco et al. 2009; Chelombitko 2018; Cui et al. 2012; Laurindo et al. 2023b; Nishikito et al. 2023; Minniti et al. 2023; McGeer et al. 1988; Pan-Montojo et al. 2012). Oxidative stress can disrupt the function and structure of mitochondria, which are organelles responsible for producing the majority of the ATP in the cell (Cui et al. 2012; Federico et al. 2012; Guo et al. 2013). In PD patients, there is mitochondrial dysfunction, which involves the impairment of the mitochondrial respiratory chain, a series of enzymes and molecules that transfer electrons from glucose and oxygen to produce ATP and water (Bose and Beal 2016; Hu and Wang 2016; Malpartida et al. 2021; Tanaka et al. 2022b). This impairment can reduce the efficiency and capacity of energy production and increase the production of ROS and other toxic metabolites (Moradi Vastegani et al. 2023; Indo et al. 2007; Lee and Kim 2022; Laurindo et al. 2023a). The dysfunction of mitochondria can initiate cell death pathways, including apoptosis and necrosis (Lemasters et al. 1999; Bock and Tait 2020; Harrington et al. 2023). Individuals with PD have genetic mutations or polymorphisms that impact the enzymes or proteins involved in energy metabolism, such as complex I of the mitochondrial respiratory chain, pyruvate dehydrogenase, or uncoupling proteins (Tryphena et al. 2023; Buchanan and Taylor 2020; Kumar et al. 2022a). These genetic factors can alter the expression, activity, or regulation of these molecules, and disrupt the equilibrium and flow of energy metabolism.
Dr. Reichmann’s comprehensive research on energy metabolism in Parkinson’s disease
Reichmann’s research explored the thermodynamic, circadian, and molecular aspects of energy metabolism in PD (Mesika and Reichmann 2019; Hermann et al. 2020; Riederer et al. 2019). He conducted research on the impact of variables such as temperature, time of day, and genetic differences on the production and consumption of energy in brain cells. Additionally, he explored the relationship between energy metabolism in brain cells and inflammation, oxidative stress, and mitochondrial dysfunction, which are factors associated with PD (Bendig et al. 2024; Mesika and Reichmann 2019; Reichmann and Janetzky 2000; Laurindo et al. 2023a).
Reichmann and Riederer’s work in 1989 provided crucial biochemical analyses of respiratory chain enzymes in various brain regions of PD patients, highlighting deficiencies in the electron transport chain (Reichmann and Riederer 1989). Building on this foundation, Schapira et al. identified a marked reduction in the 30-, 25-, and 24-kDa subunits of mitochondrial complex I specifically in the substantia nigra of PD patients. This discovery linked mitochondrial dysfunction, particularly within complex I, to the pathogenesis of PD (Schapira et al. 1989). Furthermore, Mizuno et al. (1989) confirmed these findings, solidifying our understanding that impaired energy production within neurons due to complex I deficiencies is a critical factor in the development and progression of PD (Mizuno et al. 1989; Mizuno 1990). Through his investigation of these aspects of energy metabolism, he uncovered significant findings regarding the underlying causes and consequences of PD at cellular and molecular levels.
Furthermore, his work explored the potential benefits of various interventions aimed at safeguarding neurons from harm or enhancing their energy metabolism in PD. They investigated the impact of coenzyme Q10, a natural antioxidant and cofactor of the mitochondrial respiratory chain, which is involved in energy production (Gille et al. 2004; Beal et al. 1994). He also investigated the effects of creatine, a natural compound that can increase energy storage and availability in brain cells (Löhle and Reichmann 2010). Additionally, the effects of ketogenic diets have been explored, which are high-fat, low-carbohydrate diets that can induce the production of ketone bodies that are alternative energy sources for brain cells (Włodarek 2019; Phillips et al. 2018; Shaafi et al. 2016). His research has made a substantial contribution to our understanding of the etiology and pathophysiology of PD as well as the management of its symptoms and complications, providing new avenues for the development of effective therapies for PD by modulating the energy metabolism.
Premotor symptoms
Premotor symptoms are indicators that occur prior to the emergence of the primary motor symptoms of PD, such as tremors, muscle rigidity, and slowed movement (Chen et al. 2013; Rodríguez-Violante et al. 2017; Silva et al. 2023). These symptoms may also encompass non-motor-related manifestations, such as a decreased sense of smell, constipation, rapid eye movement (REM) sleep behavior disorder, depression, anxiety, exhaustion, and cognitive decline (Lee and Koh 2015; Kumaresan and Khan 2021; Radad et al. 2023; Tanaka and Chen 2023). Additionally, subtle motor changes, such as a reduction in arm swinging motion, micrographia, and facial masking, may also be present in the early stages of PD (Wu et al. 2015; Simonet et al. 2021; Lee 2023). Prior to Reichmann’s research, it was widely acknowledged that premotor symptoms were common in PD patients and frequently went unnoticed, with some cases displaying a lag of several years or even decades before the onset of motor symptoms.
Dr. Reichmann’s contributions to the understanding of premotor symptoms in Parkinson’s disease
Reichmann has made significant contributions to the understanding of premotor symptoms associated with PD (Reichmann 2010, 2017, 2021; Maass and Reichmann 2013; Buhmann et al. 2018; Storch et al. 2013). Through careful examination and analysis of prodromal stages, prior to the onset of motor symptoms, he has unveiled a range of non-motor manifestations that can serve as early indicators of the disease. Such symptoms include olfactory dysfunction, constipation, sleep disturbances, and cognitive impairments. By identifying these premotor signs, Reichmann’s work enhances the prospects of early detection and intervention, enabling early treatment and potentially delaying the onset of motor symptoms. His expertise in the premotor diagnosis of PD was evidenced by his comprehensive review of the current knowledge and challenges associated with this area of study. He specifically focused on the most common premotor symptoms, including hyposmia, constipation, REM sleep behavior disorder, and depression (Reichmann 2010, 2017; Ziemssen and Reichmann 2007; Reichmann et al. 2009). However, he emphasized the need for further validation and standardization of these methods. He was also involved in several international collaborations and projects on premotor PD, including the European Academy of Neurology (EAN) task force on the definition of premotor and prodromal PD and the International Parkinson and Movement Disorder Society (MDS) project on the development of clinical criteria for prodromal PD.
The etiopathogenesis and treatment of Parkinson’s disease
Prior to Reichmann’s research, the etiopathogenesis of PD was primarily understood through the recognition of motor symptoms such as tremors, lack of movement, and drooling, as documented in historical medical treatises dating back to ancient times. Early treatments for PD were based on empirical observations, with anticholinergic drugs introduced as far back as the 19th century (Case 1893; Rose and Brackenridge 1881; Barrett et al. 2021). The use of levodopa by Carlsson in 1958 revolutionized PD treatment; however, limited treatment options persist (Foley 2003). By the late 1980s, deep brain stimulation had emerged as a potential therapeutic approach (Siegfried and Rea 1988; Bronstein et al. 2011; Bucur and Papagno 2023; Benabid 2003).
A number of genetic risk factors have been discovered: Mutations in genes, including SNCA, PARK2, PINK1, PRKN, and LRRK2, have been linked to familial PD, and some of these genes also play a role in sporadic cases (Cherian and Divya 2020; Tan and Skipper 2007; Cherian et al. 2023; Lesage and Brice 2012). LRRK2 mutations are the most common genetic cause of both familial and sporadic PD (Martin et al. 2014; Kluss et al. 2019). LRRK2 is a protein that has multiple functions in the cell, such as regulating the activity and recycling of lysosomes, which are involved in breaking down and disposing of waste materials (Madureira et al. 2020; Roosen and Cookson 2016; Bonet-Ponce and Cookson 2022). LRRK2 mutations can affect the function of lysosomes and cause them to release toxic substances that damage neurons (Skibinski et al. 2014; Yakhine-Diop et al. 2014; Jeong and Lee 2020). LRRK2 mutations can also activate microglia, which are immune cells in the brain that normally clear away debris and pathogens (Schapansky et al. 2015; Moehle et al. 2012; Panagiotakopoulou et al. 2020). However, when microglia are overactivated, they can produce inflammatory molecules that harm neurons and contribute to neurodegeneration (Lull and Block 2010; Song and Colonna 2018; Xu et al. 2021). Therefore, LRRK2 mutations can play a role in the etiopathogenesis of PD by impairing lysosomal function and triggering neuroinflammation. Future disease-modifying therapies may be aided by the active research of Reichmann and colleagues concerning the genetic basis of PD, including the function of LRRK2 mutations (Biskup et al. 2008; Reichmann and Jost 2023b; Schapira et al. 2009).
Dr. Reichmann’s contributions to Parkinson’s disease treatment
Reichmann has significantly advanced the treatment of PD. His innovative research has focused on developing novel treatment strategies to enhance the management of PD symptoms. One of his primary research interests is the investigation of monoamine oxidase B (MAO-B) inhibitors, including selegiline and rasagiline (Antonini et al. 2018; Reichmann 2016; Gerlach et al. 2012). MAO-B inhibitors have been shown to be neuroprotective in cell culture and animal experiments, raising the prospect that they may also be neuroprotective in humans and potentially delay disease progression (Nagatsu and Nakashima 2020; Dezsi and Vecsei 2017; Szökő et al. 2018; Tábi et al. 2020). MAO-B inhibitors can provide more sustained and continuous stimulation to post-synaptic dopaminergic receptors by increasing dopamine’s half-life in the basal ganglia (Löhle and Reichmann 2011; Reichmann et al. 2005b; Ossig and Reichmann 2015b; Nagatsu and Sawada 2006). Therapeutic strategies, such as cell-based dopamine substitution methods, are currently being researched extensively (Barker 2019; Chen et al. 2018; Rodríguez-Pallares et al. 2023). These methods are intended to improve symptom management in PD patients. Other studies have also shed light on the role of neurotrophic factors, such as brain-derived neurotrophic factor (BDNF), in the development of PD, highlighting their potential as theraeutic targets (Evans and Barker 2008; Palasz et al. 2020; Khan et al. 2023).
Reichmann’s work has not only contributed to the optimization of treatment approaches for PD but has also offered hope for better symptom control and an improved quality of life for patients (Reichmann et al. 2005a, 2020; Antonini et al. 2023). His research has addressed the current limitations in the management of PD, where available treatments mainly focus on symptomatic relief and do not alter the course of the disease. His efforts have been instrumental in paving the way for potential disease-modifying treatments that could restore dopaminergic tone in a targeted, physiological manner, as well as identify drugs capable of preventing or slowing ongoing neurodegeneration (Battaglia et al. 2024b). His work has thus been pivotal in driving the development of new and promising treatment approaches that may lead to a transformative shift in the landscape of PD management in the coming years.
Furthermore, he emphasizes the importance of individualized treatment strategies for PD patients. Patients diagnosed with PD must be treated individually based on their predominant symptoms and clinical presentation (Dias et al. 2021; Lemke et al. 2005; Antonini et al. 2023). A broad understanding of medical treatment options, as well as invasive therapeutic approaches, is required to support patients and improve their quality of life. Thus, Reichmann’s work has made significant contributions to our understanding of PD etiology and treatment approaches.
Opening future research directions: Dr. Reichmann’s insights into tryptophan metabolism in Parkinson’s disease
The pathophysiology of PD has focused on the disruption of essential amino acids, including tyrosine and tryptophan (Trp) (Nagatsu et al. 2019, 2022; Nakashima et al. 2013). Trp, an essential amino acid, plays a pivotal role in various biological processes. It is a critical component of our diet and is integral to protein synthesis (Barik 2020; Kałużna-Czaplińska et al. 2019; Fiore and Murray 2021). Beyond its nutritional role, Trp is the precursor to several important molecules, including serotonin, a neurotransmitter that regulates mood, sleep, and other functions (Peters 1991; Galla et al. 2021). Abnormal Trp metabolism has been linked to a number of neuropsychiatric disorders, highlighting its importance in maintaining mental health (Davidson et al. 2022; Comai et al. 2020; Dell’Osso et al. 2016; Fiore and Murray 2021). PD is one of the disorders in which Trp plays an important role. More and more evidence suggests that changes in Trp metabolism may contribute to the disease’s pathogenesis, opening up new avenues for treatment neuropsychiatric disorders (Heilman et al. 2020; Huang et al. 2023; Li et al. 2022; Modoux et al. 2021).
Tyrosine hydroxylase (TH) plays a crucial role as the rate-limiting enzyme in the biosynthesis of dopamine. In 1988, Mogi et al. published a significant paper that described the decrease in both the amount and function of TH in the substantia nigra of patients with PD (Mogi et al. 1988; Tábi et al. 2020). The study highlighted the loss of dopaminergic neurons and the related processes of neurodegeneration. Remarkably, the level of TH activity per enzyme was discovered to be increased in the PD-affected brain, suggesting the potential presence of a compensatory mechanism (Mogi et al. 1988). This study emphasizes how the degeneration of TH-positive neurons directly impacts dopamine production, contributing to the motor symptoms characteristic of PD. Reichmann’s research provides novel insights into the neuroprotective and neuron-differentiating properties of 9-methyl-β-carboline (9-me-BC), a Trp-derived molecule (Fig. 2) (Polanski et al. 2010; Keller et al. 2020; Wernicke et al. 2010). His findings show that 9-me-BC has the potential to increase the differentiation of dopaminergic neurons (Polanski et al. 2011; Hamann et al. 2008). This study is critical for understanding the therapeutic options for PD because it shows not only the stimulatory effects of 9-me-BC on dopaminergic neurons, but also its protective and regenerative properties. Reichmann’s research suggests a promising future for treating neurodegenerative diseases by increasing the expression of TH and its transcription factors, with 9-me-BC leading the way as a potential anti-Parkinsonian agent.
