Abstract
Serum neurofilament light chain (NfL) and chitinase 3-like 1 (CHI3L1, also called YKL-40) concentrations are attractive candidate biomarkers for neurodegenerative disorders, which include amyotrophic lateral sclerosis (ALS) and parkinsonian disorders. We aimed to assess the diagnostic power of serum NfL and CHI3L1 concentrations with regard to the early diagnosis of ALS and Parkinson’s disease (PD). We studied 157 individuals, which included 41 healthy controls, 8 patients with ALS mimics, 18 patients initially diagnosed with ALS (ID-ALS), 32 patients late-diagnosed with ALS (LD-ALS), 29 patients with PD, 12 patients with PD mimics, and 17 patients initially diagnosed with atypical parkinsonian disorders (ID-APDs) at the initial stage of diagnosis. Electrochemiluminescence was used to measure the concentrations of serum NfL and CHI3L1, the diagnostic performance of which was assessed using the area under the receiver operating curves (AUCs). The AUCs of serum NfL were 0.90 for discriminating ALS mimics from LD-ALS at the initial stage of diagnosis and 0.89 for discriminating ALS mimics from ALS (LD/ID-ALS). The AUCs of serum NfL were 0.76 for discriminating PD from PD mimics at the initial stage of diagnosis, and 0.80 for discriminating PD from APD. No significant difference existed in serum CHI3L1 concentrations between individuals with suspected ALS or parkinsonism (p = 0.14, and p = 0.44, respectively). Serum NfL had excellent and almost good diagnostic performances for patients with ALS and PD, respectively, at the initial stage of diagnosis, whereas no significant difference existed in serum CHI3L1 between any groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurofilaments are structural proteins classified as intermediate filaments with an intermediate diameter (10 nm) between actin (6 nm) and myosin (15 nm), and solely expressed in neurons as the name indicates (Khalil et al. 2018). These proteins consist of several subunits, one of which is neurofilament light chain (NfL), an attractive candidate biomarker for neurodegenerative disorders. NfL is consistently released from axons into the extracellular space in an age-dependent manner. Axonal damage induces the increased release of NfL into the extracellular space (Gaetani et al. 2019). Thus, widespread neurodegeneration in amyotrophic lateral sclerosis (ALS) and atypical parkinsonian disorders (APD) such as multiple system atrophy (MSA) and progressive supranuclear palsy (PSP) causes axonal damage, which subsequently increases the levels of NfL in the cerebrospinal fluid (CSF).
Accumulating evidence has demonstrated that CSF NfL can be helpful for the differential diagnosis of ALS or APD (Sako et al. 2015; Gaetani et al. 2019), and that moreover, the diagnostic power of blood NfL is comparable to that of CSF NfL (Hansson et al. 2017; Feneberg et al. 2018; Marques et al. 2019; Lin et al. 2019; Verde et al. 2019). Owing to the invasiveness and contraindications of obtaining CSF NfL, the use of blood NfL is expected to be extended in the clinical setting. However, blood NfL per se cannot serve as a specific diagnostic tool because NfL is only a marker for axonal damage. Although diagnostic performance of blood NfL should be tested in a clinical setting for first medical examination at the initial stage of diagnosis, only one study evaluated the diagnostic performance of blood NfL for ALS in the aforementioned setting (Feneberg et al. 2018), in addition, no study for parkinsonian disorders.
Chitinase 3-like 1 (CHI3L1), also named as YKL-40, is the other attractive candidate biomarker. CHI3L1 is produced by a variety of cells, such as astrocytes, neutrophils and monocytes/macrophages, and is involved in the activation of macrophages/microglia, the migration of macrophages, and the initial innate immune responses (Rosa et al. 2016; Sanfilippo et al. 2017). CHI3L1 is overexpressed in the primary motor cortex of patients with ALS (Sanfilippo et al. 2017), and CSF CHI3L1 was reported as useful, to some extent, in the differential diagnosis of ALS and parkinsonian disorders (Olsson et al. 2013; Thompson et al. 2018; Gille et al. 2019). Furthermore, no significant difference was observed in blood CHI3L1 levels between patients with ALS and normal controls (Vu et al. 2020). However, these studies measured the concentration of CHI3L1 in the CSF using enzyme-linked immunosorbent assay or proteomics, which are unsuitable for detecting subtle differences in proteins in the blood between the groups.
Highly sensitive methods including electrochemiluminescence (ECL) and single molecule array (Simoa) in the blood CHI3L1 are expected to allow for a good diagnostic performance similar level to CSF like NfL.
We assessed the diagnostic power of serum NfL and CHI3L1 using samples from patients with suspected ALS or parkinsonian disorders at the initial stage of diagnosis based on the findings of neurological examination, magnetic resonance imaging, single photon emission computed tomography, nerve conduction study and electromyography, to confirm the clinical importance of these blood biomarkers in the process of diagnosis.
