Abstract
Prior studies indicate more severe brainstem cholinergic deficits in Progressive Supranuclear Palsy (PSP) compared to Parkinson’s disease (PD), but the extent and topography of subcortical deficits remains poorly understood. The objective of this study is to investigate differential cholinergic systems changes in progressive supranuclear palsy (PSP, n = 8) versus Parkinson’s disease (PD, n = 107) and older controls (n = 19) using vesicular acetylcholine transporter [18F]-fluoroethoxybenzovesamicol (FEOBV) positron emission tomography (PET). A whole-brain voxel-based PET analysis using Statistical Parametric Mapping (SPM) software (SPM12) for inter-group comparisons using parametric [18F]-FEOBV DVR images. Voxel-based analyses showed lower FEOBV binding in the tectum, metathalamus, epithalamus, pulvinar, bilateral frontal opercula, anterior insulae, superior temporal pole, anterior cingulum, some striatal subregions, lower brainstem, and cerebellum in PSP versus PD (p < 0.05; false discovery rate—corrected). More severe and diffuse reductions were present in PSP vs controls. Higher frequency of midbrain cholinergic losses was seen in PSP compared to the PD participants using 5th percentile normative cut-off values (χ2 = 4.12, p < 0.05). When compared to PD, these findings suggested disease-specific cholinergic vulnerability in the tectum, striatal cholinergic interneurons, and projections from the pedunculopontine nucleus, medial vestibular nucleus, and the cholinergic forebrain in PSP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
PSP is an atypical Parkinsonian disorder characterized by more severe episodic (falls gait freezing) and non-episodic [postural instability and gait disorder (PIGD)] axial motor features experienced earlier in the disease than PD. Characteristic neuropathologic changes in PSP include cytoplasmic 4R-tau protein deposits, astrocytic changes and neuronal loss in frontal lobes, globus pallidus, subthalamic nucleus, substantia nigra, and superior colliculi (Litvan et al. 1996; Dickson 1999; Kovacs 2015; Dickson et al. 2007). Superior cerebellar peduncles and dentate nuclei often exhibit axonal and neuronal losses (Dickson et al. 2007).
Post-mortem studies indicate multiple cholinergic systems’ deficits in PSP (Hirsch et al. 1987; Ruberg et al. 1985; Suzuki et al. 2002; Kasashima and Oda 2003; Juncos et al. 1991; Whitehouse et al. 1988). Biochemical, vesicular acetylcholine transporter (VAChT) ligand autoradiographic and immunohistochemical studies of cholinergic systems’ markers indicate significant deficits in the basal forebrain corticopetal cholinergic (BFCC), PPD–LDT cholinergic projection systems, and of striatal cholinergic interneurons (SChIs). While the magnitudes of cholinergic marker deficits vary between studies, cholinergic marker deficits were reported for both nuclei containing cholinergic perikarya (BFCC, PPN–LDT, striatum) and regions receiving cholinergic afferents (cortex, thalamus, and brainstem regions). The magnitudes of cortical cholinergic marker deficits are generally reported as lower than those described in subcortical structures (Ruberg et al. 1985; Whitehouse et al. 1988).
Post-mortem studies indicate that greater pedunculopontine nucleus (PPN) cholinergic neuron may explain the greater fall frequency and more severe postural instabilities in PSP compared to PD. Post-mortem study also show evidence of cholinergic vulnerability of nuclei in the midbrain, cholinergic forebrain, and hippocampus in PSP (Warren et al. 2005; Passamonti et al. 2017).
Furthermore, in vivo acetycholinesterase (AChE) PET imaging studies suggested more severe subcortical, including cerebellar and brainstem, cholinergic deficits in PSP compared to PD (Shinotoh et al. 1999; Gilman et al. 2010; Hirano et al. 2010; Mazere et al. 2012). However, the precise topography and relative denervation levels of specific PSP cholinergic systems’ deficits remains unknown because of limitations of limited anatomic resolution of sampling of other subcortical regions in post-mortem studies and because of lower quality of previously used AChE PET and VAChT SPECT radiotracers in prior in vivo imaging studies (Bohnen et al. 2018, 2019, 2021a). In particular, the use of AChE PET limits accurate assessment of high binding subcortical regions, such as the striatum and cerebellum (Koeppe et al. 1999). Therefore, the precise extent and topography of subcortical cholinergic deficits in PSP and how this differs from PD remains poorly understood. Comparison of PSP to a PD group is of high clinical relevance to identify PSP-specific regional cholinergic vulnerability that may inform potential targets for future therapeutic interventions.
The goal of this exploratory study was to expand our understanding of the topography of differential cholinergic systems changes in PSP compared to PD and neurologically intact older adults using the spatially resolute vesicular acetylcholine transporter (VAChT) PET ligand [18F]-fluoroethoxybenzovesamicol (FEOBV). We hypothesized cholinergic deficits in PSP across the whole spectrum of brainstem, cerebellum, thalamus, basal ganglia, and cortical neural circuitry but with the most significant reductions in the tectum and projection targets of pedunculopontine–laterodorsal tegmental (PPN–PDT) cholinergic neurons.