The Trp metabolic pathways produce various metabolites that affect brain function and lead to in neurodegenerative disorders, including PD (Fig. 3) (Hestad et al. 2022; Heilman et al. 2020; Davidson et al. 2022; Tanaka et al. 2021a). PD patients exhibit altered levels of KYN metabolites in both the plasma and cerebrospinal fluid, and these changes are associated with symptom severity and nigral pathology (Heilman et al. 2020). Researchers have discovered a new mechanism by which mutations in the parkin gene, which cause familial forms of PD, disrupt the interactions between lysosomes and mitochondria, leading to impaired Trp metabolism and mitochondrial dysfunction (Peng et al. 2023; Mizuno et al. 2008; Chithra et al. 2023; Polyák et al. 2023). Scientists have identified the structures of chemicals that can inhibit tryptophan 2,3-dioxygenase (TDO) or indoleamine 2,3-dioxygenase 1 (IDO1) which convert Trp into neurotoxic kynurenine (KYN) metabolites (Table 1) (Thackray et al. 2008; Tutakhail et al. 2020; Kozlova and Frédérick 2019). The majority of the compounds tested are for cancer treatment, but at least 12 antagonists targeting TDO and IDO1 have entered clinical trials (Sari et al. 2019; Peng et al. 2022). Some substances were shown to protect neurons from degeneration in a mouse model of PD (Perez-Pardo et al. 2021; Boros and Vécsei 2021; Ning et al. 2021). In addition to TDO and IDO1, kynurenine-3-monooxygenase has the potential to be another Trp-KYN pathway target for disease intervention. Furthermore, research has also uncovered new insights into the role of α-syn, a key pathological protein in PD, in regulating dopamine and serotonin metabolisms (Perez et al. 2002; Miquel-Rio et al. 2023). These findings suggest that targeting α-syn may modulate the production of neuroprotective or neurotoxic metabolites.
Tryptophan (Trp) metabolism. More than 2%, 5%, and 90% of L-Trp is metabolized via the serotonin, gut microbial indole pyruvate, and kynurenine pathways, respectively. Tryptophan 2,3-dioxygenase, indoleamine 2,3-dioxygenase 1, and kynurenine 3-monooxygenase are major disease intervention targets that have been extensively studied
Tryptophan-kynurenine metabolism in Parkinson’s disease: emerging research and therapeutic opportunities
Meanwhile, emerging research has highlighted the dysregulation of the Trp–KYN metabolic system in the pathogenesis of PD (Lim et al. 2017; Venkatesan et al. 2020 Török et al. 2022). The metabolism of Trp along the KYN pathway plays a pivotal role in modulating neuroinflammation and oxidative stress levels within the central nervous system (Tutakhail et al. 2020; Mithaiwala et al. 2021; Mor et al. 2021; Tajti et al. 2023; Tanaka et al. 2024b). The clinical manifestations of disrupted KYN metabolism encompass a spectrum of cognitive impairments commonly observed in neurological and psychiatric disorders (Battaglia et al. 2023a, b, 2024c; Ippolito et al. 2022). These include deficiencies in memory consolidation and learning, compromised executive function leading to difficulties in planning and set-shifting, challenges in adapting behavior to environmental cues, as well as impaired working memory and emotional regulation (Battaglia et al. 2022a, b, c; Sellitto et al. 2022). Such cognitive deficits often manifest as a distinct pattern indicative of frontal lobe dysfunction (Battaglia 2022; Battaglia et al. 2022d), reflecting the intricate interplay between neurotransmitter dysregulation and neural circuitry alterations (Battaglia et al. 2022e, 2023e; Tanaka et al. 2023a; Balogh et al. 2021; Liloia et al. 2024).
Recent research has challenged the oversimplified classification of KYN metabolites as either neurotoxic or neuroprotective. Emerging evidence highlights their multifaceted roles (Leon-Letelier et al. 2023; Ramírez Ortega et al. 2021). These bioactive compounds have a wide range of properties, including the ability to modulate oxidative stress, reduce inflammation, and modulate immunity (Tan et al. 2022; Carreño et al. 2022). Their actions depend on concentration gradients and the cellular microenvironment (Valvo et al. 2022; Kaymak et al. 2021). Moreover, the metabolic system operates via complex feedback loops. Despite ongoing investigations, consensus regarding the precise functions of KYN metabolites remains elusive (Tanaka et al. 2021b). Understanding this complexity is crucial for developing targeted therapies in PD. Emerging research suggests that modulating this pathway could offer therapeutic benefits, potentially slowing disease progression and improving patient outcomes. Future studies are essential to further elucidate the mechanisms by which KYN metabolites influence neuronal survival and to develop targeted interventions. By advancing our knowledge in this area, we can pave the way for novel treatments that harness the Trp-KYN metabolism, offering hope to those affected by this debilitating condition.
Kynurenic acid and kynurenic acid analogs
Kynurenic acid (KYNA), a metabolite of the KYN pathway, has demonstrated neuroprotective effects in neurodegenerative diseases by regulating glutamatergic systems (Fig. 3) (Wang et al. 2015; Tanaka et al. 2020), and preventing dopaminergic cell death (Klein et al. 2013; Beggiato et al. 2013; Zaiter et al. 2021). Some of the potential benefits of KYNA analogs in the treatment of neuropsychiatric diseases are that they may improve the cognitive functions (Martos et al. 2022; Urbańska et al. 2021; Tanaka et al. 2022d). They may reduce oxidative stress and α-syn aggregation, which are major causes of neuronal damage and death in PD. They may modulate glutamate receptors and prevent excessive glutamate release and excitotoxicity, which can impair synaptic plasticity and learning in PD patients (Battaglia et al. 2024a). They may inhibit the formation of amyloid-beta fibrils, which are another type of toxic protein aggregates that impair neuronal function in PD. KYNA analogs are promising candidates for the development of novel drugs or supplements for PD as they may target multiple mechanisms of neurodegeneration and provide neuroprotection (Szabo et al. 2023; Majerova et al. 2022; Martos et al. 2024). However, further research is required to validate their safety and efficacy in patients with PD.
Researchers have been developing KYNA analogs to treat neurologic and psychiatric disorders (Tajti et al. 2015; Deora et al. 2017; Stone 2000; Kozak et al. 2014; Tanaka et al. 2022a). KYNA is a natural metabolite of Trp that has neuroprotective and anti-inflammatory effects, but it has low bioavailability and brain penetration (Bratek-Gerej et al. 2021; Lee et al. 2019; Ostapiuk and Urbanska 2022Török et al. 2020). KYNA analogues are specifically engineered to address these constraints and engage with KYNA receptors, including NMDA and GPR35 receptors, which play a crucial role in neuronal signaling and plasticity (Molnár et al. 2021). One of the KYNA analogs that has shown promising results is SZR-104, which can cross the blood-brain barrier and modulate the activity of KYNA receptors. SZR-104 is derived from KYNA and has similar neuroprotective and anti-inflammatory properties (Molnár et al. 2021; Szabo et al. 2023). In preclinical studies, SZR-104 improved energy homeostasis, and decreased neuroinflammation (Poles et al. 2021; Szabo et al. 2022). SZR-104 also affected the morphology and function of microglia, which are immune cells in the brain that can either protect or damage neurons (Szabo et al. 2022; Poles et al. 2021).
Another KYNA analog that has shown promising results is a series of multifunctional compounds that combine the structural features of KYNA and tacrine, a drug that inhibits cholinesterase, an enzyme that breaks down acetylcholine, a neurotransmitter that is important for memory and cognition (Gorecki et al. 2021; Lustikaiswi et al. 2021; Decker and Duncan 2020). These compounds have high brain permeability and antioxidant activity, and they can inhibit both cholinesterase and amyloid beta peptide, which are associated with AD. These compounds also have high affinity and selectivity for the alpha-7 nicotinic receptor, which is a potential target for cognitive enhancement (Cieslikiewicz-Bouet et al. 2020; Singh et al. 2023). These KYNA analogs and KYNA combinations could be potential candidates for the treatment of neurodegenerative disorders, as they have multiple beneficial effects on the brain. However, further studies are needed to confirm their safety and efficacy in humans.
Investigation into the KYN metabolism has recently emerged as a promising approach for comprehending and potentially tackling crucial aspects of PD that have been at the forefront of Reichmann’s research. The KYN metabolism, involved in the degradation of Trp and the synthesis of various metabolites, has been increasingly linked to the onset of PD, regulation of energy homeostasis, and the emergence of early symptoms. Research has emphasized the connection between KYN metabolites, the process of aging, and the onset of PD, providing insight into its possible involvement in the progression of the disease. Furthermore, the metabolic pathway’s crucial role in producing compounds that regulate energy balance has been recognized, emphasizing its broader impact on physiological functions. The study of KYNA analogs, which are artificially created molecules intended for their positive impacts, has shown promise in advancing the development of drugs for potential treatment of neuropsychiatric disorders. This line of research may reveal new targets for treating PD by exploring the complex links between the KYN pathway, energy balance, and the early stages of PD. The findings could lead to more efficient and focused interventions for PD.
Discussion
The research conducted by Reichmann has greatly enhanced our comprehension and therapeutic approaches towards PD. The researcher’s findings have provided insight into the disturbance of energy metabolism in PD, a crucial process for the optimal operation of the brain and body. This disruption is caused by the degeneration of dopamine-producing neurons in the substantia nigra, a region responsible for regulating motor control in the brain. Before his research, it was understood that there was impaired energy metabolism in PD, but the specific mechanisms causing this impairment were not yet clear. Reichmann’s research has also emphasized the frequency of premotor symptoms in PD, which manifest before the appearance of main motor symptoms. This offers valuable knowledge about the initial phases of the illness. In addition, his research has enhanced our comprehension of the function of neurotrophic factors, such as BDNF, in the development of PD and their potential as a target for therapy. His work has not only provided optimism for enhanced management of symptoms and a higher standard of living for patients with PD, but also established the foundation for the creation of novel treatment approaches and potential therapies that can modify the progression of the disease.
Reichmann, a member of the editorial board of the Journal of Neural Transmission, collaborated with other experts to advance the understanding of PD etiology and expand its treatment options (Riederer et al. 2019; Sontag et al. 1995). For instance, He collaborated extensively with Dr. Peter Riederer to investigate several topics, including the etiology and treatment of PD, the function of iron in neurodegeneration, and the use of biomarkers for early detection of PD (Riederer et al. 2006; Reichmann et al. 2012a; Gerlach et al. 2012). Reichmann has co-authored papers with Dr. Kurt A. Jellinger on a variety of topics, including mitochondrial dysfunction, particularly in the electron transport chain and mitochondrial DNA, and its potential role in the pathogenesis of PD, as investigated using postmortem brain analysis of PD patients (Janetzky et al. 1994; Lestienne et al. 1990, 1991; Reichmann et al. 1993b).
Other co-authors include Ilona Csoti on the intersection of internal medicine and neurology and lifestyle and environmental factors in PD (Csoti et al. 2016; Reichmann et al. 2022); Wolfgang H. Jost on the latest developments and controversies in PD research (Reichmann and Jost 2023b); Christiana Ossig on neuropsychiatric symptoms in movement disorders and treatment strategies for advanced PD (Ossig et al. 2015; Ossig and Reichmann 2013); Jiri Koschel on lifestyle factors related to PD (Reichmann et al. 2022); Stefan Lorenzl on the high correlation between motor functions and quality of life (Krismer et al. 2022); Moussa BH Youdim on a comprehensive overview of current clinical and basic research on PD and related disorders (Riederer et al. 2006); Sofia B Dias and José A Diniz on potential for unobtrusive remote screening and detection of early PD signs (Iakovakis et al. 2018); Björn H. Falkenburger on translational research in PD (Dinter et al. 2020): Theodoros Kalliakoudas on homeostatic plasticity in the basal ganglia, which may explain non-classical PD (Falkenburger et al. 2022); Alexander Storch on sleep disorders in PD (Schrempf et al. 2014); Peter Vieregge on executive function differences in PD (Lange et al. 2003); and Christoph Schrader on the evidence-based guidelines for laboratory testing to diagnose, monitor, and manage PD (Müller et al. 2016), among others. Additionally, he has participated in a symposium focused on this topic, showcasing his commitment to advancing knowledge and improving treatment options for PD. These collaborations serve as testament to Reichmann’s dedication and expertise in this area.
A review of Reichman’s contributions to PD research could be a valuable and timely extension of previous work, providing a comprehensive overview of his pioneering studies on the role of α-syn in the pathogenesis of this neurodegenerative disorder. He has made substantial progress in understanding the mechanisms by which α-syn interacts with mitochondrial membranes, initiates protein synthesis, and leads to the death of dopaminergic neurons. In addition, his research has discovered new therapeutic targets and approaches, such as rapamycin and gene therapy, to control the levels of α-syn and hinder its aggregation. A review article of this nature would not only provide a comprehensive overview of the existing knowledge, but also emphasize the persisting obstacles and prospective avenues for further research on PD.
Generally, PD research has faced several challenges. The use of cellular models in preclinical research relies on specific culture conditions that may not accurately mimic the natural environment. These models often lack the ability to develop neuronal networks, resulting in the absence of essential connections. In clinical studies, the variability in diagnostic criteria for PD can impact the consistency of diagnoses across studies, affecting the reproducibility of research findings. PD’s diverse range of motor and non-motor symptoms complicates the development of comprehensive treatment approaches. Long-term studies, crucial for understanding PD progression, are difficult to maintain due to patient dropout and the need for extended funding and resources. Assessing the quality of life for PD patients remains challenging due to its subjective nature and the multifaceted impact of the disease on patients and caregivers. Additionally, while exploring the gut-brain axis in PD pathogenesis holds promise, the precise mechanisms linking gut dysfunction to PD development remain unclear.