Methods
Participants
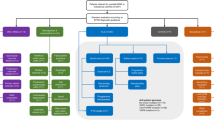
Figure 1 shows the flow diagram for the present study. A total of 331 independent subjects with available serum samples were identified. They had visited Tokushima University Hospital between July 2016 and August 2019, and were assessed for eligibility, based on the following criteria: (1) healthy controls, suspected of having ALS or parkinsonian disorders, and (2) an age of ≤ 80 years old. Among these, 157 subjects were deemed eligible for this study including 58 subjects with suspected ALS [40 men and 18 women, median age 68 years, interquartile range (IQR) 59.3–72.8], 58 subjects with parkinsonism (35 men and 23 women, median age 69 years, IQR 64–73), and 41 healthy controls (17 men and 24 women, median age 67 years, IQR 53–70). We used the International Consensus Criteria for the diagnosis of ALS (Costa et al. 2012), PD (Hughes et al. 1992), multiple system atrophy (MSA) (Gilman et al. 2008), progressive supranuclear palsy (PSP) (Hoglinger et al. 2017), and corticobasal syndrome (CBS) (Armstrong et al. 2013). All patients met the proposed criteria for “probable,” “probable laboratory-supported,” or “definite.” The control group included subjects without neurological deficits or psychiatric disorders.
A flowchart of the diagnostic process. ALS amyotrophic lateral sclerosis; APD atypical parkinsonian disorders; CBS corticobasal syndrome; Ctr healthy controls; MMN multifocal motor neuropathy; MSA-P multiple system atrophy with predominant parkinsonism; PD Parkinson’s disease; PLS primary lateral sclerosis; PSP-P progressive supranuclear palsy with predominant parkinsonism; PSP-RS progressive supranuclear palsy with Richardson’s syndrome
Of 58 patients with suspected ALS, 18 patients satisfied the criteria for ALS at the initial stage of diagnosis [initially diagnosed with ALS (ID-ALS)]. Among the remaining 40 patients, 32 patients fulfilled the criteria later [late-diagnosed with ALS (LD-ALS)], and 8 patients were diagnosed with other diseases (ALS mimics). The details of the ALS mimics were as follows: three cases of cervical spondylosis; one case of multifocal motor neuropathy; two cases of neuropathy of unknown cause with electrodiagnostic findings; one case of primary lateral sclerosis, and one case of probable PSP with Richardson’s syndrome (PSP-RS). We combined the ID-ALS group and the LD-ALS group into one group (LD/ID-ALS group), based on the final diagnosis.
Of 58 patients with parkinsonism, 17 patients were diagnosed with APD at the initial stage of diagnosis [initially diagnosed with APD (ID-APD)] including 12 cases of MSA with predominant parkinsonism and 5 cases of PSP-RS. Among the remaining 41 patients with suspected PD, 29 patients were diagnosed with PD (PD) and 12 patients received a diagnosis of APD later [PD mimics, late-diagnosed with APD (LD-APD)]. The PD mimics consisted of eight cases of PSP-RS, two cases of PSP with predominant parkinsonism, and two cases of CBS. We combined the PD mimics group and the ID-APD group into one group (PD mimics/ID-APD group), based on final diagnosis.
We evaluated age at blood sampling, sex, disease duration, follow-up period, Mini-Mental State Examination (MMSE) score, frontal assessment battery (FAB) score, and disease severity. Disease duration was the time interval from motor symptom onset to blood sampling. Disease severity in ALS and in parkinsonism were assessed using the revised ALS Functional Rating Scale (ALSFRS-R) (Cedarbaum et al. 1999) and the Hoehn–Yahr stage (HY), respectively.
Sample collection and measurement of serum concentration
Blood samples were collected between 9:00 am and 3:30 pm and centrifuged within 1 h of collection at 780 g for 10 min at 4 °C. Serum samples were aliquoted into microfuge tubes, and stored at − 80 °C until analysis. An ECL immunoassay was applied to measure the serum concentrations of NfL and CHI3L1 using the MESO QuickPlex SQ 120 (Meso Scale Discovery, Rockville, MD, USA). In this study, we used the R-PLEX Human Neurofilament L Antibody Set (Meso Scale Discovery, Rockville, MD, USA) for the analysis of NfL and the U-PLEX Human YKL-40 Assay (Meso Scale Discovery, Rockville, MD, USA) to measure the concentrations of CHI3L1 according to the manufacturer’s protocol. These kits included 96-well plates with a biotinylated capture antibody that binds to streptavidin on the plate surface. Serum samples were diluted two-fold for NfL and ten-fold for CHI3L1 with supplied assay diluent before analysis. The diluted samples were reacted with the biotinylated capture antibody and incubated for 1 h. After washing, the detection antibody conjugated with an electrochemiluminescent label (MSD GOLD SULFO-TAG) was added and incubated for 1 h. The MSD GOLD Read Buffer was added after final wash, and the MESO QuickPlex SQ 120 allowed for measurement of serum concentration of NfL and CHI3L1 by voltage-induced chemiluminescent emission. All analyses were conducted in duplicate, and the results were determined as the average. Values below the detection limit were set to zero.