Materials and methods
Study design and participants
This cross-sectional study involved 8 PSP subjects (4 males; 4 females; mean age 70.63 ± 4.75 [SD] years; range 63–76), 107 PD subjects (83 males; 24 females, mean age 68.05 ± 7.65 [SD] years; range 51–93), and 19 neurologically intact adults (8 males and 11 females; mean age 67.80 ± 7.81 years; range 51–82). PSP subjects were defined by the Movement Disorder Society clinical diagnostic criteria for PSP (MDS-PSP) criteria (Höglinger et al. 2017): 4 subjects meeting the symptoms for probable PSP Richardson syndrome (PSP-RS); 2 subjects with probable PSP predominant Parkinsonian criteria (PSP-P); 1 subject with probable PSP corticobasal syndrome criteria (PSP-CBS); 1 subject with probable PSP progressive gait freezing (PSP-PGF). Details on clinical characteristic of participants with PSP are presented in Table 1. PD subjects met the UK Parkinson’s Disease Society Brain Bank clinical diagnostic criteria (Hughes et al. 1992). Subjects completed the Montreal Cognitive Assessment with a mean score of 21.75 ± 5.99 for PSP subjects and 26.16 ± 3.26 for PD subjects (Nasreddine et al. 2005), where 20 of them had a score below 26. The mean duration of disease was 6.5 ± 4.31 years for PSP subjects and 6.02 ± 4.41 years for PD subjects. All 8 PSP subjects experienced recurrent falls and no recovery on retropulsion testing. Five PSP subjects experienced gait freezing. The mean MDS-UPDRSIII scores in the medication 'off' state were 50.75 ± 6.14 for PSP subjects and 36.14 ± 13.41 for the PD subjects (Goetz et al. 2007). PD subjects had mean stage of 2.6 ± 0.74 severity of disease according to modified Hoehn and Yahr classification (Stage 1: 6; Stage 1.5: 3; Stage 2: 22; Stage 2.5: 43; Stage 3: 27, Stage 4: 6), PSP subjects had a mean stage of 4.12 ± 0.64 (Stage 3: 1; Stage 4: 4; Stage 5: 3). The normal control subjects had no history of neurologic or psychiatric disease, no history of medications that might affect their dopaminergic or cholinergic neurotransmission and had normal neurological examination at the time of this study. Recruitment of the PSP participants was based on a convenience sample. Inclusion of PD and NC participants was based on existing subject databases in our center.
This study (ClinicalTrials.gov Identifier: NCT02458430 & NCT01754168) was approved by the Institutional Review Boards of the University and VAMC and completed in compliance with the Declaration of Helsinki guidelines. Written informed consent was obtained from all subjects.
Imaging
MRI was performed on a 3 Tesla Philips Achieva system (Philips, Best, The Netherlands) as previously described (Bohnen et al. 2021b). PET imaging was performed in 3D imaging mode with a Siemens ECAT Exact HR + tomograph (18 controls and 49 PD) or Biograph 6 TruPoint PET/CT scanner (Siemens Molecular Imaging, Inc., Knoxville, TN) (8 PSP, 1 control and 58 PD) with inter-camera harmonization using voxel-by-voxel scaling based on “ratio images”, as previously described (Kanel et al. 2022; Thiele et al. 2013). We constructed TruePoint images with a 3 mm filters to match the resolution and to account for the differences between the scanners. [18F]-FEOBV delayed dynamic imaging was performed over 30 min (in six 5-min frames) starting at 3 h after intravenous bolus dose injections of 8 mCi [18F]-FEOBV (Petrou et al. 2014). Distribution volume ratios (DVR) were calculated using white matter reference tissue (Nejad-Davarani et al. 2018; Aghourian et al. 2017). We normalize the average image from the six delayed-image frames using the reference region to create a parametric image that reflects the distribution volume ratios like those previously reported (Bohnen et al. 2021b; Kanel et al. 2020). The parametric PET images were registered with the structural MR images that went through high-dimensional DARTEL registration and spatial normalization to a template in Montreal Neurological Institute (MNI) space. We used the Müller–Gartner method (Muller-Gartner et al. 1992) for the parametric PET images to correct for partial volume effects before standardizing into MNI space. We applied a smoothing of 8 mm full width at half maximum = 8 mm to remove the random noise.