This review has the potential to yield multiple advantages for both the scientific community and patients. At first, it presents a brief summary of Reichmann’s groundbreaking research on the importance of genetics in PD, with a specific emphasis on the mutations in the LRRK2 gene that are associated with both familial and sporadic forms of the condition. Furthermore, it has the potential to emphasize the deficiencies in knowledge and the obstacles that still exist in comprehending the molecular mechanisms and clinical significance of gene-environment interaction. In addition, it can suggest possible pathways and therapeutic strategies to manipulate the activity of gene and environmental interactions, thereby impeding or decelerating the onset and progression of PD. The primary objective of this research endeavor is to discover a remedy for PD, a profoundly debilitating neurodegenerative ailment that impacts a vast number of individuals globally. However, to accomplish this goal, it is essential to develop a thorough plan that combines genetic, biochemical, cellular, animal, and human studies with advanced techniques such as gene editing, stem cells, and biomarkers. The field of study is undergoing rapid development and holds significant promise for enhancing the identification, prediction, and management of PD. An article of this nature could offer a valuable synopsis and direction for researchers and clinicians with an interest in this subject matter.
Some possible limitations are that this review article may not cover all the aspects of PD research, including other environmental factors, biomarkers, or other genetic causes. It may also not reflect the latest findings or controversies in the field, as research is constantly evolving. Some possible benefits are that this review article could provide a comprehensive and authoritative overview of Reichman’s work on PD. It could also highlight the novel therapeutic targets and strategies that Reichman has identified. Some potential translation into clinical practice is that the review article could inform and guide clinicians and researchers who are interested in this topic and inspire new collaborations and innovations (Tanaka et al. 2022c, 2023a, c, d, 2024a; Tanaka and Vécsei 2024). It could also raise awareness and interest among patients and the public and thus encourage participation in clinical trials and studies. The review article could ultimately contribute to the development of more effective and personalized treatments for PD, a devastating neurodegenerative disorder that affects millions of people worldwide.
Conclusion
Reichmann’s pioneering research in PD has significantly advanced our understanding of this complex neurodegenerative disorder. His research into energy metabolism, premotor symptoms, and the multifactorial nature of PD has yielded valuable insights that have the potential to transform the landscape of PD management. His research into the underlying mechanisms of PD paved the way for the development of novel therapeutic approaches and personalized treatment strategies. His research not only sheds light on the pathophysiology of PD, but it also provides hope for better diagnostics, symptom control, and, ultimately, an improved quality of life in patients. Future-oriented, his work emphasizes the importance of multifaceted treatment approaches that consider not only motor symptoms but also cognitive and social dysfunction that people with PD face. His findings can be expanded upon in future PD research to enhance our comprehension of the condition and create more potent therapies. By continuing to investigate new therapeutic targets and approaches, we can work toward a future in which personalized and targeted interventions open up new avenues for research on Trp metabolism and related analogs, promising better outcomes for people with PD.
Abbreviations
- α-syn:
-
Alpha-synuclein
- ATP:
-
Adenosine triphosphate
- BDNF:
-
Brain-derived neurotrophic factor
- DDI:
-
Dopa decarboxylase inhibitor
- IDO1:
-
Indoleamine 2,3-dioxygenase 1
- KYN:
-
Kynurenine
- KYNA:
-
Kynurenic acid
- MAO-B:
-
Monoamine oxidase B
- 9-me-BC:
-
9-methyl-β-carboline
- PD:
-
Parkinson’s disease
- REM:
-
Rapid eye movement
- ROS:
-
Reactive oxygen species
- TDO:
-
Tryptophan 2,3-dioxygenase
- TH:
-
Tyrosine hydroxylase
- Trp:
-
Tryptophan
References
Allam MF, Del Castillo AS, Navajas RF-C (2005) Parkinson’s disease risk factors: genetic, environmental, or both? Neurol Res 27(2):206–208
Antonini A, Moro E, Godeiro C, Reichmann H (2018) Medical and surgical management of advanced Parkinson’s disease. Mov Disord 33(6):900–908
Antonini A, Reichmann H, Gentile G, Garon M, Tedesco C, Frank A, Falkenburger B, Konitsiotis S, Tsamis K, Rigas G (2023) Toward objective monitoring of Parkinson’s disease motor symptoms using a wearable device: wearability and performance evaluation of PDMonitor®. Front Neurol 14:1080752
Arredondo-Blanco K, Zerón-Martínez R, Rodríguez-Violante M, Cervantes-Arriaga A (2018) Brief historical review of Parkinson’s disease at 200 years of its description. Gac Med Mex 154:617–623
Balbona JV, Kim Y, Keller MC (2022) The estimation of environmental and genetic parental influences. Dev Psychopathol 34(5):1876–1886
Balogh L, Tanaka M, Török N, Vécsei L, Taguchi S (2021) Crosstalk between existential phenomenological psychotherapy and neurological sciences in mood and anxiety disorders. Biomedicines 9(4):340
Barbalho SM, Direito R, Laurindo LF, Marton LT, Guiguer EL, Goulart RA, Tofano RJ, Carvalho ACA, Flato UAP, Capelluppi Tofano VA, Detregiachi CRP, Bueno PCS, Girio RSJ, Araújo AC (2022) Ginkgo biloba in the aging process: a narrative review. Antioxid (Basel) 11(3). https://doi.org/10.3390/antiox11030525
Barik S (2020) The uniqueness of tryptophan in biology: properties, metabolism, interactions and localization in proteins. Int J Mol Sci 21(22):8776
Barker RA (2019) Designing stem-cell-based dopamine cell replacement trials for Parkinson’s disease. Nat Med 25(7):1045–1053
Barrett MJ, Sargent L, Nawaz H, Weintraub D, Price ET, Willis AW (2021) Antimuscarinic anticholinergic medications in Parkinson disease: to prescribe or deprescribe? Mov Disorders Clin Pract 8(8):1181–1188
Battaglia S (2022) Neurobiological advances of learned fear in humans. Adv Clin Experimental Med 31(3):217–221
Battaglia S, Thayer JF (2022) Functional interplay between central and autonomic nervous systems in human fear conditioning. Trends Neurosci 45(7):504–506
Battaglia S, Cardellicchio P, Di Fazio C, Nazzi C, Fracasso A, Borgomaneri S (2022a) The influence of vicarious fear-learning in infecting reactive action inhibition. Front Behav Neurosci 16:946263
Battaglia S, Cardellicchio P, Di Fazio C, Nazzi C, Fracasso A, Borgomaneri S (2022b) Stopping in (e) motion: reactive action inhibition when facing valence-independent emotional stimuli. Front Behav Neurosci 16:998714
Battaglia S, Fabius JH, Moravkova K, Fracasso A, Borgomaneri S (2022c) The neurobiological correlates of gaze perception in healthy individuals and neurologic patients. Biomedicines 10(3):627
Battaglia S, Harrison BJ, Fullana MA (2022d) Does the human ventromedial prefrontal cortex support fear learning, fear extinction or both? A commentary on subregional contributions. Mol Psychiatry 27(2):784–786
Battaglia S, Orsolini S, Borgomaneri S, Barbieri R, Diciotti S, di Pellegrino G (2022e) Characterizing cardiac autonomic dynamics of fear learning in humans. Psychophysiology 59(12):e14122
Battaglia MR, Di Fazio C, Battaglia S (2023a) Activated tryptophan-kynurenine metabolic system in the human brain is associated with learned fear. Front Mol Neurosci 16:1217090
Battaglia S, Di Fazio C, Vicario CM, Avenanti A (2023b) Neuropharmacological modulation of N-methyl-D-aspartate, noradrenaline and endocannabinoid receptors in fear extinction learning: synaptic transmission and plasticity. Int J Mol Sci 24(6):5926
Battaglia S, Nazzi C, Thayer J (2023c) Fear-induced bradycardia in mental disorders: Foundations, current advances, future perspectives. Neuroscience & Biobehavioral Reviews:105163
Battaglia S, Nazzi C, Thayer J (2023d) Heart’s tale of trauma: Fear-conditioned heart rate changes in post-traumatic stress disorder. Acta Psychiatrica Scandinavica :1–4
Battaglia S, Schmidt A, Hassel S, Tanaka M (2023e) Case reports in neuroimaging and stimulation. Front Psychiatry 14:1264669
Battaglia S, Avenanti A, Vécsei L, Tanaka M (2024a) Neural correlates and Molecular mechanisms of Memory and Learning, vol 25. MDPI
Battaglia S, Avenanti A, Vécsei L, Tanaka M (2024b) Neurodegeneration in cognitive impairment and mood disorders for experimental, clinical and translational neuropsychiatry, vol 12. MDPI
Battaglia S, Di Fazio C, Mazzà M, Tamietto M, Avenanti A (2024c) Targeting human glucocorticoid receptors in fear learning: a Multiscale Integrated Approach to Study Functional Connectivity. Int J Mol Sci 25(2):864
Battaglia S, Nazzi C, Thayer JF (2024d) Genetic differences associated with dopamine and serotonin release mediate fear-induced bradycardia in the human brain. Translational Psychiatry 14(1):24
Beal MF, Henshaw DR, Jenkins BG, Rosen BR, Schulz JB (1994) Coenzyme Q10 and nicotinamide block striatal lesions produced by the mitochondrial toxin malonate. Annals Neurology: Official J Am Neurol Association Child Neurol Soc 36(6):882–888
Becker G, Seufert J, Bogdahn U, Reichmann H, Reiners K (1995) Degeneration of substantia nigra in chronic Parkinson’s disease visualized by transcranial color-coded real-time sonography. Neurology 45(1):182–184
Beggiato S, Antonelli T, Tomasini MC, Tanganelli S, Fuxe K, Schwarcz R, Ferraro L (2013) Kynurenic acid, by targeting α7 nicotinic acetylcholine receptors, modulates extracellular GABA levels in the rat striatum in vivo. Eur J Neurosci 37(9):1470–1477
Benabid AL (2003) Deep brain stimulation for Parkinson’s disease. Curr Opin Neurobiol 13(6):696–706
Bendig J, Frank A, Reichmann H (2024) Aging and Parkinson’s disease: a complex interplay of vulnerable neurons, the immune system and the blood-brain barrier
Berg D (2008) Biomarkers for the early detection of Parkinson’s and Alzheimer’s disease. Neurodegener Dis 5(3–4):133–136. https://doi.org/10.1159/000113682
Bhattacharyya KB (2017) Hallmarks of clinical aspects of Parkinson’s disease through centuries. Int Rev Neurobiol 132:1–23
Bhattacharyya KB (2022) The story of levodopa: a long and arduous journey. Ann Indian Acad Neurol 25(1):124
Bhidayasiri R, Reichmann H (2013) Different diagnostic criteria for Parkinson disease: what are the pitfalls? J Neural Transm (Vienna) 120(4):619–625. https://doi.org/10.1007/s00702-013-1007-z
Birkmayer W, Hornykiewicz O (1961) The L-3, 4-dioxyphenylalanine (DOPA)-effect in Parkinson-Akinesia. Wiener Klinische Wochenschrift 73:787–788
Birkmayer W, Mentasti M (1967) Further experimental studies on the catecholamine metabolism in extrapyramidal diseases (Parkinson and chorea syndromes). Archiv fur Psychiatrie und Nervenkrankheiten 210(1):29–35
Biskup S, Gerlach M, Kupsch A, Reichmann H, Riederer P, Vieregge P, Wüllner U, Gasser T (2008) Genes associated with Parkinson syndrome. J Neurol 255:8–17
Bjorklund A, Kordower JH (2013) Cell therapy for Parkinson’s disease: what next? Mov Disord 28(1):110–115
Blattspieler LH (1946) Physiological and pathological aspects of tremor
Bloem BR, Marks WJ Jr., Silva de Lima AL, Kuijf ML, van Laar T, Jacobs BPF, Verbeek MM, Helmich RC, van de Warrenburg BP, Evers LJW, intHout J, van de Zande T, Snyder TM, Kapur R, Meinders MJ (2019) The personalized Parkinson Project: examining disease progression through broad biomarkers in early Parkinson’s disease. BMC Neurol 19(1):160. https://doi.org/10.1186/s12883-019-1394-3
Blonder LX (2018) Historical and cross-cultural perspectives on Parkinson’s disease. J Complement Integr Med 15(3):20160065