Statistical analysis
The clinical characteristics and concentrations of NfL and CHI3L1 were compared between groups using the Kruskal–Wallis nonparametric analysis of variance, followed by a Dunn’s post hoc test corrected for multiple comparisons with Bonferroni adjustment for continuous variables, or Fisher’s exact test for categorical variables. Spearman correlation coefficient was assessed between serum concentration of NfL and CHI3L1 in the all subjects and each subgroup. Receiver operating characteristic (ROC) curves were generated and the area under the curve (AUC) was measured to assess the diagnostic accuracy. We used multivariate logistic analysis to combine concentration of NfL and CHI3L1. Diagnostic performances were compared on the basis of AUC using DeLong’s test. Findings were significant for a value of p < 0.05. All analyses were conducted with R software (version 3.5.2; http://www.r-project.org/).
Availability of supporting data
Anonymized data are available on request after ethical approval and permission from the responsible committee.
Results
Subject characteristics
No between-group differences existed in age at blood sampling, sex, follow-up period, or score on the ALSFRS-R, MMSE, or FAB. The median (IQR) disease durations were 15 (9–47.8) months in the ALS mimics group, 8 (5–12) months in the LD-ALS, and 14 (7.5–27) months in the ID-ALS group, 43 (24–72) months in the PD group, 29 (9–67) months in the PD mimics group, and 33 (13–50) months in the ID-APD group. Disease duration was significantly different between the groups (p < 0.001, Kruskal–Wallis test).
The median (IQR) HY scores were 2 (2–3) in the PD group, 3.5 (2–4) in the PD mimics group, and 4 (3–4) in the ID-APD group. The HY stage was significantly different between patients with parkinsonism (p < 0.001, Kruskal–Wallis test). The HY stages were higher in the PD mimics group (p < 0.01, Dunn’s test) and ID-APD group (p < 0.001, Dunn’s test) than in the PD group. Subject characteristics were summarized in Table 1.
Serum NfL and CHI3L1 concentrations
A significant difference in the serum NfL concentration existed between subjects with suspected ALS, including the control group (p < 0.001, Kruskal–Wallis test; Fig. 2A, left). Compared to the control and the ALS mimics groups, the LD-ALS group (p < 0.001 and p < 0.05, respectively, Dunn’s test) and the ID-ALS group (p < 0.001 and p < 0.05, respectively, Dunn’s test) had higher serum concentrations of NfL (Fig. 2A, left). A significant difference was observed in the serum NfL concentration between subjects with parkinsonism including the control group (p < 0.001, Kruskal–Wallis test; Fig. 2A, right). Post hoc Dunn’s test revealed significantly higher serum concentrations of NfL in the PD mimics group (p < 0.05 and p < 0.05, respectively, Dunn’s test) and ID-APD (p < 0.001 and p < 0.001, respectively, Dunn’s test) than in the control group or PD group (Fig. 2A, right).
Serum concentration of NfL and CHI3L1. A In the group with suspected ALS, the serum NfL concentration is increased in the LD-ALS and ID-ALS groups, compared to the control group or ALS mimics group. In the parkinsonism group, the serum NfL concentration is decreased in the PD group, compared to the PD mimics group and ID-APD group. B No significant difference in CHI3L1 exists between any groups. CHI3L1 chitinase 3-like 1; Ctr healthy control; ID-ALS initially diagnosed with amyotrophic lateral sclerosis; ID-APD initially diagnosed with atypical parkinsonian disorders; LD-ALS late-diagnosed with amyotrophic lateral sclerosis; LD-APD late-diagnosed with atypical parkinsonian disorders; NfL neurofilament light chain; NS not significant; PD Parkinson’s disease. The significant levels are *(p < 0.05), **(p < 0.01), and ***(p < 0.001)
No significant difference existed in the serum CHI3L1 concentrations between subjects with suspected ALS (p = 0.14, Kruskal–Wallis test; Fig. 2B, left) or parkinsonism (p = 0.44, Kruskal–Wallis test; Fig. 2B, right).
Serum concentration of NfL was correlated with that of CHI3L1 in parkinsonism (parkinsonism, rho = 0.46, p < 0.001; PD, rho = 0.48. p = 0.01; LD-APD, rho = 0.64, p = 0.03; ID-APD, rho = 0.53, p = 0.03; Figure S1G–J), whereas serum concentration of NfL was not correlated with that of CHI3L1 in other groups (all subjects, rho = 0.16, p = 0.04; Ctr, rho = 0.00, p = 0.99; suspected ALS, rho = 0.12, p = 0.37; ALS mimics, rho = 0.05. p = 0.93; LD-ALS, rho = 0.04, p = 0.84; ID-ALS, rho = 0.40, p = 0.10; Figure S1A–F).
Diagnostic accuracy of serum NfL and CHI3L1 concentration
Serum NfL had high AUC for differentiating ALS mimics from LD-ALS at the initial stage of diagnosis (AUC = 0.90, p < 0.001; Fig. 3A), as well as from ALS (LD/ID-ALS; AUC = 0.89, p < 0.001; Fig. 3B). The diagnostic accuracy of serum NfL for PD versus PD mimics was almost high at the initial stage of diagnosis (AUC = 0.76, p < 0.01; Fig. 3C), and the serum NfL was good for distinguishing PD from APD (AUC = 0.80, p < 0.001; Fig. 3D).