Whole-brain voxel-based [18F]-FEOBV PET analyses were conducted using Statistical Parametric Mapping (SPM) software (SPM12; Wellcome Trust Centre for Neuroimaging, University College, London, England [https://www.fil.ion.ucl.ac.uk/spm/software/spm12/]) for inter-group comparisons using parametric [18F] -FEOBV DVR images. The false discovery rate (FDR) to correct for multiple testing effects was employed. As a post hoc analysis, we performed a volume-of-interest (VOI) group analysis using Freesurfer (http://surfer.nmr.mgh.harvard.edu) Mindboggle-101 atlas. Group comparison t tests were performed to determine the percentage differences of group means for the VOIs in python using the statsmodels module.
A 2 × 2 contingency table of abnormally low midbrain cholinergic nerve terminal by group (PD or PSP) was constructed based on the lower cutoff of midbrain cholinergic nerve terminal density using 5th percentile of normative FEOBV DVR data in 19 age- and gender-matched controls. Age and gender matching was achieved by dropping male PD participants on the extreme ends of combined PSP and NC participant age distribution. All PD participants that showed abnormally low midbrain FEOBV DVR were retained, and only those without abnormally low midbrain FEOBV DVR were subject to exclusion for matching purposes. Chi-square test of independence was applied to the contingency table to determine whether there was an association between abnormally low midbrain cholinergic nerve terminal densities in PSP relative to PD.
We calculated the Magnetic Resonance Parkinsonism Index (MRPI) 2.0 scores using T1-weighted structural images of our PSP participants as previously described (Quattrone et al. 2018).
Results
Clinical and MRPI information of the PSP participants
The table lists clinical information and MRPI 2.0 scores for each of the (variant) PSP patients. Using the MRPI 2.0 cutoff of 2.18—the optimal cut-off score for the PSP-P subtype—7 out of 8 participants in our group meet this criterion, but use a cut-off of 2.5—the optimal cut-off score for the PSP-RS subtype—6 out of 8 participants met the cut-off scores.
PSP vs. PD voxel-based group comparison
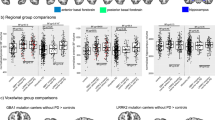
Significantly lower FEOBV bindings in the PSP subjects compared to the PD subjects were found in multiple cortical and subcortical regions (p < 0.05, FDR-corrected; Fig. 1). Neocortical regions with significant reductions in the PSP participants included bilateral frontal opercula and prefrontal cortex. Significant paralimbic cortex PSP group reductions were present in bilateral superior temporal poles, bilateral insula, left more than right parahippocampal gyrus, bilateral fusiform gyrus, bilateral orbitofrontal cortices, and anterior cingulum. Limbic structures with reductions found include left more than right nucleus accumbens (limbic striatum), left posterior hippocampus, and left more than right septal nuclei. Reductions were also seen in the caudate nucleus, putamen, and anterior external globus pallidus. Other subcortical regions with reduced FEOBV binding included the metathalamus (lateral and medial geniculate nucleus), pulvinar, and the epithalamus. Brainstem regions with reduced FEOBV binding included the superior colliculus, inferior colliculus and inferior olivary nuclei. Cerebellar FEOBV-binding reductions were also seen, including the bilateral tonsils, bilateral crus I, II and bilateral cerebellar lobules III, IV, V, VI, VIIB, VIII, & IX. Similar topography was seen in analysis corrected for MDS-UPDRSIII scores.
FEOBV PET-binding differences between the PSP versus the PD patients in MNI space. Relative to PD, voxel-based analyses showed lower FEOBV binding in the tectum, metathalamus, epithalamus, pulvinar, bilateral frontal opercula, anterior insulae, superior temporal pole, anterior cingulum, some striatal subregions, lower brainstem, and cerebellum in patients with PSP (p < 0.05; FDR-corrected)
PSP vs NC voxel-based group comparison
When compared to control adults, PSP subjects exhibited diffuse reductions in FEOBV binding in the cortical mantle, with the left hemisphere more decreased compared to the right hemisphere (p < 0.05, FDR-corrected; Fig. 2). Relatively more prominent reductions were found in the left more than right inferior temporal lobe. Relatively spared cortical regions included the right more than left superior frontal cortices and bilateral visual cortical association areas. Mid-cingulum FEOBV binding was preserved. Reduced FEOBV binding was found in the bilateral hippocampi and the left amygdala. Prominent FEOBV-binding reductions were present in the tectum, including the inferior and superior colliculi, bilateral metathalamus, epithalamus, and thalamus. Subcortical reductions greater in PSP participants were also seen in the caudate nuclei, bilateral anterior putamina, and left anterior external globus pallidus. Reduced FEOBV bindings were present in the inferior olivary nuclei and in the dorsal medulla oblongata in the PSP participants. Cerebellar hemispheric-binding reductions were also greater on the left compared to the right side, including crus I and crus VIIB in the PSP participants.
