Abstract
Depression in Parkinson’s Disorder (DPD) has been estimated to appear in up to 40% of people with PD and negatively impacts quality of life, motor and cognitive deficits and functional disability. Knowledge of the pathophysiology of DPD is unclear, DPD may be related to dysfunction in subcortical nuclei and the prefrontal cortex, striatal–thalamic–prefrontal and basotemporal limbic circuits, brainstem monoamine, and indolamine (i.e. dopamine, serotonin, and norepinephrine) systems. DPD is characterized by sadness, loss of interest, increased exhaustibility, feelings of helplessness, reduced drive, dysphoria, irritability, and pessimism about future. The diagnosis is complicated by overlap with PD symptoms, Detection of depression in PD should be made by psychometric depression scales. DPD is underrecognized and undertreated in clinical practice. Treatment mainly includes antidepressive medications and behavioral interventions as psychotherapy. Dopamine agonists showed some antidepressant effects, there are no sufficient numbers of RCTs. Important randomized clinical trials (RCTs) are summarized. SSRIs and SNRIs have a satisfying efficacy in DPD. TCAs are also good for improving depression. Side effects of different antidepressants (e.g. TCAs, SSRIs, SNRIs, bupropion, MAOIs) and potential interactions should be considered. In existing guidelines so far no statements, algorithms and recommendations are given for diagnosis and treatment of DPD. Methodologically adequate designed RCTs and comparative studies (NIS) which offer evidence-based results are urgently needed having the impact of DPD in mind.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction—epidemiology
Parkinson's disease (PD) is considered as the second most prevalent neurodegenerative disorder after Alzheimer disease (Feigin et al. 2019) consisting of multiple disease variants (Armstrong and Okun 2020). Age is the greatest risk factor of PD, so according to sociodemographic developments the incidence rate of PD increases. Many patients, over the course of their PD, experience neuropsychiatric disturbances, including depression, anxiety, sleep disturbances, psychosis, and behavioral and cognitive changes (Aarsland et al. 2009). For patients and families, these neuropsychiatric disturbances are often more problematic and distressing than the motor aspects of PD (Han et al. 2018).
The estimated prevalence of depression as a symptom of PD ranges from 7 to 76%, as a result of inconsistent sampling procedures, assessment techniques, and definitions of depression. PD depression (DPD) appears in up to 40% of people with PD, onset can be at any stage of the disease and premorbid (Aarsland et al. 2011; McDonald et al. 2003). An older systematic review found significant depressive symptoms in 35% (Reijnders et al. 2008). In a nationwide German sample of 1449 PD outpts with the Montgomery–Asberg Depression Rating Scale (MADRS) a depression prevalence was found in 25% (Riedel et al. 2010). Depressive disturbances are common in patients with PD and influence many other clinical aspects of the disease. In addition to causing inherent emotional distress, depressive disorders negatively impact quality of life, motor and cognitive deficits, functional disability, and other psychiatric comorbidities (Marsh 2013). Depression and anxiety are the strongest predictors of quality of life in PD (Barone et al. 2009). Before onset of PD motor symptoms premorbid depression is common (Ishihara and Brayne 2006).
There is evidence that depression is underrecognized and undertreated in clinical practice (Weintraub et al. 2003).
Pathophysiology—neurochemistry, neuropathology
PD is a heterogenous, multi-system, complex disorder, with both environmental and genetic factors converging on a common set of pathways including cell deaths, mitochondrial dysfunction, oxidative stress, protein aggregation, impaired autophagy, and neuroinflammation affecting numerous fundamental cellular processes (Kalia and Lang 2015; Simon et al. 2020).
The main pathological characteristics of PD are cell death in the brain's basal ganglia (affecting up to 70% of the dopamine-secreting neurons in the substantia nigra pars compacta). Alpha-synuclein becomes misfolded and clump together, becomes cytotoxic. Loss of neurons is accompanied by the death of astrocytes, neuroinflammation might be a significant and essential upstream contributor to alpha-synuclein aggregation and to the neurodegenerative process (Simon et al. 2020).
Epidemiological studies have provided evidence of associations between diseases with peripheral inflammation (e.g. type 2 diabetes and inflammatory bowel disease) and elevated PD risk and depression (Riederer et al. 2011).