The ROC curve analysis results of serum NfL. A, B The AUCs are significant and approximately 0.9 for ALS mimics versus LD-ALS or ALS (LD/ID ALS). C, D The AUCs are significant and approximately 0.8 for PD versus PD mimics (LD-APD) or APD (PD mimics/ID-APD). AUCs area under the curves; ID-ALS initially diagnosed with amyotrophic lateral sclerosis; ID-APD initially diagnosed with atypical parkinsonian disorders; LD-ALS late-diagnosed with amyotrophic lateral sclerosis; LD-APD late-diagnosed with atypical parkinsonian disorders; NfL neurofilament light chain; PD Parkinson’s disease; ROC receiver operating characteristic
Serum CHI3L1 had a low AUC for differentiating ALS mimics from LD-ALS (AUC = 0.55, p = 0.33; Figure S2A) and ALS (LD/ID-ALS) (AUC = 0.50, p = 0.50; Figure S2B). Furthermore, CHI3L1 had a low AUC for differentiating PD from PD mimics (AUC = 0.64, p = 0.08; Figure S2C) and APD (PD mimics/ID-APD) (AUC = 0.59, p = 0.12, Figure S2D). These results reflected no significant differences in the serum concentrations of CHI3L1 between subjects with suspected ALS or parkinsonism.
The AUCs of the serum NfL and the combination of serum NfL and serum CHI3L1 were 0.90 and 0.89 for discriminating ALS mimics from LD-ALS at the initial stage of diagnosis (p = 0.48, Figure S3A). The AUCs of the serum NfL and the combination of NfL and CHI3L1 were 0.89 and 0.88 for discriminating ALS mimics from ALS (p = 0.57, Figure S3B). The AUCs of the serum NfL and the combination of NfL and CHI3L1 were 0.76 and 0.80 for discriminating PD from PD mimics at the initial stage of diagnosis (p = 0.36, Figure S3C). The AUCs of the serum NfL and combination of NfL and CHI3L1 were 0.80 and 0.83 for discriminating PD from APD (p = 0.39, Figure S3D).
Discussion
Our findings suggested that serum NfL can be helpful in the differential diagnosis of ALS and to some extent of parkinsonian disorders at the initial stage of diagnosis. By contrast, serum CHI3L1 did not show a difference between clinically mimicking and true ALS/PD.
In studies comparing of PD with MSA or PSP, the AUC of the ROC curve exceeded at least 0.8, albeit not mimicking PD (Hansson et al. 2017; Marques et al. 2019; Lin et al. 2019), which corresponded to our finding. Likewise, in a study comparing patients with ALS onset within 6 months and those with an ALS-mimicking disease, the AUC of the ROC curve was more than 0.9 (Feneberg et al. 2018), which agreed with our finding. In addition, the AUC exceeded 0.85 in another study that compared ALS with other disorders (Verde et al. 2019), and the diagnostic power of our study was not inferior to that of the previous studies. Serum NfL is increased at least 6 months before phenoconversion in inherited ALS (Benatar et al. 2018, 2019). Given these results, serum NfL allows for an early differential diagnosis between ALS and other mimicking disorders. However, to the best of our knowledge, no reports exist in which the diagnostic accuracy of serum NfL in patients with APD was tested at the initial stage of diagnosis, which was found to be almost good in this study. Serum NfL may help differentiate parkinsonian syndromes, even in the early stage of the diseases.
Plasma NfL has been associated with progression of cognitive impairment in PD (Lin et al. 2019). Patients with the postural instability and gait difficulty subtype have a higher concentration of plasma NfL than those with the tremor-dominant subtype (Ng et al. 2020). Cognitive impairment and the postural instability and gait difficulty subtype are recognized risk factors for mortality (Kempster et al. 2010; Forsaa et al. 2010), and thus, an increased level of blood NfL may increase mortality because blood NfL was associated with these two factors (i.e., cognitive impairment, postural instability and gait difficulty). An increased level of blood NfL is related to poor survival in patients with ALS (Lu et al. 2015). Patients with typical ALS or progressive bulbar palsy exhibit a higher level of CSF NfL than those with a frail arm, leg syndrome or progressive muscular atrophy in a subtype-dependent manner (Gaiani et al. 2017), and tend to have a poor prognosis. Collectively, blood NfL can allow us to predict the prognosis in PD and ALS, and the patients enrolled in this study will be followed up to determine the usefulness of serum NfL for predicting a prognosis.