FEOBV PET-binding differences between the PSP patients and neurologically intact adults in MNI space. When compared to the PSP versus PD group comparison, more severe and more diffuse cortical and subcortical VAChT-binding reductions were present in the PSP patients compared to healthy control persons (p < 0.05, FDR-corrected). When compared to control adults, PSP subjects exhibited diffuse reductions in FEOBV binding in the cortical mantle, with the left hemisphere more decreased compared to the right hemisphere (p < 0.05, FDR-corrected; Fig. 2). Relatively more prominent reductions were found in the left more than right inferior temporal lobe. Relatively spared cortical regions included the right more than left superior frontal cortices and bilateral visual cortical association areas. Mid-cingulum FEOBV binding was preserved. Reduced FEOBV binding was found in the bilateral hippocampi and the left amygdala. Prominent FEOBV-binding reductions were present in the tectum, including the inferior and superior colliculi, bilateral metathalamus, epithalamus, and thalamus. Subcortical reductions greater in PSP participants were also seen in the caudate nuclei, bilateral anterior putamina, and left anterior external globus pallidus. Reduced FEOBV bindings were present in the inferior olivary nuclei and in the dorsal medulla oblongata in the PSP participants. Cerebellar hemispheric-binding reductions were also greater on the left compared to the right side, including crus I and crus VIIB in the PSP participants
Post hoc analysis 1: quantitative differences in the PSP versus PD VOI-based analyses
VOI-based group comparisons were carried out using bilaterally averaged Freesurfer VOIs that corresponded to regions of the voxel-based findings above. Subjects with PSP had lower FEOBV bindings in the bilateral thalamus-proper (12.06%), bilateral caudate (16.50%), bilateral putamen (13.83%), bilateral pars opercularis (8.34%), bilateral rostral anterior cingulate (11.15%), midbrain (12.34%), pons (17.93%), and medulla (19.20%) compared to PD (all p < 0.01).
Post hoc analysis 2: PSP versus PD midbrain VOI diagnostic comparison
Age and gender matching was confirmed by performing a one-way ANOVA and Chi-square test of independence, respectively, neither of which showed significant difference in age (F = 0.96, p = 0.38) or gender (χ2 = 3.59, p = 0.17) distribution across the three groups. After age and gender matching, 53 PD participants (male: 35, age: 68.86 ± 5.30) and 8 PSP participants (male: 4, age: 70.62 ± 4.75) were retained for this post hoc analysis. The FEOBV DVR lower cutoff based on 5th percentile of NC participants (male: 8, age: 69.89 ± 7.40) was set at 1.3033. Chi-square test of independence demonstrated a significant association between PSP group versus PD group for abnormally low midbrain FEOBV DVR (χ2 = 4.12, p = 0.042). In total, 50% of PSP participants and only 13.2% of PD participants demonstrated abnormally low midbrain cholinergic nerve terminal density using this cut-off level. In contrast, analysis based on the MRI atrophy measures (Table 1) failed to achieve statistical significance.
Post hoc analysis 3: effects of partial volume correction in the PSP vs. PD voxel-based comparison.
To assess the performance of PVC methods in our analysis, we performed a voxel-based analysis without PVC. In PVC-corrected analysis using SPM-based VBM, we found the number of voxels with statistically significant PSP vs PD differences within the cortical gray matter decreased (Fig. 3). This reduction in the cluster size might be due to PVC's ability to correct spill‐over or the tissue fraction effects within the analysis focusing only on the gray matter. Although the number of voxels in each cluster increased in the non-PVC method, the confidence in those voxels decreased (given by their t value). We believe that applying PVC may enable the discovery of effects specific for PSP that would not be considered statistically significant without PVC method when corrected for multiple comparison.
FEOBV PET-binding differences between the PSP versus the PD patients in MNI space using partial volume uncorrected parametric images. More severe and diffuse brainstem VAChT-binding reductions were present in the PSP patients compared to more localized tectum/superior colliculus deficits seen with PVC-corrected analysis (p < 0.05, FDR-corrected)
Post hoc analysis 4: PD vs NC voxel-based group comparison
Results of a voxel-based PD patient versus NC group comparison are shown in Fig. 4 (p < 0.05, FDR-corrected). Findings show relative preservation of VAChT binding in the brainstem in PD. Cortical VAChT-binding losses in PD predominantly affect posterior cortical regions, including the occipital and parietal and posterior superior temporal cortices. When compared to the PSP vs. PD comparison from Fig. 2, there is relative sparing of the prefrontal and inferior temporal cortices in PD contrast with prominent denervation findings seen in these regions with PSP. Unlike our findings seen in PSP, there is no apparent hemispheric asymmetry in PD-binding losses compared to NC. Striatal losses are relatively more prominent in PD compared to PSP.