Bock FJ, Tait SWG (2020) Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol 21(2):85–100. https://doi.org/10.1038/s41580-019-0173-8
Bogers JS, Bloem BR, Den Heijer JM (2023) The etiology of Parkinson’s Disease: New perspectives from Gene-Environment interactions. J Parkinson’s Disease (Preprint) :1–8
Bonet-Ponce L, Cookson MR (2022) LRRK2 recruitment, activity, and function in organelles. FEBS J 289(22):6871–6890
Boros FA, Vécsei L (2021) Tryptophan 2, 3-dioxygenase, a novel therapeutic target for Parkinson’s disease. Expert Opin Ther Targets 25(10):877–888
Bose A, Beal MF (2016) Mitochondrial dysfunction in Parkinson’s disease. J Neurochem 139:216–231
Bosso H, Barbalho SM, de Alvares Goulart R, Otoboni AMMB (2023) Green coffee: economic relevance and a systematic review of the effects on human health. Crit Rev Food Sci Nutr 63(3):394–410
Bratek-Gerej E, Ziembowicz A, Godlewski J, Salinska E (2021) The mechanism of the neuroprotective effect of kynurenic acid in the experimental model of neonatal hypoxia–ischemia: the link to oxidative stress. Antioxidants 10(11):1775
Bronstein JM, Tagliati M, Alterman RL, Lozano AM, Volkmann J, Stefani A, Horak FB, Okun MS, Foote KD, Krack P (2011) Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues. Arch Neurol 68(2):165–165
Buchanan JL, Taylor EB (2020) Mitochondrial pyruvate carrier function in Health and Disease across the Lifespan. Biomolecules 10(8). https://doi.org/10.3390/biom10081162
Bucur M, Papagno C (2023) Deep brain stimulation in Parkinson disease: a meta-analysis of the long-term neuropsychological outcomes. Neuropsychol Rev 33(2):307–346
Buglio DS, Marton LT, Laurindo LF, Guiguer EL, Araújo AC, Buchaim RL, Goulart RA, Rubira CJ, Barbalho SM (2022) The role of resveratrol in mild cognitive impairment and Alzheimer’s Disease: a systematic review. J Med Food 25(8):797–806. https://doi.org/10.1089/jmf.2021.0084
Buhmann C, Ip CW, Oehlwein C, Toenges L, Wolz M, Reichmann H, Kassubek J (2018) Parkinson disease and pain-diagnostic and therapeutic approaches to a challenging non-motor symptom. Fortschr Neurol Psychiatr 86(01):S48–S58
Burn DJ, Landau S, Hindle JV, Samuel M, Wilson KC, Hurt CS, Brown RG, Group PPS (2012) Parkinson’s disease motor subtypes and mood. Mov Disord 27(3):379–386
Camilleri M (1990) Disorders of gastrointestinal motility in neurologie diseases. In: Mayo Clinic Proceedings, vol 6. Elsevier, pp 825–846
Candini M, Battaglia S, Benassi M, di Pellegrino G, Frassinetti F (2021) The physiological correlates of interpersonal space. Sci Rep 11(1):2611
Carlsson A (1964) Evidence for a role of dopamine in extrapyramidal functions. Acta Neurovegetativa 26:484–493
Carlsson A (2001) A half-century of neurotransmitter research: impact on neurology and psychiatry (Nobel lecture). ChemBioChem 2(7–8):484–493
Carlsson A, Lindqvist M, Magnusson T (1957) 3, 4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature 180(4596):1200–1200
Carlsson A, Lindqvist M, Magnusson T, Waldeck B (1958) On the presence of 3-hydroxytyramine in brain. Science 127(3296):471–471
Carreño M, Pires MF, Woodcock SR, Brzoska T, Ghosh S, Salvatore SR, Chang F, Khoo NK, Dunn M, Connors N (2022) Immunomodulatory actions of a kynurenine-derived endogenous electrophile. Sci Adv 8(26):eabm9138
Case EE (1893) Clinical cases. Homoeopathic Physician 13(2):76–80
Castrioto A, Thobois S, Carnicella S, Maillet A, Krack P (2016) Emotional manifestations of PD: neurobiological basis. Mov Disord 31(8):1103–1113
Chang K-H, Chen C-M (2020) The role of oxidative stress in Parkinson’s disease. Antioxidants 9(7):597
Charcot J (1877) On Parkinson’s disease. Lectures on diseases of the nervous system delivered at the Salpêtrière. (Transl Sigerson G):129–156
Chelombitko M (2018) Role of reactive oxygen species in inflammation: a minireview. Mosc Univ Biol Sci Bull 73:199–202
Chen H, Burton EA, Ross GW, Huang X, Savica R, Abbott RD, Ascherio A, Caviness JN, Gao X, Gray KA (2013) Research on the premotor symptoms of Parkinson’s disease: clinical and etiological implications. Environ Health Perspect 121(11–12):1245–1252
Chen W, Huang Q, Ma S, Li M (2018) Progress in dopaminergic cell replacement and regenerative strategies for Parkinson’s disease. ACS Chem Neurosci 10(2):839–851
Cherian A, Divya K (2020) Genetics of Parkinson’s disease. Acta Neurol Belgica :1–9
Cherian A, Divya K, Vijayaraghavan A (2023) Parkinson’s disease–genetic cause. Curr Opin Neurol 36(4):292–301
Chithra Y, Dey G, Ghose V, Chandramohan V, Gowthami N, Vasudev V, Srinivas Bharath M (2023) Mitochondrial complex I inhibition in dopaminergic neurons causes altered protein profile and protein oxidation: implications for Parkinson’s disease. Neurochem Res 48(8):2360–2389
Cieslikiewicz-Bouet M, Naldi M, Bartolini M, Pérez B, Servent D, Jean L, Aráoz R, Renard P-Y (2020) Functional characterization of multifunctional ligands targeting acetylcholinesterase and alpha 7 nicotinic acetylcholine receptor. Biochem Pharmacol 177:114010
Cohen G (1983) Catalase, glutathione peroxidase, superoxide dismutase, and cytochrome P-450. Handbook of Neurochemistry: volume 4 enzymes in the nervous system. Springer, pp 315–330
Colombo D, Pnevmatikou P, Melloni E, Keywood C (2020) Therapeutic innovation in Parkinson’s disease: a 2020 update on disease-modifying approaches. Expert Rev Neurother 20(10):1047–1064
Comai S, Bertazzo A, Brughera M, Crotti S (2020) Tryptophan in health and disease. Adv Clin Chem 95:165–218
Cotzias GC (1971) Levodopa in the treatment of parkinsonism. JAMA 218(13):1903–1908
Cotzias GC, Van Woert MH, Schiffer LM (1967) Aromatic amino acids and modification of parkinsonism. N Engl J Med 276(7):374–379
Cotzias GC, Papavasiliou PS, Gellene R (1969) Modification of parkinsonism—chronic treatment with L-dopa. N Engl J Med 280(7):337–345
Cotzias GC, Papavasiliou PS, Fehling C, Kaufman B, Mena I (1970) Similarities between neurologic effects of L-dopa and of apomorphine. N Engl J Med 282(1):31–33
Csoti I, Jost WH, Reichmann H (2016) Parkinson’s disease between internal medicine and neurology. J Neural Transm 123:3–17
Cui H, Kong Y, Zhang H (2012) Oxidative stress, mitochondrial dysfunction, and aging. Journal of signal transduction 2012
Davidson M, Rashidi N, Nurgali K, Apostolopoulos V (2022) The role of tryptophan metabolites in neuropsychiatric disorders. Int J Mol Sci 23(17):9968
de Oliveira Zanuso B, de Oliveira Dos Santos AR, Miola VFB, Guissoni Campos LM, Spilla CSG, Barbalho SM (2022) Panax ginseng and aging related disorders: a systematic review. Exp Gerontol 161:111731. https://doi.org/10.1016/j.exger.2022.111731
de Souza GA, de Marqui SV, Matias JN, Guiguer EL, Barbalho SM (2020) Effects of Ginkgo biloba on diseases related to oxidative stress. Planta Med 86(06):376–386
Decker AL, Duncan K (2020) Acetylcholine and the complex interdependence of memory and attention. Curr Opin Behav Sci 32:21–28
Dell’Osso L, Carmassi C, Mucci F, Marazziti D (2016) Depression, serotonin and tryptophan. Curr Pharm Design 22(8):949–954
Deora GS, Kantham S, Chan S, Dighe SN, Veliyath SK, McColl G, Parat M-O, McGeary RP, Ross BP (2017) Multifunctional analogs of kynurenic acid for the treatment of Alzheimer’s disease: synthesis, pharmacology, and molecular modeling studies. ACS Chem Neurosci 8(12):2667–2675
Dexter DT, Jenner P (2013) Parkinson disease: from pathology to molecular disease mechanisms. Free Radic Biol Med 62:132–144
Dezsi L, Vecsei L (2017) Monoamine oxidase B inhibitors in Parkinson’s disease. CNS & neurological disorders-drug targets (formerly current drug Targets-CNS &. Neurol Disorders) 16(4):425–439
Di Gregorio F, Battaglia S (2023) Advances in EEG-based functional connectivity approaches to the study of the central nervous system in health and disease. Adv Clin Experimental Med 32(6):607–612
Di Gregorio F, Steinhauser M, Maier ME, Thayer JF, Battaglia S (2024) Error-related cardiac deceleration: functional interplay between error-related brain activity and autonomic nervous system in performance monitoring. Neurosci Biobehavioral Reviews :105542
Dias SB, Diniz JA, Konstantinidis E, Savvidis T, Zilidou V, Bamidis PD, Grammatikopoulou A, Dimitropoulos K, Grammalidis N, Jaeger H (2021) Assistive HCI-serious games co-design insights: the case study of i-PROGNOSIS personalized game suite for Parkinson’s disease. Front Psychol 11:612835
Dinter E, Saridaki T, Diederichs L, Reichmann H, Falkenburger BH (2020) Parkinson’s disease and translational research. Translational Neurodegeneration 9:1–11
Dong J, Cui Y, Li S, Le W (2016) Current pharmaceutical treatments and alternative therapies of Parkinson’s disease. Curr Neuropharmacol 14(4):339–355
Dorszewska J, Kowalska M, Prendecki M, Piekut T, Kozłowska J, Kozubski W (2021) Oxidative stress factors in Parkinson’s disease. Neural Regeneration Res 16(7):1383–1391
Emamzadeh FN, Surguchov A (2018) Parkinson’s disease: biomarkers, treatment, and risk factors. Front NeuroSci 12:394054
Evans JR, Barker RA (2008) Neurotrophic factors as a therapeutic target for Parkinson’s disease. Expert Opin Ther Targets 12(4):437–447
Falkenburger B, Kalliakoudas T, Reichmann H (2022) Adaptive changes in striatal projection neurons explain the long duration response and the emergence of dyskinesias in patients with Parkinson’s disease. J Neural Transm 129(5):497–503
Federico A, Cardaioli E, Da Pozzo P, Formichi P, Gallus GN, Radi E (2012) Mitochondria, oxidative stress and neurodegeneration. J Neurol Sci 322(1–2):254–262
Fiore A, Murray PJ (2021) Tryptophan and indole metabolism in immune regulation. Curr Opin Immunol 70:7–14
Fleming SM (2017) Mechanisms of gene-environment interactions in Parkinson’s disease. Curr Environ Health Rep 4:192–199
Foley PB (2003) Beans, roots and leaves: a history of the chemical therapy of parkinsonism. Tectum Verlag DE
Fornari Laurindo L, Aparecido Dias J, Cressoni Araújo A, Torres Pomini K, Machado Galhardi C, Rucco Penteado Detregiachi C, Santos de Argollo Haber L, Donizeti Roque D, Dib Bechara M, Vialogo Marques de Castro M, de Souza Bastos Mazuqueli Pereira E, José Tofano R, Jasmin Santos German Borgo I, Maria Barbalho S (2023) Immunological dimensions of neuroinflammation and microglial activation: exploring innovative immunomodulatory approaches to mitigate neuroinflammatory progression. Front Immunol 14:1305933. https://doi.org/10.3389/fimmu.2023.1305933
Franco R, Sánchez-Olea R, Reyes-Reyes EM, Panayiotidis MI (2009) Environmental toxicity, oxidative stress and apoptosis: menage a trois. Mutat Research/Genetic Toxicol Environ Mutagen 674(1–2):3–22
Galla Z, Rajda C, Rácz G, Grecsó N, Baráth Á, Vécsei L, Bereczki C, Monostori P (2021) Simultaneous determination of 30 neurologically and metabolically important molecules: a sensitive and selective way to measure tyrosine and tryptophan pathway metabolites and other biomarkers in human serum and cerebrospinal fluid. J Chromatogr A 1635:461775
García Ruiz PJ (2004) Prehistory of Parkinson’s disease. Neurologia 19(10):735–737
Gerlach M, Reichmann H, Riederer P (2012) A critical review of evidence for preclinical differences between rasagiline and selegiline. Basal Ganglia 2(4):S9–S15
Gille G, HUNG ST, Reichmann H, RAUSCH WD (2004) Oxidative stress to dopaminergic neurons as models of Parkinson’s disease. Ann N Y Acad Sci 1018(1):533–540
Goetz CG (2011) The history of Parkinson’s disease: early clinical descriptions and neurological therapies. Cold Spring Harbor Perspect Med 1 (1)
Goetz CG, Lutge W, Tanner CM (1986) Autonomic dysfunction in Parkinson’s disease. Neurology 36(1):73–73
Gorecki L, Misiachna A, Damborsky J, Dolezal R, Korabecny J, Cejkova L, Hakenova K, Chvojkova M, Karasova JZ, Prchal L (2021) Structure-activity relationships of dually-acting acetylcholinesterase inhibitors derived from tacrine on N-methyl-d-Aspartate receptors. Eur J Med Chem 219:113434