The CSF CHI3L1 concentration has been reported as decreased or unchanged in patients with PD (Olsson et al. 2013; Hall et al. 2018; Magdalinou et al. 2014; Wennström et al. 2015), and increased in patients with MSA, PSP (Magdalinou et al. 2014; Alcolea et al. 2017; Hall et al. 2018), and ALS (Illán-Gala et al. 2018; Thompson et al. 2018; Gille et al. 2019; Gray et al. 2020; Vu et al. 2020); however, the blood CHI3L1 concentration was not significantly different between any groups in this study-even with ECL, which is a highly sensitive method. This result was in agreement with that of a previous study (Vu et al. 2020). Using difference in blood CHI3L1 to reflect pathology in the central nervous system may be difficult because various types of peripheral cells contribute to the serum concentration of CHI3L1 and decrease the signal-to-noise ratio (Rosa et al. 2016; Sanfilippo et al. 2017). Patients with ALS nevertheless tended to have a slightly high concentration of serum CHI3L1 compared to patients with ALS-mimic diseases. Further studies are required to determine the significance of the increased level of serum CHI3L1 in ALS.
Serum NfL was found to be significantly correlated with serum CHI3L1 not in ALS but in parkinsonian disorders, suggesting neuronal damage might be associated with innate immunity in parkinsonian disorders. Toll-like receptors, involved in innate immunity, were thought to play an important role in pro-inflammation in the presence of alpha-synuclein (Kouli et al. 2019). In contrast, recent study demonstrated that Toll-like receptor 4 deficiency facilitates alpha-synuclein propagation and neurodegeneration in a mouse model of prodromal PD (Venezia et al. 2021). Collectively, it remains controversial how innate immunity contributes to pathology of parkinsonian disorders. Further studies need to be performed to confirm our findings.
A major limitation of this study is the small number of subjects at the initial stage of diagnosis due to the single-center design. In addition, single-molecule assay (Simoa) allows to capture single protein molecules using antibody-coated beads, and has been accepted as an optimal method to measure concentration of NfL in the CSF compared to ECL (Alagaratnam et al. 2021). Simoa could improve sensitivity to detect NfL in serum and plasma (Kuhle et al. 2016; Rissin et al. 2010; Wilson et al. 2016). To the best of our knowledge, this is the first study to assess the discrimination ability of the serum NfL concentration for parkinsonism at the initial stage of diagnosis, and additional studies are needed to confirm our findings.
In conclusion, the serum NfL concentration had an excellent and an almost good diagnostic performance for patients with ALS and PD, respectively, at the initial stage of diagnosis, whereas no significant difference existed in serum CHI3L1 between any groups. These findings contribute to development of a diagnostic biomarker for ALS and parkinsonian disorders in the early stage of the diseases.
References
Alagaratnam J, von Widekind S, De Francesco D, Underwood J, Edison P, Winston A, Zetterberg H, Fidler S (2021) Correlation between CSF and blood neurofilament light chain protein: a systematic review and meta-analysis. BMJ Neurol Open 3(1):e000143. https://doi.org/10.1136/bmjno-2021-000143
Alcolea D, Vilaplana E, Suárez-Calvet M, Illán-Gala I, Blesa R, Clarimón J, Lladó A, Sánchez-Valle R, Molinuevo JL, García-Ribas G, Compta Y, Martí MJ, Piñol-Ripoll G, Amer-Ferrer G, Noguera A, García-Martín A, Fortea J, Lleó A (2017) CSF sAPPβ, YKL-40, and neurofilament light in frontotemporal lobar degeneration. Neurology 89(2):178–188. https://doi.org/10.1212/wnl.0000000000004088
Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, Boxer AL, Dickson DW, Grossman M, Hallett M, Josephs KA, Kertesz A, Lee SE, Miller BL, Reich SG, Riley DE, Tolosa E, Troster AI, Vidailhet M, Weiner WJ (2013) Criteria for the diagnosis of corticobasal degeneration. Neurology 80(5):496–503. https://doi.org/10.1212/WNL.0b013e31827f0fd1
Benatar M, Wuu J, Andersen PM, Lombardi V, Malaspina A (2018) Neurofilament light: a candidate biomarker of presymptomatic amyotrophic lateral sclerosis and phenoconversion. Ann Neurol 84(1):130–139. https://doi.org/10.1002/ana.25276
Benatar M, Wuu J, Lombardi V, Jeromin A, Bowser R, Andersen PM, Malaspina A (2019) Neurofilaments in pre-symptomatic ALS and the impact of genotype. Amyotroph Lateral Scler Front Degener 20(7–8):538–548. https://doi.org/10.1080/21678421.2019.1646769
Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, Nakanishi A (1999) The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci 169(1–2):13–21