FEOBV PET-binding differences between the PD patients and neurologically intact adults in MNI space using partial volume corrected parametric images. Relative to NC, voxel-based analyses showed lower FEOBV binding predominantly in the posterior cortical regions, including the occipital, parietal, and posterior superior temporal cortices in patients with PD (p < 0.05; FDR-corrected)
Discussion
Prior molecular imaging studies confirmed the presence of significant cholinergic systems’ deficits in living PSP subjects, also suggesting greater subcortical than cortical deficits. A [123I]-iodobenzovesamicol VAChT single-photon emission computerized tomography (SPECT) imaging study comparing PSP subjects (N = 10) to controls found non-significant differences in ligand binding in striatum, hippocampus, and most cortical regions (Mazere et al. 2012). Thalamic ligand binding was reduced by approximately 50% (Mazere et al. 2012). AChE PET studies also found evidence of greater cholinergic denervation of subcortical structures than cortex in PSP (Shinotoh et al. 1999; Gilman et al. 2010; Hirano et al. 2010). Shinotoh and colleagues compared regional AChE activity between PSP, PD, and control subjects, and found significant deficits in both disease groups but greater cortical deficits in PD (− 17%, significant) than in PSP (− 10%; not significant) subjects (Shinotoh et al. 1999). Thalamic AChE deficits, in contrast, were significantly greater in PSP (− 38%) than PD (− 13%) subjects. Gilman et al. found significant percentage reductions in AChE hydrolysis rates in several subcortical regions (striatum 41.3%; cerebellum 21.1%; thalamus 20%; midbrain 17.6% and pons 22.9%) in 4 PSP subjects compared to control subjects (Gilman et al. 2010). Hirano and colleagues described cortical AChE activity reductions (− 9.4%; significant) with more severe thalamic deficits (− 24%; significant) in 12 PSP subjects compared to controls (Hirano et al. 2010). Voxel-based analysis of this dataset suggested a regional topography of significant losses in the left thalamus, left peri-central cortex, insula, and temporal lobe with right hemisphere precentral losses (Hirano et al. 2010).
The limited resolution of VAChT SPECT and AChE PET precluded detailed evaluation of cholinergic deficit topography. Our in vivo VAChT PET study also found distinct topographies of cholinergic deficits in PSP and PD, with greater subcortical deficits in PSP and added considerable anatomic detail. Novel observations include severe losses in the tectum, in particular the superior colliculus, in the epithalamus, relatively more severe losses in the metathalamus (lateral and medial geniculate nuclei), pulvinar, superior temporal poles, parahippocampal gyrus, inferior olivary nuclei, several cerebellar regions (including the flocculonodular lobules and tonsils), anterior cingulum, and left septal nuclei. Similar as reported by Hirano et al., excepting symmetric inferior temporal and cuneus reductions, cortical reductions were more prominent in the left hemisphere (Hirano et al. 2010). We also found that single brain region analysis of midbrain VACHT binding may aid the distinction between PSP versus PD suggesting possible clinical utility of this ligand. However, analysis of the MRI atrophy measures failed to achieve statistical significance in this sample.
Our prior work described distinct cholinergic systems’ deficit patterns associated with episodic (falls and gait freezing) and non-episodic (PIGD) axial motor features of PD (Bohnen et al. 2021b, 2019). All PSP subjects studied had a history of falls and cholinergic deficits in the visual thalamus and caudate nucleus suggesting shared fall pathophysiology in PD and PSP (Bohnen et al. 2019). We discovered limited overlaps with the cholinergic deficit pattern associated with PD gait freezing (bilateral striatum, hippocampus, amygdala, and prefrontal cortex). Our PSP findings show significant overlaps with the cholinergic deficit patterns associated with non-episodic PIGD in PD (metathalamus and mesiotemporal lobe) (Bohnen et al. 2021b). An intriguing, distinctive, regional cholinergic deficit in our PSP subjects was reduced tectal (superior and inferior colliculus) FEOBV binding. The superior colliculus is important for initiation and execution of orienting movements toward salient objects in space (Myoga 2021; Sparks 1986). While saccade control is the best-characterized aspect of superior collicular function, tectal dysfunction could underlie multiple aspects of visuomotor integration deficits in PSP (Pretegiani et al. 2019).
While our results confirm greater deficits of PPN–LDT cholinergic projection systems than BFCC projection cholinergic systems in PSP compared to PD, our PSP to control comparisons shows that all major CNS cholinergic systems were affected in PSP. These include the BFCC and PPN–LDT cholinergic projection systems and SChIs. Cerebellar cholinergic deficits likely implicate cholinergic medial vestibular neurons innervating the cerebellum. The superior colliculus received cholinergic afferents from both PPN–LDT cholinergic neurons and cholinergic parabigeminal nucleus neurons, suggesting that both these neuron populations are affected in PSP.