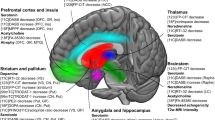
Knowledge of the pathophysiology of DPD is unclear (Ryan et al. 2019). DPD may be related to dysfunction in subcortical nuclei and the prefrontal cortex, striatal–thalamic–prefrontal and basotemporal limbic circuits, brainstem monoamine, and indolamine (i.e. dopamine, serotonin, and norepinephrine) systems (Richelson 2002). Beside degeneration of neurotransmitter systems in many cases psychosocial factors may play a role (Wolters and Braak 2006; Weintraub and Mamikonyan 2019).
Early neurobiological investigations suggest that DPD may mediated by dysfunction in mesocortical/prefrontal reward and dopaminergic and noradrenergic stress-response systems compared to nondepressed pts (Cummings 1992). In pre-clinical, pre- and early motor phases early dysfunction in neural circuity, the limbic loop of the basal ganglia and the lateral habenula has proposed to play a key role for depression (Borgonovo et al. 2017). The mesolimbic dopaminergic pathway and the complex network of interrelated systems, such as serotonergic, noradrenergic, and opioid, among others, have been suggested to contribute to the development of depressive symptomatology. Reduction of connectivity in cortical–subcortical limbic circuities and increased connectivity between specific limbic areas (amygdala, limbic thalamus, temporal cortex) have been identified in DPD. The imbalance and changes in dopamine, serotonin, and noradrenergic hormones are known to be a primary cause of depression in DPD. Post-mortem binding studies and in vivo imaging studies have suggested an involvement of the 5-HT system in DPD (Huot and Fox 2013).
SPECT and PET studies using tracers for dopamine system provided evidence of degeneration of the mesocorticolimbic dopaminergic projections to the ventral striatum, orbitofrontal cortex, anterior cingulate cortex, and thalamus in DPD. Dopamine transporter imaging with [123I]FP-CIT (DaTSCAN) in Parkinson's disease with depressive symptoms has been proposed as biological marker (Grachev et al. 2013).
Beside neurobiological factors psychological, psychodynamic, and personality factors are also involved in etiopathogenesis of DPD, elaborated studies are missing in this field, however.
Clinical features, diagnosis
Major depressive disorder (MDD) is a common psychiatric condition characterized by depressed mood, loss of interest, and energy combined with psychological and vegetative changes, such as sleep and/or appetite disturbances, fatigue, loss of motivation, feelings of guilt and despair, difficulties in maintaining mental focus, and suicidal thinking and behavior.
Depression in PD (DPD) is characterized by sadness, loss of interest, increased exhaustibility, feelings of helplessness/ hopelessness, reduced drive and energy, elevated degree of dysphoria, irritability and pessimism about future, with low levels of inadequacy, feelings of failures, and sense of guilt (Han et al. 2018). Off-dose depressions are characterized by dysphoria, irritability, and pessimism. Depressive disorders involve prominent somatic signs and complaints, cognitive changes, and vegetative symptoms that overlap with the features of PD itself. So, the diagnosis of depression in PD may be complicated by overlap of symptoms between the two conditions since they both include fatigue, loss of energy, psychomotor retardation, hypomimia, slowing of intellectual functions, difficulty in concentration, reduced appetite, and insomnia. All of these symptoms, especially cognitive decline, greatly increase disability. In a survey of 1072 Italian PD pts 98,6% reported nonmotor symptoms, most common fatigue, anxiety, insomnia, difficulties in maintaining concentration (Barone et al. 2009). Fatigue and apathy are the most common and most disabling nonmotor symptoms of PD (Skorvanek et al. 2015).
Overlapping clinical features of DPD and Parkinson’s disease are shown in Table 1.
Some studies indicate that self-blame, negative self-attitude, delusions, and suicidality are less common in DPD patients. Although suicide is reported as uncommon in PD, suicidal ideation in PD patients is higher than in the general population, but suicidal attempts themselves are lower than in patients with depression without PD. In one study, 28% of 116 outpatients had current death ideation, 11% suicide ideation, and 4% had a lifetime suicide attempt (Nazem et al. 2008).
Assessment of clinical psychopathology is complicated by antiparkinson medication effects on motor and mood states.
Regarding clinical classification organic affective disorder, major depression, dysthymia, minor or subsyndromal depression or reactive adjustment disorder (grief, demoralization to the diagnosis of PD—depressive disturbances as “understandable” emotional reactions) can be differentiated. The frequency of major depressions seems to be 5–20% (Weintraub and Mamikonyan 2019).