Guo C, Sun L, Chen X, Zhang D (2013) Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regeneration Res 8(21):2003–2014
Halliday G, Lees A, Stern M (2011) Milestones in Parkinson’s disease—clinical and pathologic features. Mov Disord 26(6):1015–1021
Hamann J, Wernicke C, Lehmann J, Reichmann H, Rommelspacher H, Gille G (2008) 9-Methyl-β-carboline up-regulates the appearance of differentiated dopaminergic neurones in primary mesencephalic culture. Neurochem Int 52(4–5):688–700
Harrington JS, Ryter SW, Plataki M, Price DR, Choi AMK (2023) Mitochondria in health, disease, and aging. Physiol Rev 103(4):2349–2422. https://doi.org/10.1152/physrev.00058.2021
Heikkila RE, Manzino L, Cabbat FS, Duvoisin RC (1984) Protection against the dopaminergic neurotoxicity of 1-methyl-4-phenyl-1, 2, 5, 6-tetrahydropyridine by monoamine oxidase inhibitors. Nature 311(5985):467–469
Heilman PL, Wang EW, Lewis MM, Krzyzanowski S, Capan CD, Burmeister AR, Du G, Escobar Galvis ML, Brundin P, Huang X (2020) Tryptophan metabolites are associated with symptoms and nigral pathology in Parkinson’s disease. Mov Disord 35(11):2028–2037
Herman T, Weiss A, Brozgol M, Wilf-Yarkoni A, Giladi N, Hausdorff JM (2015) Cognitive function and other non-motor features in non-demented Parkinson’s disease motor subtypes. J Neural Transm 122:1115–1124
Hermann W, Flemming T, Brandt MD, Langner S, Reichmann H, Storch A (2020) Asymmetry of periodic Leg movements in Sleep (PLMS) in Parkinson’s Disease. J Parkinsons Dis 10(1):255–266. https://doi.org/10.3233/jpd-191667
Hestad K, Alexander J, Rootwelt H, Aaseth JO (2022) The role of tryptophan dysmetabolism and quinolinic acid in depressive and neurodegenerative diseases. Biomolecules 12(7):998
Höglinger GU, Lang AE (2024) The why and how of the SynNerGe criteria of Parkinson´ s disease. J Neural Transm :1–6
Höglinger GU, Adler CH, Berg D, Klein C, Outeiro TF, Poewe W, Postuma R, Stoessl AJ, Lang AE (2024) A biological classification of Parkinson’s disease: the SynNeurGe research diagnostic criteria. Lancet Neurol 23(2):191–204
Holzer P, Farzi A, Hassan AM, Zenz G, Jačan A, Reichmann F (2017) Visceral inflammation and immune activation stress the brain. Front Immunol 8:1613
Hu Q, Wang G (2016) Mitochondrial dysfunction in Parkinson’s disease. Translational Neurodegeneration 5:1–8
Huang Y, Zhao M, Chen X, Zhang R, Le A, Hong M, Zhang Y, Jia L, Zang W, Jiang C (2023) Tryptophan metabolism in central nervous system diseases: pathophysiology and potential therapeutic strategies. Aging Disease 14(3):858
Hughes TD, Güner OF, Iradukunda EC, Phillips RS, Bowen JP (2022) The kynurenine pathway and kynurenine 3-monooxygenase inhibitors. Molecules 27(1):273
Hunt L (1988) Continuity of care maximizes autonomy of the elderly. Am J Occup Ther 42(6):391–393
Iakovakis D, Hadjidimitriou S, Charisis V, Bostantjopoulou S, Katsarou Z, Klingelhoefer L, Reichmann H, Dias SB, Diniz JA, Trivedi D (2018) Motor impairment estimates via touchscreen typing dynamics toward Parkinson’s disease detection from data harvested in-the-wild. Front ICT 5:28
Indo HP, Davidson M, Yen HC, Suenaga S, Tomita K, Nishii T, Higuchi M, Koga Y, Ozawa T, Majima HJ (2007) Evidence of ROS generation by mitochondria in cells with impaired electron transport chain and mitochondrial DNA damage. Mitochondrion 7(1–2):106–118. https://doi.org/10.1016/j.mito.2006.11.026
Ingvarsson PE, Gordon AM, Forssberg H (1997) Coordination of manipulative forces in Parkinson’s disease. Exp Neurol 145(2):489–501
Ippolito G, Bertaccini R, Tarasi L, Di Gregorio F, Trajkovic J, Battaglia S, Romei V (2022) The role of alpha oscillations among the main neuropsychiatric disorders in the adult and developing human brain: evidence from the last 10 years of research. Biomedicines 10(12):3189
Janetzky B, Hauck S, Youdim MB, Riederer P, Jellinger K, Pantucek F, Zo R, Boissl KW, Reichmann H (1994) Unaltered aconitase activity, but decreased complex I activity in substantia nigra pars compacta of patients with Parkinson’s disease. Neurosci Lett 169(1–2):126–128
Jászberényi M, Thurzó B, Bagosi Z, Vécsei L, Tanaka M (2024) The Orexin/Hypocretin System, the Peptidergic Regulator of Vigilance, orchestrates adaptation to stress. Biomedicines 12(2):448
Jeong GR, Lee BD (2020) Pathological functions of LRRK2 in Parkinson’s disease. Cells 9(12):2565
Jiang T, Sun Q, Chen S (2016) Oxidative stress: a major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Prog Neurobiol 147:1–19. https://doi.org/10.1016/j.pneurobio.2016.07.005
Kałużna-Czaplińska J, Gątarek P, Chirumbolo S, Chartrand MS, Bjørklund G (2019) How important is tryptophan in human health? Crit Rev Food Sci Nutr 59(1):72–88
Kaymak I, Williams KS, Cantor JR, Jones RG (2021) Immunometabolic interplay in the tumor microenvironment. Cancer Cell 39(1):28–37
Keller GA, Czerniuk P, Bertuola R, Spatz JG, Assefi AR, Di Girolamo G (2011) Comparative bioavailability of 2 tablet formulations of levodopa/benserazide in healthy, fasting volunteers: a single-dose, randomized-sequence, open-label crossover study. Clin Ther 33(4):500–510
Keller S, Polanski WH, Enzensperger C, Reichmann H, Hermann A, Gille G (2020) 9-Methyl-β-carboline inhibits monoamine oxidase activity and stimulates the expression of neurotrophic factors by astrocytes. J Neural Transm 127:999–1012
Khan MA, Haider N, Singh T, Bandopadhyay R, Ghoneim MM, Alshehri S, Taha M, Ahmad J, Mishra A (2023) Promising biomarkers and therapeutic targets for the management of Parkinson’s disease: recent advancements and contemporary research. Metab Brain Dis 38(3):873–919
Kidd PM (2000) Parkinson’s disease as multifactorial oxidative neurodegeneration: implications for integrative management. Altern Med Rev 5(6):502–529
Klein C, Patte-Mensah C, Taleb O, Bourguignon J-J, Schmitt M, Bihel F, Maitre M, Mensah-Nyagan AG (2013) The neuroprotector kynurenic acid increases neuronal cell survival through neprilysin induction. Neuropharmacology 70:254–260
Klingelhoefer L, Reichmann H (2015) Pathogenesis of Parkinson disease—the gut–brain axis and environmental factors. Nat Reviews Neurol 11(11):625–636
Klingelhoefer L, Reichmann H (2017) Parkinson’s disease as a multisystem disorder. J Neural Transm 124:709–713
Kluss JH, Mamais A, Cookson MR (2019) LRRK2 links genetic and sporadic Parkinson’s disease. Biochem Soc Trans 47(2):651–661
Komiya T, Huang CH (2018) Updates in the clinical development of epacadostat and other indoleamine 2, 3-dioxygenase 1 inhibitors (IDO1) for human cancers. Front Oncol 8:423
Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, Halliday GM, Bartus RT (2013) Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain 136(8):2419–2431
Kouli A, Torsney KM, Kuan W-L (2018) Parkinson’s disease: etiology, neuropathology, and pathogenesis. Exon Publications:3–26
Kozak R, Campbell BM, Strick CA, Horner W, Hoffmann WE, Kiss T, Chapin DS, McGinnis D, Abbott AL, Roberts BM (2014) Reduction of brain kynurenic acid improves cognitive function. J Neurosci 34(32):10592–10602
Kozlova A, Frédérick R (2019) Current state on tryptophan 2, 3-dioxygenase inhibitors: a patent review. Expert Opin Ther Pat 29(1):11–23
Krismer F, Seppi K, Jönsson L, Åström DO, Berger AK, Simonsen J, Gordon MF, Wenning GK, Poewe W, Investigators EMSASGNHS (2022) Sensitivity to change and patient-centricity of the unified multiple system atrophy rating scale items: a data‐driven analysis. Mov Disord 37(7):1425–1431
Kuhl DE, Metter EJ, Riege WH (1984) Patterns of local cerebral glucose utilization determined in Parkinson’s disease by the [18F] fluorodeoxyglucose method. Annals Neurology: Official J Am Neurol Association Child Neurol Soc 15(5):419–424
Kumar R, Singothu TA, Singh S, Bhandari SB V (2022a) Uncoupling proteins as a therapeutic target for the development of new era drugs against neurodegenerative disorder. Biomed Pharmacother 147:112656. https://doi.org/10.1016/j.biopha.2022.112656
Kumar S, Goyal L, Singh S (2022b) Tremor and rigidity in patients with Parkinson’s disease: emphasis on epidemiology, pathophysiology and contributing factors. CNS & neurological disorders-drug targets (formerly current drug Targets-CNS &. Neurol Disorders) 21(7):596–609
Kumaresan M, Khan S (2021) Spectrum of non-motor symptoms in Parkinson’s disease. Cureus 13 (2)
Kwok JYY, Choi KC, Chan HYL (2016) Effects of mind–body exercises on the physiological and psychosocial well-being of individuals with Parkinson’s disease: a systematic review and meta-analysis. Complement Ther Med 29:121–131
Lange K, Tucha O, Alders G, Preier M, Csoti I, Merz B, Mark G, Herting B, Fornadi F, Reichmann H (2003) Differentiation of parkinsonian syndromes according to differences in executive functions. J Neural Transm 110:983–995
Lanska DJ (2009) The history of movement disorders. Handb Clin Neurol 95:501–546
Laurindo LF, Barbalho SM, Araújo AC, Guiguer EL, Mondal A, Bachtel G, Bishayee A (2023a) Açaí (Euterpe oleracea Mart.) In health and disease: a critical review. Nutrients 15(4):989
Laurindo LF, de Carvalho GM, de Oliveira Zanuso B, Figueira ME, Direito R, de Alvares Goulart R, Buglio DS, Barbalho SM (2023b) Curcumin-based nanomedicines in the treatment of inflammatory and immunomodulated diseases: an evidence-based comprehensive review. Pharmaceutics 15(1):229
Laurindo LF, Rodrigues VD, Minniti G, de Carvalho ACA, Zutin TLM, DeLiberto LK, Bishayee A, Barbalho SM (2024) Pomegranate (Punica granatum L.) phytochemicals target the components of metabolic syndrome. J Nutr Biochem :109670
Lee J-H (2023) Understanding Parkinson’s disorders: classification and evaluation methods, Movement disorders, and treatment methods. Int J Adv Cult Technol 11(3):9–17
Lee N, Kim D (2022) Toxic metabolites and inborn errors of amino acid metabolism: what one informs about the other. Metabolites 12(6). https://doi.org/10.3390/metabo12060527
Lee HM, Koh S-B (2015) Many faces of Parkinson’s disease: non-motor symptoms of Parkinson’s disease. J Mov Disorders 8(2):92
Lee T, Park HS, Jeong JH, Jung TW (2019) Kynurenic acid attenuates pro-inflammatory reactions in lipopolysaccharide-stimulated endothelial cells through the PPARδ/HO-1-dependent pathway. Mol Cell Endocrinol 495:110510
Lees AJ (2007) Unresolved issues relating to the shaking palsy on the celebration of James Parkinson’s 250th birthday. Mov Disorders: Official J Mov Disorder Soc 22(S17):S327–S334
Lemasters JJ, Qian T, Bradham CA, Brenner DA, Cascio WE, Trost LC, Nishimura Y, Nieminen AL, Herman B (1999) Mitochondrial dysfunction in the pathogenesis of necrotic and apoptotic cell death. J Bioenerg Biomembr 31(4):305–319. https://doi.org/10.1023/a:1005419617371
Lemke MR, Brecht HM, Koester J, Kraus PH, Reichmann H (2005) Anhedonia, depression, and motor functioning in Parkinson’s disease during treatment with pramipexole. J Neuropsychiatry Clin Neurosci 17(2):214–220
Leon-Letelier RA, Dou R, Vykoukal J, Sater AHA, Ostrin E, Hanash S, Fahrmann JF (2023) The kynurenine pathway presents multi-faceted metabolic vulnerabilities in cancer. Front Oncol 13:1256769
Lesage S, Brice A (2012) Role of mendelian genes in sporadic Parkinson’s disease. Parkinsonism Relat Disord 18:S66–S70
Lestienne P, Nelson J, Riederer P, Jellinger K, Reichmann H (1990) Normal mitochondrial genome in brain from patients with Parkinson’s disease and complex I defect. J Neurochem 55(5):1810–1812
Lestienne P, Nelson I, Riederer P, Reichmann H, Jellinger K (1991) Mitochondrial DNA in postmortem brain from patients with Parkinson’s disease. J Neurochem 56(5):1819–1819
Li D, Yu S, Long Y, Shi A, Deng J, Ma Y, Wen J, Li X, Liu S, Zhang Y (2022) Tryptophan metabolism: mechanism-oriented therapy for neurological and psychiatric disorders. Front Immunol 13:985378