Costa J, Swash M, de Carvalho M (2012) Awaji criteria for the diagnosis of amyotrophic lateral sclerosis:a systematic review. Arch Neurol 69(11):1410–1416. https://doi.org/10.1001/archneurol.2012.254
Di Rosa M, Distefano G, Zorena K, Malaguarnera L (2016) Chitinases and immunity: ancestral molecules with new functions. Immunobiology 221(3):399–411. https://doi.org/10.1016/j.imbio.2015.11.014
Feneberg E, Oeckl P, Steinacker P, Verde F, Barro C, Van Damme P, Gray E, Grosskreutz J, Jardel C, Kuhle J, Koerner S, Lamari F, Amador MDM, Mayer B, Morelli C, Muckova P, Petri S, Poesen K, Raaphorst J, Salachas F, Silani V, Stubendorff B, Turner MR, Verbeek MM, Weishaupt JH, Weydt P, Ludolph AC, Otto M (2018) Multicenter evaluation of neurofilaments in early symptom onset amyotrophic lateral sclerosis. Neurology 90(1):e22–e30. https://doi.org/10.1212/wnl.0000000000004761
Forsaa EB, Larsen JP, Wentzel-Larsen T, Alves G (2010) What predicts mortality in Parkinson disease?: a prospective population-based long-term study. Neurology 75(14):1270–1276. https://doi.org/10.1212/WNL.0b013e3181f61311
Gaetani L, Blennow K, Calabresi P, Di Filippo M, Parnetti L, Zetterberg H (2019) Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry 90(8):870–881. https://doi.org/10.1136/jnnp-2018-320106
Gaiani A, Martinelli I, Bello L, Querin G, Puthenparampil M, Ruggero S, Toffanin E, Cagnin A, Briani C, Pegoraro E, Sorarù G (2017) Diagnostic and prognostic biomarkers in amyotrophic lateral sclerosis: neurofilament light chain levels in definite subtypes of disease. JAMA Neurol 74(5):525–532. https://doi.org/10.1001/jamaneurol.2016.5398
Gille B, De Schaepdryver M, Dedeene L, Goossens J, Claeys KG, Van Den Bosch L, Tournoy J, Van Damme P, Poesen K (2019) Inflammatory markers in cerebrospinal fluid: independent prognostic biomarkers in amyotrophic lateral sclerosis? J Neurol Neurosurg Psychiatry 90(12):1338–1346. https://doi.org/10.1136/jnnp-2018-319586
Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Durr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K, Vidailhet M (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71(9):670–676. https://doi.org/10.1212/01.wnl.0000324625.00404.15
Gray E, Thompson AG, Wuu J, Pelt J, Talbot K, Benatar M, Turner MR (2020) CSF chitinases before and after symptom onset in amyotrophic lateral sclerosis. Ann Clin Transl Neurol. https://doi.org/10.1002/acn3.51114
Hall S, Janelidze S, Surova Y, Widner H, Zetterberg H, Hansson O (2018) Cerebrospinal fluid concentrations of inflammatory markers in Parkinson’s disease and atypical parkinsonian disorders. Sci Rep 8(1):13276. https://doi.org/10.1038/s41598-018-31517-z
Hansson O, Janelidze S, Hall S, Magdalinou N, Lees AJ, Andreasson U, Norgren N, Linder J, Forsgren L, Constantinescu R, Zetterberg H, Blennow K (2017) Blood-based NfL: a biomarker for differential diagnosis of parkinsonian disorder. Neurology 88(10):930–937. https://doi.org/10.1212/wnl.0000000000003680
Hoglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, Mollenhauer B, Muller U, Nilsson C, Whitwell JL, Arzberger T, Englund E, Gelpi E, Giese A, Irwin DJ, Meissner WG, Pantelyat A, Rajput A, van Swieten JC, Troakes C, Antonini A, Bhatia KP, Bordelon Y, Compta Y, Corvol JC, Colosimo C, Dickson DW, Dodel R, Ferguson L, Grossman M, Kassubek J, Krismer F, Levin J, Lorenzl S, Morris HR, Nestor P, Oertel WH, Poewe W, Rabinovici G, Rowe JB, Schellenberg GD, Seppi K, van Eimeren T, Wenning GK, Boxer AL, Golbe LI, Litvan I (2017) Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord 32(6):853–864. https://doi.org/10.1002/mds.26987
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55(3):181–184. https://doi.org/10.1136/jnnp.55.3.181
Illán-Gala I, Alcolea D, Montal V, Dols-Icardo O, Muñoz L, de Luna N, Turón-Sans J, Cortés-Vicente E, Sánchez-Saudinós MB, Subirana A, Sala I, Blesa R, Clarimón J, Fortea J, Rojas-García R, Lleó A (2018) CSF sAPPβ, YKL-40, and NfL along the ALS-FTD spectrum. Neurology 91(17):e1619–e1628. https://doi.org/10.1212/wnl.0000000000006383
Kempster PA, O’Sullivan SS, Holton JL, Revesz T, Lees AJ (2010) Relationships between age and late progression of Parkinson’s disease: a clinico-pathological study. Brain 133(Pt 6):1755–1762. https://doi.org/10.1093/brain/awq059
Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, Barro C, Kappos L, Comabella M, Fazekas F, Petzold A, Blennow K, Zetterberg H, Kuhle J (2018) Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol 14(10):577–589. https://doi.org/10.1038/s41582-018-0058-z
Kouli A, Horne CB, Williams-Gray CH (2019) Toll-like receptors and their therapeutic potential in Parkinson’s disease and α-synucleinopathies. Brain Behav Immun 81:41–51. https://doi.org/10.1016/j.bbi.2019.06.042