Limitations of our study include the small sample size of the PSP group based on a convenience sample, the use of a single presynaptic cholinergic marker, and the lack of available neuropathological correlate available at the time of this study making this an exploratory study. Another limitation is that not all of our PSP participants were of the classical Richardson’s subtype but included variant PSP syndromes that are now recognized by the Movement Disorder Society-revised diagnostic criteria (Hoglinger et al. 2017). This resulted in a range of clinical PSP-related spectra at various levels of diagnostic sensitivity and specificity. We believe that using individualized cut-off scores for each subtype of PSP, as shown in (Quattrone et al. 2018), would yield better accuracy in recognizing PSP subtypes. A further limitation might be the use white matter as a reference region, since oligodendroglial tau pathology may be present in the white matter of the PSP patients. However, FEOBV binding is specific to the vesicular acetylcholine transporter and does not bind to tau. The more diffuse-binding differences between PSP and the normal control may possibly be influenced by a systemic factor. However, no global topographic-binding differences were seen between the PSP and PD groups. The presence of group differences’ localization to specific and limited topographic patterns confirms that the group differences cannot be explained by differences in non-specific white matter binding of the radioligand.
We conclude that there is disease-specific cholinergic vulnerability of the tectum, metathalamus, epithalamus, and paralimbic and frontal opercular regions with additional but more limited involvement of the striatum in PSP in comparison with PD. These cholinergic deficits may contribute to some of the prominent axial motor deficits of PSP.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request by qualified investigators.
Abbreviations
- AChE:
-
Acetylcholinesterase
- BFCC:
-
Basal forebrain cholinergic corticopetal
- FEOBV:
-
[18F]-fluoroethoxybenzovesamicol
- LGN:
-
Lateral geniculate nucleus
- MVN:
-
Medial vestibular nucleus
- MGN:
-
Medial geniculate nucleus
- PIGD:
-
Postural instability and gait difficulties
- PPN–LDT:
-
Pedunculopontine nucleus-laterodorsal tegmental complex
- SC:
-
Superior colliculus
- VAChT:
-
Vesicular acetylcholine transporter
References
Aghourian M, Legault-Denis C, Soucy JP, Rosa-Neto P, Gauthier S, Kostikov A, Gravel P, Bedard MA (2017) Quantification of brain cholinergic denervation in Alzheimer’s disease using PET imaging with [(18)F]-FEOBV. Mol Psychiatry 22(11):1531–1538. https://doi.org/10.1038/mp.2017.183
Bohnen NI, Kanel P, Muller M (2018) Molecular imaging of the cholinergic system in Parkinson’s disease. Int Rev Neurobiol 141:211–250. https://doi.org/10.1016/bs.irn.2018.07.027
Bohnen NI, Kanel P, Zhou Z, Koeppe RA, Frey KA, Dauer WT, Albin RL, Muller M (2019) Cholinergic system changes of falls and freezing of gait in Parkinson’s disease. Ann Neurol 85(4):538–549. https://doi.org/10.1002/ana.25430
Bohnen NI, Kanel P, Koeppe RA, Sanchez-Catasus CA, Frey KA, Scott P, Constantine GM, Albin RL, Muller M (2021a) Regional cerebral cholinergic nerve terminal integrity and cardinal motor features in Parkinson’s disease. Brain Commun 3(2):fcab109. https://doi.org/10.1093/braincomms/fcab109
Bohnen NI, Kanel P, Koeppe RA, Sanchez-Catasus CA, Frey KA, Scott P, Constantine GM, Albin RL, Muller MLTM (2021b) Regional cerebral cholinergic nerve terminal integrity and cardinal motor features in Parkinson’s disease. Brain Commun 3(2):fcab109. https://doi.org/10.1093/braincomms/fcab109
Dickson DW (1999) Neuropathologic differentiation of progressive supranuclear palsy and corticobasal degeneration. J Neurol 246(Suppl 2):6–15. https://doi.org/10.1007/bf03161076
Dickson DW, Rademakers R, Hutton ML (2007) Progressive supranuclear palsy: pathology and genetics. Brain Pathol 17(1):74–82. https://doi.org/10.1111/j.1750-3639.2007.00054.x
Gilman S, Koeppe RA, Nan B, Wang CN, Wang X, Junck L, Chervin RD, Consens F, Bhaumik A (2010) Cerebral cortical and subcortical cholinergic deficits in parkinsonian syndromes. Neurology 74(18):1416–1423
Goetz CG, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stebbins GT, Stern MB, Tilley BC, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, Lewitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, Van Hilten JJ, Lapelle N (2007) Movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): process, format, and clinimetric testing plan. Mov Disord 22:41–47