DPD is frequently associated with other psychopathologic symptoms like alexithymia, defined as the inability to mentalize and symbolize emotions. The prevalence of apathy, defined as reduced initiative/interests in new activities or the world around them, emotional indifference and reduced energy, ranges between 17 and 70% of PD patients. Anhedonia, defined as the inability to experience pleasure from activities usually found enjoyable, is a key symptom of depression and apathy in PD patients.
Anxiety has been estimated to have a prevalence in PD-affected people usually around 30–60%. Anxiety can often be found during "off" periods. PD pts suffer from panic attacks more frequently compared to the general population. Both anxiety and depression have been found to be associated with decreased quality of life.
In PD, isolated manic phenomena most often occur as the result of dopaminergic medications or neurosurgical treatments. Some patients manifest hypomanic features in the “on state” with increased irritability and goal-directed activities. Aside from fluctuating mood states, mania and hypomania occur similarly as in non-PD patients. The features include elevated mood and sense of self, irritability hyperactivity, increased goal-directed activity, including risky behaviors, and mood-congruent psychotic phenomena.
Psychometric scales
Detection of depression in PD can and should be made by psychometric depression scales. Self-report scales are Beck Depression Inventory (BDI-II), Geriatric Depression Scale (GDS), Inventory of Depressive Symptoms–Patient (IDS-SR). Clinician-rated scales are Hamilton Depression Rating Scale (HAMD-17) and Montgomery–Asberg Depression Rating Scale (MADRS). A comparison found valid screening when PD-specific cutoff scores are used and favoured GDS because of its brevity (Williams et al. 2012).
Therapy
Psychoeducation, supportive psychosocial interventions, relaxation techniques and exercises belong to the basics of depression treatment, there are only few studies on psychosocial treatments that specifically assess depression in DPD patients, however (Tursi et al. 2013; Yang et al. 2012).
Currently, treatment for depression of PD includes antidepressive medications, behavioral interventions as psychotherapy, electroconvulsive therapy, repetitive transcranial magnetic stimulation, and deep brain stimulation. DPD is frequently not diagnosed, many DPD pts are not treated.
Pharmacotherapy
Typically used antidepression medication includes tricyclic antidepressants (TCA), selective serotonin reuptake inhibitors (SSRI), serotonin and norepinephrine reuptake inhibitors (SNRI), monoamine-oxidase inhibitors (MAOI), and dopamine agonists (DA).
Dopamine agonists
Dopamine agonists (DA) have been explored as a primary treatment for DPD with mixed results. DA showed some antidepressant effects, there are no sufficient numbers of RCTs. They may cause side effects like dizziness, confusion or even hallucinations (Raskin and Durst 2010).
Leentjens (2011) concluded in a systematic review of ten studies (none RCT) inconclusive results and insufficient evidence.
An 8-month multicentre prospective randomized study investigated the effects of pramipexole as add-on to L-dopa in 41 non-demented patients (25 men, 16 women) suffering from both mild or moderate depression and advanced PD. A significant decrease in the average value of MADRS scores was present, the total daily dose of L-dopa decreased significantly (Rektorova et al. 2003). Despite small effect sizes, pramipexole showed efficacy for reducing depressive symptoms over 12 weeks. In the ACCORDO study, Barone and collaborators (2015) investigated the effects of rasagiline on depressive symptoms and cognition in non-demented PD patients displaying depressive symptoms. PD patients (N = 123) were randomized to either rasagiline or placebo. Treatment was carried out for 12 weeks, and the primary endpoint was the change of the BDI score. There was no significant difference in the primary efficacy variable neither at any cognitive test. Post hoc analyses signaled some improvement in patient-rated cognitive and depression outcomes.
Antidepressants
Studies investigating the tolerability, safety, and efficacy of antidepressant drugs in PD patients have been limited for long. A Cochrane Review based of three RCTs with 106 pts only concluded 2003 for insufficient data on the efficacy and safety of any antidepressant drug in PD (Shabnam et al. 2003).