Liloia D, Zamfira DA, Tanaka M, Manuello J, Crocetta A, Keller R, Cozzolino M, Duca S, Cauda F, Costa T (2024) Disentangling the role of gray matter volume and concentration in autism spectrum disorder: a meta-analytic investigation of 25 years of voxel-based morphometry research. Neurosci Biobehav Rev 164:105791
Lim CK, Fernandez-Gomez FJ, Braidy N, Estrada C, Costa C, Costa S, Bessede A, Fernandez-Villalba E, Zinger A, Herrero MT (2017) Involvement of the kynurenine pathway in the pathogenesis of Parkinson’s disease. Prog Neurobiol 155:76–95
Lindvall O, Brundin P, Widner H, Rehncrona S, Gustavii B, Frackowiak R, Leenders KL, Sawle G, Rothwell JC, Marsden CD (1990) Grafts of fetal dopamine neurons survive and improve motor function in Parkinson’s disease. Science 247(4942):574–577
Löhle M, Reichmann H (2010) Clinical neuroprotection in Parkinson’s disease—still waiting for the breakthrough. J Neurol Sci 289(1–2):104–114
Löhle M, Reichmann H (2011) Controversies in neurology: why monoamine oxidase B inhibitors could be a good choice for the initial treatment of Parkinson’s disease. BMC Neurol 11(1):112
Lotankar S, Prabhavalkar KS, Bhatt LK (2017) Biomarkers for Parkinson’s Disease: recent Advancement. Neurosci Bull 33(5):585–597. https://doi.org/10.1007/s12264-017-0183-5
Lull ME, Block ML (2010) Microglial activation and chronic neurodegeneration. Neurotherapeutics 7(4):354–365
Lustikaiswi DK, Yuliani S, Annura R, Rahmadani E (2021) Tryptophan in banana peel (Musa Paradisiaca) as an anti-dementia alternative treatment: a narrative review. JKKI: Jurnal Kedokteran Dan Kesehatan Indonesia :175–181
Maass A, Reichmann H (2013) Sleep and non-motor symptoms in Parkinson’s disease. J Neural Transm 120:565–569
Madureira M, Connor-Robson N, Wade-Martins R (2020) LRRK2: autophagy and lysosomal activity. Front NeuroSci 14:536324
Majerova P, Olesova D, Golisova G, Buralova M, Michalicova A, Vegh J, Piestansky J, Bhide M, Hanes J, Kovac A (2022) Analog of kynurenic acid decreases tau pathology by modulating astrogliosis in rat model for tauopathy. Biomed Pharmacother 152:113257
Malpartida AB, Williamson M, Narendra DP, Wade-Martins R, Ryan BJ (2021) Mitochondrial dysfunction and mitophagy in Parkinson’s disease: from mechanism to therapy. Trends Biochem Sci 46(4):329–343
Martin I, Kim JW, Dawson VL, Dawson TM (2014) LRRK2 pathobiology in Parkinson’s disease. J Neurochem 131(5):554–565
Martos D, Tuka B, Tanaka M, Vécsei L, Telegdy G (2022) Memory enhancement with kynurenic acid and its mechanisms in neurotransmission. Biomedicines 10(4):849
Martos D, Lőrinczi B, Szatmári I, Vécsei L, Tanaka M (2024) The impact of C-3 side chain modifications on Kynurenic Acid: a behavioral analysis of its analogs in the Motor Domain. Int J Mol Sci 25(6):3394
Matias JN, Achete G, Campanari G, Guiguer ÉL, Araújo AC, Buglio DS, Barbalho SM (2021) A systematic review of the antidepressant effects of curcumin: beyond monoamines theory. Aust N Z J Psychiatry 55(5):451–462. https://doi.org/10.1177/0004867421998795
Mazzoni P, Shabbott B, Cortés JC (2012) Motor control abnormalities in Parkinson’s disease. Cold Spring Harbor perspectives in medicine 2 (6)
McDonald C, Gordon G, Hand A, Walker RW, Fisher JM (2018) 200 years of Parkinson’s disease: what have we learnt from James Parkinson? Age Ageing 47(2):209–214
McGeer P, Itagaki S, McGeer E (1988) Expression of the histocompatibility glycoprotein HLA-DR in neurological disease. Acta Neuropathol 76(6):550–557
McKenna MC, Dienel GA, Sonnewald U, Waagepetersen HS, Schousboe A (2012) Energy metabolism of the brain. Basic neurochemistry. Elsevier, pp 200–231
McKinnon C, Gros P, Lee DJ, Hamani C, Lozano AM, Kalia LV, Kalia SK (2019) Deep brain stimulation: potential for neuroprotection. Ann Clin Transl Neurol 6(1):174–185. https://doi.org/10.1002/acn3.682
Meder D, Herz DM, Rowe JB, Lehéricy S, Siebner HR (2019) The role of dopamine in the brain-lessons learned from Parkinson’s disease. NeuroImage 190:79–93
Mesika R, Reichmann D (2019) When safeguarding goes wrong: impact of oxidative stress on protein homeostasis in health and neurodegenerative disorders. Adv Protein Chem Struct Biol 114:221–264. https://doi.org/10.1016/bs.apcsb.2018.11.001
Minniti G, Laurindo LF, Machado NM, Duarte LG, Guiguer EL, Araujo AC, Dias JA, Lamas CB, Nunes YC, Bechara MD (2023) Mangifera indica L., By-Products, and Mangiferin on Cardio-Metabolic and Other Health conditions: a systematic review. Life 13(12):2270
Miquel-Rio L, Sarriés-Serrano U, Pavia-Collado R, Meana JJ, Bortolozzi A (2023) The role of α-Synuclein in the regulation of Serotonin System: physiological and pathological features. Biomedicines 11(2):541
Mithaiwala MN, Santana-Coelho D, Porter GA, O’connor JC (2021) Neuroinflammation and the kynurenine pathway in CNS disease: molecular mechanisms and therapeutic implications. Cells 10(6):1548
Mizuno Y (1990) Deficiencies in complex I subunits of the respiratory chain in Parkinson’s disease. Biochem Biophys Res Commun 169:1293–1298
Mizuno Y, Ohta S, Tanaka M, Takamiya S, Suzuki K, Sato T, Oya H, Ozawa T, Kagawa Y (1989) Deficiencies in complex I subunits of the respiratory chain in Parkinson’s disease. Biochem Biophys Res Commun 163(3):1450–1455
Mizuno Y, Hattori N, Kubo S-i, Sato S, Nishioka K, Hatano T, Tomiyama H, Funayama M, Machida Y, Mochizuki H (2008) Progress in the pathogenesis and genetics of Parkinson’s disease. Philosophical Trans Royal Soc B: Biol Sci 363(1500):2215–2227
Modoux M, Rolhion N, Mani S, Sokol H (2021) Tryptophan metabolism as a pharmacological target. Trends Pharmacol Sci 42(1):60–73
Moehle MS, Webber PJ, Tse T, Sukar N, Standaert DG, DeSilva TM, Cowell RM, West AB (2012) LRRK2 inhibition attenuates microglial inflammatory responses. J Neurosci 32(5):1602–1611
Mogi M, Harada M, Kiuchi K, Kojima K, Kondo T, Narabayashi H, Rausch D, Riederer P, Jellinger K, Nagatsu T (1988) Homospecific activity (activity per enzyme protein) of tyrosine hydroxylase increases in parkinsonian brain. J Neural Transm 72:77–82
Molina-Negro P, Hardy J (1975) Semiology of tremors. Can J Neurol Sci 2(1):23–29
Molnár K, Lőrinczi B, Fazakas C, Szatmári I, Fülöp F, Kmetykó N, Berkecz R, Ilisz I, Krizbai IA, Wilhelm I (2021) Szr-104, a novel kynurenic acid analogue with high permeability through the blood–brain barrier. Pharmaceutics 13(1):61
Molochnikov L, Rabey JM, Dobronevsky E, Bonuccelli U, Ceravolo R, Frosini D, Grünblatt E, Riederer P, Jacob C, Aharon-Peretz J (2012) A molecular signature in blood identifies early Parkinson’s disease. Mol Neurodegeneration 7:1–10
Mor A, Tankiewicz-Kwedlo A, Krupa A, Pawlak D (2021) Role of kynurenine pathway in oxidative stress during neurodegenerative disorders. Cells 10(7):1603
Moradi Vastegani S, Nasrolahi A, Ghaderi S, Belali R, Rashno M, Farzaneh M, Khoshnam SE (2023) Mitochondrial dysfunction and Parkinson’s disease: pathogenesis and therapeutic strategies. Neurochem Res 48(8):2285–2308
Moustafa AA, Chakravarthy S, Phillips JR, Gupta A, Keri S, Polner B, Frank MJ, Jahanshahi M (2016) Motor symptoms in Parkinson’s disease: a unified framework. Neurosci Biobehavioral Reviews 68:727–740
Müller T, Baas H, Kassubek J, Riederer P, Urban PP, Schrader C, Reichmann H, Woitalla D, Gerlach M (2016) Laboratory assessments in the course of Parkinson’s disease: a clinician’s perspective. J Neural Transm 123:65–71
Nagatsu T, Nakashima A (2020) Monoamine oxidase inhibitor (MAO-I)-mediated neuroprotection for treating Parkinson’s disease. NeuroPsychopharmacotherapy:1–21
Nagatsu T, Sawada M (2005) Inflammatory process in Parkinson’s disease: role for cytokines. Curr Pharm Design 11(8):999–1016
Nagatsu T, Sawada M (2006) Molecular mechanism of the relation of monoamine oxidase B and its inhibitors to Parkinson’s disease: possible implications of glial cells. Oxidative Stress Neuroprotection :53–65
Nagatsu T, Mogi M, Ichinose H, Togari A (2000) Changes in cytokines and neurotrophins in Parkinson’s disease. Advances in research on neurodegeneration:277–290
Nagatsu T, Nakashima A, Ichinose H, Kobayashi K (2019) Human tyrosine hydroxylase in Parkinson’s disease and in related disorders. J Neural Transm 126:397–409
Nagatsu T, Nakashima A, Watanabe H, Ito S, Wakamatsu K (2022) Neuromelanin in Parkinson’s disease: tyrosine hydroxylase and tyrosinase. Int J Mol Sci 23(8):4176
Nakashima A, Ota A, S Kaneko Y, Mori K, Nagasaki H, Nagatsu T (2013) A possible pathophysiological role of tyrosine hydroxylase in Parkinson’s disease suggested by postmortem brain biochemistry: a contribution for the special 70th birthday symposium in honor of Prof. Peter Riederer. J Neural Transm 120:49–54
Ning X-L, Li Y-Z, Huo C, Deng J, Gao C, Zhu K-R, Wang M, Wu Y-X, Yu J-L, Ren Y-L (2021) X-ray structure-guided discovery of a potent, orally bioavailable, dual human indoleamine/tryptophan 2, 3-dioxygenase (hIDO/hTDO) inhibitor that shows activity in a mouse model of Parkinson’s Disease. J Med Chem 64(12):8303–8332
Nishikito DF, Borges ACA, Laurindo LF, Otoboni AMB, Direito R, Goulart RA, Nicolau CC, Fiorini AM, Sinatora RV, Barbalho SM (2023) Anti-inflammatory, antioxidant, and other health effects of dragon fruit and potential delivery systems for its bioactive compounds. Pharmaceutics 15(1):159
Ntetsika T, Papathoma P-E, Markaki I (2021) Novel targeted therapies for Parkinson’s disease. Mol Med 27:1–20
Nunes YC, de Oliveira Santos G, Machado NM, Otoboni AM, Laurindo LF, Bishayee A, Fimognari C, Bishayee A, Barbalho SM (2023) Peanut (Arachis hypogaea L.) seeds and by-products in metabolic syndrome and cardiovascular disorders: a systematic review of clinical studies. Phytomedicine:155170
Oertel W, Schulz JB (2016) Current and experimental treatments of Parkinson disease: a guide for neuroscientists. J Neurochem 139:325–337
Ossig C, Reichmann H (2013) Treatment of Parkinson’s disease in the advanced stage. J Neural Transm 120:523–529
Ossig C, Reichmann H (2015a) Treatment strategies in early and advanced Parkinson disease. Neurol Clin 33(1):19–37. https://doi.org/10.1016/j.ncl.2014.09.009
Ossig C, Reichmann H (2015b) Treatment strategies in early and advanced Parkinson disease. Neurol Clin 33(1):19–37
Ossig C, Storch A, Reichmann H (2015) Neuropsychiatric Symptoms of Movement Disorders
Ostapiuk A, Urbanska EM (2022) Kynurenic acid in neurodegenerative disorders—unique neuroprotection or double-edged sword? CNS Neurosci Ther 28(1):19–35
Ovallath S, Deepa P (2013) The history of parkinsonism: descriptions in ancient Indian medical literature, vol 28. Wiley Online Library
Pagotto GLO, LMOd S, Osman N, Lamas CB, Laurindo LF, Pomini KT, Guissoni LM, Lima EPd VMS (2024) Ginkgo biloba: A Leaf of Hope in the Fight against Alzheimer’s Dementia: Clinical Trial Systematic Review. Antioxidants 13 (6):651
Pahwa R, Lyons KE (2010) Early diagnosis of Parkinson’s disease: recommendations from diagnostic clinical guidelines. Am J Manag Care 16(Suppl Implications):S94–99
Pajares M, Rojo I, Manda A, Boscá G, Cuadrado L A (2020) Inflammation in Parkinson’s disease: mechanisms and therapeutic implications. Cells 9(7):1687
Palasz E, Wysocka A, Gasiorowska A, Chalimoniuk M, Niewiadomski W, Niewiadomska G (2020) BDNF as a promising therapeutic agent in Parkinson’s disease. Int J Mol Sci 21(3):1170
Pallotta MT, Rossini S, Suvieri C, Coletti A, Orabona C, Macchiarulo A, Volpi C, Grohmann U (2022) Indoleamine 2, 3-dioxygenase 1 (IDO1): an up‐to‐date overview of an eclectic immunoregulatory enzyme. FEBS J 289(20):6099–6118
Pan-Montojo F, Schwarz M, Winkler C, Arnhold M, O’Sullivan GA, Pal A, Said J, Marsico G, Verbavatz J-M, Rodrigo-Angulo M (2012) Environmental toxins trigger PD-like progression via increased alpha-synuclein release from enteric neurons in mice. Sci Rep 2(1):898