Hirano S, Shinotoh H, Shimada H, Aotsuka A, Tanaka N, Ota T, Sato K, Ito H, Kuwabara S, Fukushi K, Irie T, Suhara T (2010) Cholinergic imaging in corticobasal syndrome, progressive supranuclear palsy and frontotemporal dementia. Brain 133(Pt 7):2058–2068. https://doi.org/10.1093/brain/awq120
Hirsch EC, Graybiel AM, Duyckaerts C, Javoy-Agid F (1987) Neuronal loss in the pedunculopontine tegmental nucleus in Parkinson disease and in progressive supranuclear palsy. Proc Natl Acad Sci USA 84(16):5976–5980
Höglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, Mollenhauer B, Müller U, Nilsson C, Whitwell JL, Arzberger T, Englund E, Gelpi E, Giese A, Irwin DJ, Meissner WG, Pantelyat A, Rajput A, van Swieten JC, Troakes C, Antonini A, Bhatia KP, Bordelon Y, Compta Y, Corvol JC, Colosimo C, Dickson DW, Dodel R, Ferguson L, Grossman M, Kassubek J, Krismer F, Levin J, Lorenzl S, Morris HR, Nestor P, Oertel WH, Poewe W, Rabinovici G, Rowe JB, Schellenberg GD, Seppi K, van Eimeren T, Wenning GK, Boxer AL, Golbe LI, Litvan I (2017) Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov Disord 32(6):853–864. https://doi.org/10.1002/mds.26987
Hoglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, Mollenhauer B, Muller U, Nilsson C, Whitwell JL, Arzberger T, Englund E, Gelpi E, Giese A, Irwin DJ, Meissner WG, Pantelyat A, Rajput A, van Swieten JC, Troakes C, Antonini A, Bhatia KP, Bordelon Y, Compta Y, Corvol JC, Colosimo C, Dickson DW, Dodel R, Ferguson L, Grossman M, Kassubek J, Krismer F, Levin J, Lorenzl S, Morris HR, Nestor P, Oertel WH, Poewe W, Rabinovici G, Rowe JB, Schellenberg GD, Seppi K, van Eimeren T, Wenning GK, Boxer AL, Golbe LI, Litvan I, Movement Disorder Society-endorsed PSPSG (2017) Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov Disorders 32(6):853–864. https://doi.org/10.1002/mds.26987
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55(3):181–184
Juncos JL, Hirsch EC, Malessa S, Duyckaerts C, Hersh LB, Agid Y (1991) Mesencephalic cholinergic nuclei in progressive supranuclear palsy. Neurology 41(1):25–30. https://doi.org/10.1212/wnl.41.1.25
Kanel P, Müller M, van der Zee S, Sanchez-Catasus CA, Koeppe RA, Frey KA, Bohnen NI (2020) Topography of cholinergic changes in dementia with Lewy bodies and key neural network hubs. J Neuropsychiatry Clin Neurosci 32(4):370–375. https://doi.org/10.1176/appi.neuropsych.19070165
Kanel P, van der Zee S, Sanchez-Catasus CA, Koeppe RA, Scott PJH, van Laar T, Albin RL, Bohnen NI (2022) Cerebral topography of vesicular cholinergic transporter changes in neurologically intact adults: A [18F]FEOBV PET study. Aging Brain 2:100039. https://doi.org/10.1016/j.nbas.2022.100039
Kasashima S, Oda Y (2003) Cholinergic neuronal loss in the basal forebrain and mesopontine tegmentum of progressive supranuclear palsy and corticobasal degeneration. Acta Neuropathol 105(2):117–124. https://doi.org/10.1007/s00401-002-0621-x
Koeppe RA, Frey KA, Snyder SE, Meyer P, Kilbourn MR, Kuhl DE (1999) Kinetic modeling of N-[11C]methylpiperidin-4-yl propionate: alternatives for analysis of an irreversible positron emission tomography trace for measurement of acetylcholinesterase activity in human brain. J Cerebral Blood Flow Metabol 19(10):1150–1163. https://doi.org/10.1097/00004647-199910000-00012
Kovacs GG (2015) Invited review: Neuropathology of tauopathies: principles and practice. Neuropathol Appl Neurobiol 41(1):3–23. https://doi.org/10.1111/nan.12208
Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, Goetz CG, Golbe LI, Grafman J, Growdon JH, Hallett M, Jankovic J, Quinn NP, Tolosa E, Zee DS (1996) Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 47(1):1–9. https://doi.org/10.1212/wnl.47.1.1
Mazere J, Meissner WG, Mayo W, Sibon I, Lamare F, Guilloteau D, Tison F, Allard M (2012) Progressive supranuclear palsy: in vivo SPECT imaging of presynaptic vesicular acetylcholine transporter with [123I]-iodobenzovesamicol. Radiology 265(2):537–543. https://doi.org/10.1148/radiol.12112650
Muller-Gartner HW, Links JM, Prince JL, Bryan RN, McVeigh E, Leal JP, Davatzikos C, Frost JJ (1992) Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI-based correction for partial volume effects. J Cerebral Blood Flow Metabol 12(4):571–583. https://doi.org/10.1038/jcbfm.1992.81