Kuhle J, Barro C, Andreasson U, Derfuss T, Lindberg R, Sandelius Å, Liman V, Norgren N, Blennow K, Zetterberg H (2016) Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med 54(10):1655–1661. https://doi.org/10.1515/cclm-2015-1195
Lin CH, Li CH, Yang KC, Lin FJ, Wu CC, Chieh JJ, Chiu MJ (2019) Blood NfL: a biomarker for disease severity and progression in Parkinson disease. Neurology 93(11):e1104–e1111. https://doi.org/10.1212/wnl.0000000000008088
Lu CH, Macdonald-Wallis C, Gray E, Pearce N, Petzold A, Norgren N, Giovannoni G, Fratta P, Sidle K, Fish M, Orrell R, Howard R, Talbot K, Greensmith L, Kuhle J, Turner MR, Malaspina A (2015) Neurofilament light chain: a prognostic biomarker in amyotrophic lateral sclerosis. Neurology 84(22):2247–2257. https://doi.org/10.1212/wnl.0000000000001642
Magdalinou N, Lees AJ, Zetterberg H (2014) Cerebrospinal fluid biomarkers in parkinsonian conditions: an update and future directions. J Neurol Neurosurg Psychiatry 85(10):1065–1075. https://doi.org/10.1136/jnnp-2013-307539
Marques TM, van Rumund A, Oeckl P, Kuiperij HB, Esselink RAJ, Bloem BR, Otto M, Verbeek MM (2019) Serum NFL discriminates Parkinson disease from atypical parkinsonisms. Neurology 92(13):e1479–e1486. https://doi.org/10.1212/wnl.0000000000007179
Ng ASL, Tan YJ, Yong ACW, Saffari SE, Lu Z, Ng EY, Ng SYE, Chia NSY, Choi X, Heng D, Neo S, Xu Z, Keong NCH, Tay KY, Au WL, Tan LCS, Tan EK (2020) Utility of plasma Neurofilament light as a diagnostic and prognostic biomarker of the postural instability gait disorder motor subtype in early Parkinson’s disease. Mol Neurodegener 15(1):33. https://doi.org/10.1186/s13024-020-00385-5
Olsson B, Constantinescu R, Holmberg B, Andreasen N, Blennow K, Zetterberg H (2013) The glial marker YKL-40 is decreased in synucleinopathies. Mov Disord 28(13):1882–1885. https://doi.org/10.1002/mds.25589
Rissin DM, Kan CW, Campbell TG, Howes SC, Fournier DR, Song L, Piech T, Patel PP, Chang L, Rivnak AJ, Ferrell EP, Randall JD, Provuncher GK, Walt DR, Duffy DC (2010) Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotechnol 28(6):595–599. https://doi.org/10.1038/nbt.1641
Sako W, Murakami N, Izumi Y, Kaji R (2015) Neurofilament light chain level in cerebrospinal fluid can differentiate Parkinson’s disease from atypical parkinsonism: evidence from a meta-analysis. J Neurol Sci 352(1–2):84–87. https://doi.org/10.1016/j.jns.2015.03.041
Sanfilippo C, Longo A, Lazzara F, Cambria D, Distefano G, Palumbo M, Cantarella A, Malaguarnera L, Di Rosa M (2017) CHI3L1 and CHI3L2 overexpression in motor cortex and spinal cord of sALS patients. Mol Cell Neurosci 85:162–169. https://doi.org/10.1016/j.mcn.2017.10.001
Thompson AG, Gray E, Thézénas ML, Charles PD, Evetts S, Hu MT, Talbot K, Fischer R, Kessler BM, Turner MR (2018) Cerebrospinal fluid macrophage biomarkers in amyotrophic lateral sclerosis. Ann Neurol 83(2):258–268. https://doi.org/10.1002/ana.25143
Venezia S, Kaufmann WA, Wenning GK, Stefanova N (2021) Toll-like receptor 4 deficiency facilitates α-synuclein propagation and neurodegeneration in a mouse model of prodromal Parkinson’s disease. Parkinsonism Relat Disord 91:59–65. https://doi.org/10.1016/j.parkreldis.2021.09.007
Verde F, Steinacker P, Weishaupt JH, Kassubek J, Oeckl P, Halbgebauer S, Tumani H, von Arnim CAF, Dorst J, Feneberg E, Mayer B, Müller HP, Gorges M, Rosenbohm A, Volk AE, Silani V, Ludolph AC, Otto M (2019) Neurofilament light chain in serum for the diagnosis of amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 90(2):157–164. https://doi.org/10.1136/jnnp-2018-318704
Vu L, An J, Kovalik T, Gendron T, Petrucelli L, Bowser R (2020) Cross-sectional and longitudinal measures of chitinase proteins in amyotrophic lateral sclerosis and expression of CHI3L1 in activated astrocytes. J Neurol Neurosurg Psychiatry 91(4):350–358. https://doi.org/10.1136/jnnp-2019-321916
Wennström M, Surova Y, Hall S, Nilsson C, Minthon L, Hansson O, Nielsen HM (2015) The inflammatory marker YKL-40 is elevated in cerebrospinal fluid from patients with Alzheimer’s but not Parkinson’s disease or Dementia with Lewy bodies. PLoS ONE 10(8):e0135458. https://doi.org/10.1371/journal.pone.0135458
Wilson DH, Rissin DM, Kan CW, Fournier DR, Piech T, Campbell TG, Meyer RE, Fishburn MW, Cabrera C, Patel PP, Frew E, Chen Y, Chang L, Ferrell EP, von Einem V, McGuigan W, Reinhardt M, Sayer H, Vielsack C, Duffy DC (2016) The Simoa HD-1 analyzer: a novel fully automated digital immunoassay analyzer with single-molecule sensitivity and multiplexing. J Lab Autom 21(4):533–547. https://doi.org/10.1177/2211068215589580
Acknowledgements
We would like to thank Dr. Takahiro Furukawa for contribution to establishment of dataset of the present study.