Panagiotakopoulou V, Ivanyuk D, De Cicco S, Haq W, Arsić A, Yu C, Messelodi D, Oldrati M, Schöndorf DC, Perez M-J (2020) Interferon-γ signaling synergizes with LRRK2 in neurons and microglia derived from human induced pluripotent stem cells. Nat Commun 11(1):5163
Pang SY-Y, Ho PW-L, Liu H-F, Leung C-T, Li L, Chang EES, Ramsden DB, Ho S-L (2019) The interplay of aging, genetics and environmental factors in the pathogenesis of Parkinson’s disease. Translational Neurodegeneration 8:1–11
Parkinson J (1817) An essay on the shaking palsy: London: Whittingham and Rowland for Sherwood. Neely and jones
Parkinson J (2002) An essay on the shaking palsy. J Neuropsychiatry Clin Neurosci 14(2):223–236
Peng X, Zhao Z, Liu L, Bai L, Tong R, Yang H, Zhong L (2022) Targeting indoleamine dioxygenase and tryptophan dioxygenase in cancer immunotherapy: clinical progress and challenges. Drug Design, Development and Therapy, pp 2639–2657
Peng W, Schröder LF, Song P, Wong YC, Krainc D (2023) Parkin regulates amino acid homeostasis at mitochondria-lysosome (M/L) contact sites in Parkinson’s disease. Sci Adv 9(29):eadh3347
Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ (2002) A role for α-synuclein in the regulation of dopamine biosynthesis. J Neurosci 22(8):3090–3099
Perez-Pardo P, Grobben Y, Willemsen‐Seegers N, Hartog M, Tutone M, Muller M, Adolfs Y, Pasterkamp RJ, Vu‐Pham D, van Doornmalen AM (2021) Pharmacological validation of TDO as a target for Parkinson’s disease. FEBS J 288(14):4311–4331
Peters J (1991) Tryptophan nutrition and metabolism: an overview. Kynurenine and serotonin pathways: Progress in Tryptophan research:345–358
Phillips MC, Murtagh DK, Gilbertson LJ, Asztely FJ, Lynch CD (2018) Low-fat versus ketogenic diet in Parkinson’s disease: a pilot randomized controlled trial. Mov Disord 33(8):1306–1314
Phillips RS, Iradukunda EC, Hughes T, Bowen JP (2019) Modulation of enzyme activity in the kynurenine pathway by kynurenine monooxygenase inhibition. Front Mol Biosci 6:3
Poewe W (2008) Non-motor symptoms in Parkinson’s disease. Eur J Neurol 15:14–20
Poewe W, Mahlknecht P (2009) The clinical progression of Parkinson’s disease. Parkinsonism Relat Disord 15:S28–S32
Polanski W, Enzensperger C, Reichmann H, Gille G (2010) The exceptional properties of 9-methyl‐β‐carboline: stimulation, protection and regeneration of dopaminergic neurons coupled with anti‐inflammatory effects. J Neurochem 113(6):1659–1675
Polanski W, Reichmann H, Gille G (2011) Stimulation, protection and regeneration of dopaminergic neurons by 9-methyl-β-carboline: a new anti-parkinson drug? Expert Rev Neurother 11(6):845–860
Poles MZ, Nászai A, Gulácsi L, Czakó BL, Gál KG, Glenz RJ, Dookhun D, Rutai A, Tallósy SP, Szabó A (2021) Kynurenic acid and its synthetic derivatives protect against sepsis-associated neutrophil activation and brain mitochondrial dysfunction in rats. Front Immunol 12:717157
Polito L, Greco A, Seripa D (2016) Genetic profile, environmental exposure, and their interaction in Parkinson’s disease. Parkinson’s disease 2016
Polyák H, Galla Z, Nánási N, Cseh EK, Rajda C, Veres G, Spekker E, Szabó Á, Klivényi P, Tanaka M (2023) The tryptophan-kynurenine metabolic system is suppressed in cuprizone-induced model of demyelination simulating progressive multiple sclerosis. Biomedicines 11(3):945
Prendergast GC, Malachowski WJ, Mondal A, Scherle P, Muller AJ (2018) Indoleamine 2, 3-dioxygenase and its therapeutic inhibition in cancer. Int Rev cell Mol Biology 336:175–203
Radad K, Moldzio R, Krewenka C, Kranner B, Rausch W-D (2023) Pathophysiology of non-motor signs in parkinsons disease: some recent updating with brief presentation. Explor Neuroprotective Therapy 3(1):24–46
Ramírez Ortega D, Ugalde Muñiz PE, Blanco Ayala T, Vázquez Cervantes GI, Lugo Huitrón R, Pineda B, González Esquivel DF, Pérez, de la Cruz G, Pedraza Chaverrí J, Sánchez, Chapul L (2021) On the antioxidant properties of L-kynurenine: An efficient ROS scavenger and enhancer of rat brain antioxidant defense. Antioxidants 11 (1):31
Reichmann H (2010) Clinical criteria for the diagnosis of Parkinson’s disease. Neurodegenerative Dis 7(5):284–290
Reichmann H (2011) View point: etiology in Parkinson’s disease. Dual hit or spreading intoxication. J Neurol Sci 310(1–2):9–11
Reichmann H (2016) Modern treatment in Parkinson’s disease, a personal approach. J Neural Transm 123:73–80
Reichmann H (2017) Premotor diagnosis of Parkinson’s disease. Neurosci Bull 33:526–534
Reichmann H (2021) Neue Prodromale Symptome Bei Parkinson-Patienten identifiziert. InFo Neurologie + Psychiatrie 23(9):27–27
Reichmann H, Janetzky B (2000) Mitochondrial dysfunction–a pathogenetic factor in Parkinson’s disease. J Neurol 247:II63–II68
Reichmann H, Jost W (2023a) Parkinson’s disease: a never ending story. J Neural Transmission:1–2
Reichmann H, Jost W (2023b) Parkinson’s disease: a never ending story. J Neural Transm 130(6):735–736
Reichmann H, Riederer P (1989) Biochemical analyses of respiratory chain enzymes in different brain regions of patients with Parkinson’s disease. In: BMBF Symposium Morbus Parkinson und andere Basalganglienerkrankungen, Bad Kissingen, abstract, p 44
Reichmann H, Gold R, Meurers B, Naumann M, Seibel P, Walter U, Klopstock T (1993a) Progression of myopathology in Kearns-Sayre syndrome: a morphological follow-up study. Acta Neuropathol 85(6):679–681
Reichmann H, Lestienne P, Jellinger K, Riederer P (1993b) Parkinson’s disease and the electron transport chain in postmortem brain. Adv Neurol 60:297–299
Reichmann H, Schalke B, Seibel P, Naumann M, Toyka K (1995) Sarcoid myopathy and mitochondrial respiratory chain defects: clinicopathological, biochemical and molecular biological analyses. Neuromuscul Disord 5(4):277–283
Reichmann G, Schroeter M, Jander S, Fischer H-G (2002) Dendritic cells and dendritic-like microglia in focal cortical ischemia of the mouse brain. J Neuroimmunol 129(1–2):125–132
Reichmann H, Boas J, Macmahon D, Myllyla V, Hakala A, Reinikainen K, Group CS (2005a) Efficacy of combining levodopa with entacapone on quality of life and activities of daily living in patients experiencing wearing-off type fluctuations. Acta Neurol Scand 111(1):21–28
Reichmann H, Janetzky B, Riederer P (2005b) Dopamine and glutamate in Parkinson’s Disease: Biochemistry, clinical aspects, and treatment. Dopamine and glutamate in psychiatric disorders. Springer, pp 503–522
Reichmann H, Schneider C, Löhle M (2009) Non-motor features of Parkinson’s disease: depression and dementia. Parkinsonism Relat Disord 15:S87–S92
Reichmann H, Janetzky B, Riederer P (2012a) ADVANCES IN RESEARCH ON NEURODEGENERATION. II Etiopathogenesis 2:169
Reichmann H, Janetzky B, Riederer P (2012b) Mitochondrial disturbances in Parkinson’s. Etiopathogenesis 2:169
Reichmann H, Lees A, Rocha J-F, Magalhães D, Soares-da-Silva P (2020) Effectiveness and safety of opicapone in Parkinson’s disease patients with motor fluctuations: the OPTIPARK open-label study. Translational Neurodegeneration 9:1–9
Reichmann H, Csoti I, Koschel J, Lorenzl S, Schrader C, Winkler J, Wüllner U (2022) Life style and Parkinson’s disease. J Neural Transm 129(9):1235–1245
Riederer P, Reichmann H, Youdim MB, Gerlach M (2006) Parkinson’s disease and related disorders. Springer Science & Business Media
Riederer P, Berg D, Casadei N, Cheng F, Classen J, Dresel C, Jost W, Krüger R, Müller T, Reichmann H (2019) α-Synuclein in Parkinson’s disease: causal or bystander? J Neural Transm 126:815–840
Riederer P, Monoranu C, Strobel S, Iordache T, Sian-Hülsmann J (2021) Iron as the concert master in the pathogenic orchestra playing in sporadic Parkinson’s disease. J Neural Transm :1–22
Riklan M, Cooper I (1961) Rehabilitation and research in Parkinson’s disease. J Rehabilitation 27(2):22
Rodriguez-Oroz MC, Jahanshahi M, Krack P, Litvan I, Macias R, Bezard E, Obeso JA (2009) Initial clinical manifestations of Parkinson’s disease: features and pathophysiological mechanisms. Lancet Neurol 8(12):1128–1139
Rodríguez-Pallares J, García-Garrote M, Parga JA, Labandeira-García JL (2023) Combined cell-based therapy strategies for the treatment of Parkinson’s disease: focus on mesenchymal stromal cells. Neural Regeneration Res 18(3):478–484
Rodríguez-Violante M, Zerón-Martínez R, Cervantes-Arriaga A, Corona T (2017) Who can diagnose Parkinson’s disease first? Role of pre-motor symptoms. Arch Med Res 48(3):221–227
Roosen DA, Cookson MR (2016) LRRK2 at the interface of autophagosomes, endosomes and lysosomes. Mol Neurodegeneration 11:1–10
Rose FC, Brackenridge R (1881) Diseases of the nervous system. Brackenridge’s Medical Selection of Life Risks. Springer, pp 799–838
Salter M, Hazelwood R, Pogson CI, Iyer R, Madge DJ (1995) The effects of a novel and selective inhibitor of tryptophan 2, 3-dioxygenase on tryptophan and serotonin metabolism in the rat. Biochem Pharmacol 49(10):1435–1442
Sardi SP, Cedarbaum JM, Brundin P (2018) Targeted therapies for Parkinson’s disease: from genetics to the clinic. Mov Disord 33(5):684–696
Sari S, Tomek P, Leung E, Reynisson J (2019) Discovery and characterisation of dual inhibitors of tryptophan 2, 3-dioxygenase (TDO2) and indoleamine 2, 3-dioxygenase 1 (IDO1) using virtual screening. Molecules 24(23):4346
Sarkar S, Raymick J, Imam S (2016) Neuroprotective and Therapeutic Strategies against Parkinson’s Disease: Recent Perspectives. Int J Mol Sci 17(6). https://doi.org/10.3390/ijms17060904
Schapansky J, Nardozzi JD, LaVoie MJ (2015) The complex relationships between microglia, alpha-synuclein, and LRRK2 in Parkinson’s disease. Neuroscience 302:74–88
Schapira A, Cooper J, Dexter D, Jenner P, Clark J, Marsden C (1989) Mitochondrial complex I defi ciency in Parkinson’s disease
Schapira A, Agid Y, Barone P, Jenner P, Lemke M, Poewe W, Rascol O, Reichmann H, Tolosa E (2009) Perspectives on recent advances in the understanding and treatment of Parkinson’s disease. Eur J Neurol 16(10):1090–1099
Schiller F (1986) Parkinsonian rigidity: the first hundred-and-one years 1817–1918. History and Philosophy of the Life Sciences, pp 221–236
Schrempf W, Brandt MD, Storch A, Reichmann H (2014) Sleep disorders in Parkinson’s disease. J Parkinson’s Disease 4(2):211–221
Schulz JB, Gerlach M, Gille G, Kuhn W, Müngersdorf M, Riederer P, Südmeyer M, Ludolph A (2011) Basic science in Parkinson’s disease: its impact on clinical practice. J Neurol 258:299–306
Sellitto M, Terenzi D, Starita F, di Pellegrino G, Battaglia S (2022) The cost of imagined actions in a reward-valuation Task. Brain Sci 12(5):582
Shaafi S, Najmi S, Aliasgharpour H, Mahmoudi J, Sadigh-Etemad S, Farhoudi M, Baniasadi N (2016) The efficacy of the ketogenic diet on motor functions in Parkinson’s disease: a rat model. Iran J Neurol 15(2):63
Siegfried J, Rea G (1988) Deep brain stimulation for the treatment of motor disorders. Mod Stereotact Neurosurg :409–412
Silva ABRL, de Oliveira RWG, Diógenes GP, de Castro Aguiar MF, Sallem CC, Lima MPP, de Albuquerque Filho LB, de Medeiros SDP, de Mendonça LLP, de Santiago Filho PC (2023) Premotor, nonmotor and motor symptoms of Parkinson’s disease: a new clinical state of the art. Ageing Res Rev 84:101834
Simonet C, Schrag A, Lees A, Noyce A (2021) The motor prodromes of parkinson’s disease: from bedside observation to large-scale application. J Neurol 268:2099–2108
Singh K, Ngo A, Keerthisinghe OV, Patel KK, Liang C, Mukherjee J (2023) Synthesis and evaluation of compound targeting α7 and β2 subunits in Nicotinic Acetylcholinergic receptor. Molecules 28(24):8128
Skibinski G, Nakamura K, Cookson MR, Finkbeiner S (2014) Mutant LRRK2 toxicity in neurons depends on LRRK2 levels and synuclein but not kinase activity or inclusion bodies. J Neurosci 34(2):418–433
Song WM, Colonna M (2018) The identity and function of microglia in neurodegeneration. Nat Immunol 19(10):1048–1058