Myoga M (2021) The superior colliculus: auditory inputs integrate with other senses to coordinate orientationg movements. In: The senses: a comprehensive assessment, vol 2. Elsevier Inc., New York, pp 601–622
Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53(4):695–699
Nejad-Davarani S, Koeppe RA, Albin RL, Frey KA, Muller M, Bohnen NI (2018) Quantification of brain cholinergic denervation in dementia with Lewy bodies using PET imaging with [(18)F]-FEOBV. Mol Psychiatry. https://doi.org/10.1038/s41380-018-0130-5
Passamonti L, Vazquez Rodriguez P, Hong YT, Allinson KS, Williamson D, Borchert RJ, Sami S, Cope TE, Bevan-Jones WR, Jones PS, Arnold R, Surendranathan A, Mak E, Su L, Fryer TD, Aigbirhio FI, O’Brien JT, Rowe JB (2017) 18F-AV-1451 positron emission tomography in Alzheimer’s disease and progressive supranuclear palsy. Brain 140(3):781–791. https://doi.org/10.1093/brain/aww340
Petrou M, Frey KA, Kilbourn MR, Scott PJ, Raffel DM, Bohnen NI, Muller ML, Albin RL, Koeppe RA (2014) In vivo imaging of human cholinergic nerve terminals with (-)-5-18F-fluoroethoxybenzovesamicol: biodistribution, dosimetry, and tracer kinetic analyses. J Nucl Med 55(3):396–404. https://doi.org/10.2967/jnumed.113.124792
Pretegiani E, Vanegas-Arroyave N, FitzGibbon EJ, Hallett M, Optican LM (2019) Evidence from Parkinson’s disease that the superior colliculus couples action and perception. Mov Disord 34(11):1680–1689. https://doi.org/10.1002/mds.27861
Quattrone A, Morelli M, Nigro S, Quattrone A, Vescio B, Arabia G, Nicoletti G, Nistico R, Salsone M, Novellino F, Barbagallo G, Le Piane E, Pugliese P, Bosco D, Vaccaro MG, Chiriaco C, Sabatini U, Vescio V, Stana C, Rocca F, Gulla D, Caracciolo M (2018) A new MR imaging index for differentiation of progressive supranuclear palsy-parkinsonism from Parkinson’s disease. Parkinsonism Relat Disord 54:3–8. https://doi.org/10.1016/j.parkreldis.2018.07.016
Ruberg M, Javoy-Agid F, Hirsch E, Scatton B, Hauw JJ, Duyckaerts C, Gray F, Morel-Maroger A, Rascol A et al (1985) Dopaminergic and cholinergic lesions in progressive supranuclear palsy. Ann Neurol 18(5):523–529. https://doi.org/10.1002/ana.410180503
Shinotoh H, Namba H, Yamaguchi M, Fukushi K, Nagatsuka S, Iyo M, Asahina M, Hattori T, Tanada S, Irie T (1999) Positron emission tomographic measurement of acetylcholinesterase activity reveals differential loss of ascending cholinergic systems in Parkinson’s disease and progressive supranuclear palsy. Ann Neurol 46:62–69
Sparks DL (1986) Translation of sensory signals into commands for control of saccadic eye movements: role of primate superior colliculus. Physiol Rev 66(1):118–171. https://doi.org/10.1152/physrev.1986.66.1.118
Suzuki M, Desmond TJ, Albin RL, Frey KA (2002) Cholinergic vesicular transporters in progressive supranuclear palsy. Neurology 58(7):1013–1018
Thiele F, Young S, Buchert R, Wenzel F (2013) Voxel-based classification of FDG PET in dementia using inter-scanner normalization. Neuroimage 77:62–69. https://doi.org/10.1016/j.neuroimage.2013.03.031
Warren NM, Piggott MA, Perry EK, Burn DJ (2005) Cholinergic systems in progressive supranuclear palsy. Brain 128(Pt 2):239–249. https://doi.org/10.1093/brain/awh391
Whitehouse PJ, Martino AM, Marcus KA, Zweig RM, Singer HS, Price DL, Kellar KJ (1988) Reductions in acetylcholine and nicotine binding in several degenerative diseases. Arch Neurol 45(7):722–724. https://doi.org/10.1001/archneur.1988.00520310028012
Funding
This study was funded by National Institutes of Health (P01 NS015655, RO1 NS070856, P50 NS091856, and P50 NS123067), Department of Veterans Affairs grant (I01 RX001631), the Michael J. Fox Foundation, and the Parkinson’s Foundation. None of the funding agencies had a role in the design and conduct of the study, in the collection, management, analysis and interpretation of the data, in the preparation, review or approval of the manuscript, nor in the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or conflict of interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kanel, P., Spears, C.C., Roytman, S. et al. Differential cholinergic systems’ changes in progressive supranuclear palsy versus Parkinson’s disease: an exploratory analysis. J Neural Transm 129, 1469–1479 (2022). https://doi.org/10.1007/s00702-022-02547-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-022-02547-9