Funding
This study was supported by Japan Society for Promotion of Science (JSPS) KAKENHI Grant Number 20K12670, Japan Intractable Disease (Nanbyo) Research Foundation, and Grants-in Aid from the Research Committee of CNS Degenerative Diseases, Research on Policy Planning and Evaluation for Rare and Intractable Diseases, Health, Labour and Welfare Sciences Research Grants, the Ministry of Health, Labour and Welfare, Japan.
Author information
Authors and Affiliations
Contributions
WS conceived the idea for this research. WS, SH, and NM designed the experiments. WS, YO, and YI recruited the patients. NM measured concentrations of NfL and CHI3L1. SH analyzed the data. WS wrote the first draft of the manuscript, with important contributions from SH, NM, YO, and YI. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical approval
This study was approved by the ethics committee of Tokushima University Hospital (#2572).
Consent to participate
Written informed consent was obtained from all patients prior to participating in the study.
Consent for publication
All the authors of the manuscript have read the paper and agree to its submission.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
702_2022_2470_MOESM1_ESM.tif
Supplementary file 1 Figure S1. A–F NfL was not correlated with CHI3L1 in other groups (all subjects, rho = 0.16, p = 0.04; Ctr, rho = 0.00, p = 0.99; suspected ALS, rho = 0.12, p = 0.37; ALS mimics, rho = 0.05. p = 0.93; ID-ALS, rho = 0.40, p = 0.10; LD-ALS, rho = 0.04, p = 0.84). G–J NfL was correlated with CHI3L1 in parkinsonism (parkinsonism, rho = 0.46, p < 0.001; PD, rho = 0.48. p = 0.01; LD-APD, rho = 0.64, p = 0.03; ID-APD, rho = 0.53, p = 0.03). (TIF 329 KB)
702_2022_2470_MOESM2_ESM.tif
Supplementary file 2 Figure S2. The ROC curve analysis results of serum CHI3L1. A, B The AUCs are not significant for ALS mimics versus LD-ALS or ALS (LD/ID ALS). C, D The AUCs are not significant and around 0.6 for PD versus PD mimics (LD-APD) or APD (PD mimics/ID-APD). AUCs area under the curves; CHI3L1 chitinase 3-like 1; ID-ALS initially diagnosed with amyotrophic lateral sclerosis; ID-APD initially diagnosed with atypical parkinsonian disorders; LD-ALS late diagnosed with amyotrophic lateral sclerosis; LD-APD late diagnosed with atypical parkinsonian disorders; NfL neurofilament light chain; PD Parkinson’s disease; ROC, receiver operating characteristic. (TIF 789 KB)
702_2022_2470_MOESM3_ESM.tif
Supplementary file 3 Figure S3. The ROC curve analysis results of serum NfL and the combination of NfL and CHI3L1. Solid line indicates the ROC curve results of serum NfL, and dotted line indicates those of the combination of NfL and CHI3L1. A The AUCs of NfL and the combination of NfL and CHI3L1 were 0.90 and 0.89 for discriminating ALS mimics from LD-ALS at the initial stage of diagnosis (p = 0.48). B The AUCs of NfL only and the combination of NfL and CHI3L1 were 0.89 and 0.88 for discriminating ALS mimics from ALS (p = 0.57). C The AUCs of NfL only and the combination of NfL and CHI3L1 were 0.76 and 0.80 for discriminating PD from PD mimics at the initial stage of diagnosis (p = 0.36). D The AUCs of NfL only and combination of NfL and CHI3L1 were 0.80 and 0.83 for discriminating PD from APD (p = 0.39). The combination of NfL and CHI3L1 did not improve diagnostic performance in ALS and PD. (TIF 310 KB)
Rights and permissions
About this article
Cite this article
Haji, S., Sako, W., Murakami, N. et al. Serum NfL and CHI3L1 for ALS and parkinsonian disorders in the process of diagnosis. J Neural Transm 129, 301–309 (2022). https://doi.org/10.1007/s00702-022-02470-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-022-02470-z