Sontag K, Heim C, Sontag T, God R, Reichmann H, Wesemann W, Rausch W, Riederer P, Bringmann G (1995) Long-term behavioural effects of TaClo (1-trichloromethyl-1, 2, 3, 4-tetrahydro-beta-carboline) after subchronic treatment in rats. J Neural Transmission Suppl 46:283–289
Stone TW (2000) Development and therapeutic potential of kynurenic acid and kynurenine derivatives for neuroprotection. Trends Pharmacol Sci 21(4):149–154
Storch A, Schneider CB, Wolz M, Stürwald Y, Nebe A, Odin P, Mahler A, Fuchs G, Jost WH, Chaudhuri KR (2013) Nonmotor fluctuations in Parkinson disease: severity and correlation with motor complications. Neurology 80(9):800–809
Szabo M, Lajkó N, Dulka K, Szatmári I, Fülöp F, Mihály A, Vécsei L, Gulya K (2022) Kynurenic acid and its analog SZR104 exhibit strong antiinflammatory effects and alter the intracellular distribution and methylation patterns of H3 histones in immunochallenged microglia-enriched cultures of newborn rat brains. Int J Mol Sci 23(3):1079
Szabo M, Lajkó N, Dulka K, Barczánfalvi G, Lőrinczi B, Szatmári I, Mihály A, Vécsei L, Gulya K (2023) The kynurenic acid analog SZR104 induces cytomorphological changes associated with the anti-inflammatory phenotype in cultured microglia. Sci Rep 13(1):11328
Szökő É, Tábi T, Riederer P, Vécsei L, Magyar K (2018) Pharmacological aspects of the neuroprotective effects of irreversible MAO-B inhibitors, selegiline and rasagiline, in Parkinson’s disease. J Neural Transm 125:1735–1749
Tábi T, Vécsei L, Youdim MB, Riederer P, Szökő É (2020) Selegiline: a molecule with innovative potential. J Neural Transm 127(5):831–842
Tajti J, Majlath Z, Szok D, Csati A, Toldi J, Fulop F, Vecsei L (2015) Novel kynurenic acid analogues in the treatment of migraine and neurodegenerative disorders: preclinical studies and pharmaceutical design. Curr Pharm Design 21(17):2250–2258
Tajti J, Szok D, Csáti A, Szabó Á, Tanaka M, Vécsei L (2023) Exploring novel therapeutic targets in the common pathogenic factors in migraine and neuropathic pain. Int J Mol Sci 24(4):4114
Tan EK, Skipper LM (2007) Pathogenic mutations in Parkinson disease. Hum Mutat 28(7):641–653
Tan Y-Q, Wang Y-N, Feng H-Y, Guo Z-Y, Li X, Nie X-L, Zhao Y-Y (2022) Host/microbiota interactions-derived tryptophan metabolites modulate oxidative stress and inflammation via aryl hydrocarbon receptor signaling. Free Radic Biol Med 184:30–41
Tanaka M, Chen C (2023) Towards a mechanistic understanding of depression, anxiety, and their comorbidity: perspectives from cognitive neuroscience. Front Behav Neurosci 17
Tanaka M, Vécsei L (2020) Monitoring the redox status in multiple sclerosis. Biomedicines 8(10):406
Tanaka M, Vécsei L (2021) Editorial of Special Issue Crosstalk between Depression, anxiety, and dementia: comorbidity in behavioral neurology and neuropsychiatry. Biomedicines 9(5). https://doi.org/10.3390/biomedicines9050517
Tanaka M, Vécsei L (2024) From lab to life: exploring cutting-Edge models for Neurological and Psychiatric disorders, vol 12. MDPI
Tanaka M, Bohár Z, Vécsei L (2020) Are kynurenines accomplices or principal villains in dementia? Maintenance of kynurenine metabolism. Molecules 25(3):564
Tanaka M, Török N, Tóth F, Szabó Á, Vécsei L (2021a) Co-players in chronic pain: neuroinflammation and the tryptophan-kynurenine metabolic pathway. Biomedicines 9(8):897
Tanaka M, Tóth F, Polyák H, Szabó Á, Mándi Y, Vécsei L (2021b) Immune influencers in action: metabolites and enzymes of the tryptophan-kynurenine metabolic pathway. Biomedicines 9(7):734
Tanaka M, Spekker E, Szabó Á, Polyák H, Vécsei L (2022a) Modelling the neurodevelopmental pathogenesis in neuropsychiatric disorders. Bioactive kynurenines and their analogues as neuroprotective agents-in celebration of 80th birthday of Professor Peter Riederer. J Neural Transm (Vienna) 129(5–6):627–642. https://doi.org/10.1007/s00702-022-02513-5
Tanaka M, Szabó Á, Spekker E, Polyák H, Tóth F, Vécsei L (2022b) Mitochondrial impairment: a common motif in neuropsychiatric presentation? The link to the tryptophan–kynurenine metabolic system. Cells 11(16):2607
Tanaka M, Szabó Á, Vécsei L (2022c) Integrating armchair, bench, and bedside research for behavioral neurology and neuropsychiatry, vol 10. MDPI
Tanaka M, Török N, Vécsei L (2022d) Novel pharmaceutical approaches in dementia. In: NeuroPsychopharmacotherapy. Springer, pp 2803–2820
Tanaka M, Diano M, Battaglia S (2023a) Insights into structural and functional organization of the brain: evidence from neuroimaging and non-invasive brain stimulation techniques. Front Psychiatry 14:1225755
Tanaka M, Szabó Á, Körtési T, Szok D, Tajti J, Vécsei L (2023b) From CGRP to PACAP, VIP, and Beyond: unraveling the Next chapters in Migraine Treatment. Cells 12(22):2649
Tanaka M, Szabó Á, Vécsei L (2023c) Preclinical modeling in depression and anxiety: current challenges and future research directions. Adv Clin Experimental Med 32(5):505–509
Tanaka M, Szabó Á, Vécsei L, Giménez-Llort L (2023d) Emerging translational research in neurological and psychiatric diseases: from in vitro to in vivo models, vol 24. MDPI
Tanaka M, Battaglia S, Giménez-Llort L, Chen C, Hepsomali P, Avenanti A, Vécsei L (2024a) Innovation at the intersection: Emerging Translational Research in Neurology and Psychiatry, vol 13. MDPI
Tanaka M, Tuka B, Vécsei L (2024b) Navigating the Neurobiology of Migraine: from pathways to potential therapies, vol 13. Multidisciplinary Digital Publishing Institute
Tang K, Wu Y-H, Song Y, Yu B (2021) Indoleamine 2, 3-dioxygenase 1 (IDO1) inhibitors in clinical trials for cancer immunotherapy. J Hematol Oncol 14:1–21
Thackray SJ, Mowat CG, Chapman SK (2008) Exploring the mechanism of tryptophan 2, 3-dioxygenase. Biochem Soc Trans 36(6):1120–1123
Török N, Tanaka M, Vécsei L (2020) Searching for peripheral biomarkers in neurodegenerative diseases: the tryptophan-kynurenine metabolic pathway. Int J Mol Sci 21(24):9338
Török N, Török R, Molnár K, Szolnoki Z, Somogyvári F, Boda K, Tanaka M, Klivényi P, Vécsei L (2022) Single nucleotide polymorphisms of indoleamine 2, 3-dioxygenase 1 influenced the age onset of Parkinson’s disease. Front Bioscience-Landmark 27 (9)
Tortora F, Hadipour AL, Battaglia S, Falzone A, Avenanti A, Vicario CM (2023) The role of serotonin in fear learning and memory: a systematic review of human studies. Brain Sci 13(8):1197
Triarhou LC (2013) Dopamine and Parkinson’s disease. Madame Curie Bioscience database [Internet]. Landes Bioscience
Tryphena KP, Nikhil US, Pinjala P, Srivastava S, Singh SB, Khatri DK (2023) Mitochondrial complex I as a pathologic and therapeutic target for Parkinson’s Disease. ACS Chem Neurosci. https://doi.org/10.1021/acschemneuro.2c00819
Tsuboi Y (2012) Environmental-genetic interactions in the pathogenesis of Parkinson’s disease. Experimental Neurobiol 21(3):123
Tutakhail A, Boulet L, Khabil S, Nazari QA, Hamid H, Coudoré F (2020) Neuropathology of kynurenine pathway of tryptophan metabolism. Curr Pharmacol Rep 6:8–23
Urbańska EM, Chmiel-Perzyńska I, Perzyński A, Derkacz M, Owe-Larsson B (2021) Endogenous kynurenic acid and neurotoxicity. Handb Neurotox :1–31
Vadivel V, Pugalenthi M (2008) Effect of various processing methods on the levels of antinutritional constituents and protein digestibility of Mucuna pruriens (L.) DC. Var. Utilis (Wall. Ex Wight) Baker ex Burck (velvet bean) seeds. J Food Biochem 32(6):795–812
Valotto Neto LJ, Reverete de Araujo M, Moretti Junior RC, Mendes Machado N, Joshi RK, dos Santos Buglio D, Barbalho Lamas C, Direito R, Fornari Laurindo L, Tanaka M (2024) Investigating the neuroprotective and cognitive-enhancing effects of Bacopa monnieri: a systematic review focused on inflammation, oxidative stress, mitochondrial dysfunction, and apoptosis. Antioxidants 13(4):393
Valvo V, Parietti E, Deans K, Ahn SW, Park NR, Ferland B, Thompson D, Dominas C, Bhagavatula SK, Davidson S (2022) High-throughput in situ perturbation of metabolite levels in the tumor micro-environment reveals favorable metabolic condition for increased fitness of infiltrated T-cells. Front Cell Dev Biology 10:1032360
Venkatesan D, Iyer M, Narayanasamy A, Siva K, Vellingiri B (2020) Kynurenine pathway in Parkinson’s disease—An update. Eneurologicalsci 21:100270
Verma S, Vashishth E, Singh R, Pant P, Padhi M (2014) A review on phytochemistry and pharmacological activity of parts of Mucuna Pruriens used as an ayurvedic medicine. World J Pharm Res 3(5):138–158
Wagner BA, Venkataraman S, Buettner GR (2011) The rate of oxygen utilization by cells. Free Radic Biol Med 51(3):700–712
Wang Q, Liu D, Song P, Zou M-H (2015) Deregulated tryptophan-kynurenine pathway is linked to inflammation, oxidative stress, and immune activation pathway in cardiovascular diseases. Front Bioscience (Landmark Edition) 20:1116
Weiner WJ, Singer C (1989) Parkinson’s disease and nonpharmacologic treatment programs. J Am Geriatr Soc 37(4):359–363
Weng M, Xie X, Liu C, Lim K-L, Zhang C-w, Li L (2018) The sources of reactive oxygen species and its possible role in the pathogenesis of Parkinson’s disease. Parkinson’s disease 2018
Wernicke C, Hellmann J, Zięba B, Kuter K, Ossowska K, Frenzel M, Dencher NA, Rommelspacher H (2010) 9-Methyl-β-carboline has restorative effects in an animal model of Parkinson’s disease. Pharmacol Rep 62(1):35–53
Włodarek D (2019) Role of ketogenic diets in neurodegenerative diseases (Alzheimer’s disease and Parkinson’s disease). Nutrients 11(1):169
Wolfson LI, Leenders KL, Brown LL, Jones T (1985) Alterations of regional cerebral blood flow and oxygen metabolism in Parkinson’s disease. Neurology 35(10):1399–1399
Wu T, Hallett M, Chan P (2015) Motor automaticity in Parkinson’s disease. Neurobiol Dis 82:226–234
Xu Y, Jin M-Z, Yang Z-Y, Jin W-L (2021) Microglia in neurodegenerative diseases. Neural Regeneration Res 16(2):270–280
Yakhine-Diop SM, Bravo-San Pedro JM, Gómez-Sánchez R, Pizarro-Estrella E, Rodríguez-Arribas M, Climent V, Aiastui A, de Munain AL, Fuentes JM, González-Polo RA (2014) G2019S LRRK2 mutant fibroblasts from Parkinson’s disease patients show increased sensitivity to neurotoxin 1-methyl-4-phenylpyridinium dependent of autophagy. Toxicology 324:1–9
Yuan H, Zhang Z-W, Liang L-W, Shen Q, Wang X-D, Ren S-M, Ma H-J, Jiao S-J, Liu P (2010) Treatment strategies for Parkinson’s disease. Neurosci Bull 26(1):66
Zaiter J, Hibot A, Hafid A, Khouili M, Neves CM, Simoes MM, Neves MGP, Faustino MAF, Dagci T, Saso L (2021) Evaluation of the cellular protection by novel spiropyrazole compounds in dopaminergic cell death. Eur J Med Chem 213:113140
Zgaljardic DJ, Borod JC, Foldi NS, Mattis P (2003) A review of the cognitive and behavioral sequelae of Parkinson’s disease: relationship to frontostriatal circuitry. Cogn Behav Neurol 16(4):193–210
Zhang S, Sakuma M, Deora GS, Levy CW, Klausing A, Breda C, Read KD, Edlin CD, Ross BP, Wright Muelas M (2019) A brain-permeable inhibitor of the neurodegenerative disease target kynurenine 3-monooxygenase prevents accumulation of neurotoxic metabolites. Commun Biology 2(1):271
Zhang Y, Hu Z, Zhang J, Ren C, Wang Y (2022) Dual-target inhibitors of indoleamine 2, 3 dioxygenase 1 (Ido1): a promising direction in cancer immunotherapy. Eur J Med Chem 238:114524
Ziemssen T, Reichmann H (2007) Non-motor dysfunction in Parkinson’s disease. Parkinsonism Relat Disord 13(6):323–332
Acknowledgements
Figures are created by biorender.com.
Funding
This work was supported by the National Research, Development, and Innovation Office—NKFIH K138125, SZTE SZAOK-KKA No: 2022/5S729, and the HUN-REN Hungarian Research Network.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tanaka, M., Vécsei, L. Revolutionizing our understanding of Parkinson’s disease: Dr. Heinz Reichmann’s pioneering research and future research direction. J Neural Transm (2024). https://doi.org/10.1007/s00702-024-02812-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00702-024-02812-z