Frequently cited is the placebo-controlled positive study with nortriptyline (Andersen et al. 1980), only N = 19 pts have been included, however. Small studies have been undertaken with reboxetine (N = 16) (Lemke et al. 2002) and bupropion. Raskin and Durst (2010) proposed bupropion due to its norepinephrine and dopamine profile without serotonin associated side effects reporting positive effects. Goetz et al. (1984) found in 20 PD patients depression alleviation in only 5 of 12 DPD patients, however. Controlled studies are missing so far. Possible dopamine induced psychosis (hallucinations) should be mentioned, additionally.
MAO-B inhibitors (safinamide, selegiline, and rasagiline) may reduce physical fatigue in PD. The reversible MAO-A inhibitor moclobemide (600 mg/d) improved depression in 10 pts, even more combined with selegiline (Steur Ballering 1997).
Today, all traditional antidepressants studied in DPD have been found to be safe and well tolerated; efficacy, relative to placebo, has been demonstrated for nortriptyline, venlafaxine extended release, desipramine, citalopram, and paroxetine. Most treatment trials last 8–12 weeks.
On the other hand, nine placebo-controlled RCTs investigating different pharmacological treatment for depression and anxiety in PD were cumulatively reviewed by Troeung et al. (2013). The pooled effect of antidepressants was moderate but non-significant (d = 0.71). When pooled across, citalopram, sertraline, desipramine, nortriptyline, paroxetine, and venlafaxine, the standard mean difference (SMD) for antidepressant vs. placebo was 0.69. Tricyclic antidepressant (TCAs), however, had a stronger effect. CBT showed in a single trial significant effects (d = 1.57). The poverty of controlled trials for both pharmacological and non-pharmacological treatment of depression and anxiety in PD did not allow clear conclusions.
Liu et al. 2013 found in a network meta-analysis comparing TCAs, SSRIs, SNRIs, pramipexole with placebo sufficient evidence of antidepressant efficacy for TCAs only, SSRIs last choice.
Rocha et al. (2013) reviewed all randomized controlled trials that studied the efficacy of antidepressants for depression in PD. Out of the 1438 studies retrieved, only 6 could be included. The overall risk ratio (RR) for response was 1.36, indicating no statistically significant superiority of antidepressants over placebo. No specific antidepressant class was superior to placebo. The results suggest antidepressants may be efficacious in the treatment of DPD. However, the results were unstable. In fact, the small number of trials and methodological drawbacks preclude definitive conclusions about their efficacy and tolerability in DPD. Mills et al. (2018) complained in a meta-analysis of 20 studies considerable heterogeneity in the definition of depression and data of only 5 studies regarding tolerability and adverse events. In an open study with 52 Japanese pts SSRIs (paroxetine, escitalopram) and the SNRI duloxetine were equal effective, similar dropout rates due to side effects (24 vs 26% and side effects (36% SSRIs mainly nausea, 37% duloxetine mainly tremor) were seen (Takahashi et al. 2019).
Depression onset is often before onset of PD, so preventive or early studies are necessary. In this aspect one meta-analysis reported, that in N = 2064 early PD pts TCAs delayed the need for dopaminergic therapy (Paumier et al. 2012).
Clinical studies
Important randomized clinical trials (RCTs) are summarized in Table 2.
Neuropsychiatric complications frequently lead to disability. In a cross-sectional study of 1449 PD outpatients care-dependency was present in 18, 3% of all pts. 18, 8% had depression, 14, and 3% depression and dementia. Regression analyses revealed increasing effects of age, PD duration and severity with strongest effects in pts with depression. Antidepressive treatment was associated with lower rates of care-dependency (Riedel et al. 2012).
Summarizing the following can be concluded
SSRIs have a satisfying efficacy in DPD and are first-line treatment, SNRIs have high efficacy as well, improvements in motor symptoms remain poor, however. TCAs are also good for improving depression. MAOI-B may be considered as therapeutic alternative, showing better performance in motor symptoms, DA as well (Han et al. 2018). According Parkinson's Disease Update on Non‐Motor Symptoms Study Group on behalf of the Movement Disorders Society Evidence‐Based Medicine Committee (Seppi et al. 2019) can be stated: Tricyclic Antidepressants (TCAs) “insufficient evidence”, venlafaxine “clinically useful”, “insufficient evidence” for paroxetine, citalopram, paroxetine, and sertraline “possibly useful”. SSRIs may worsen PD tremor in up to 5% of patients and occasionally worsen parkinsonism.
Recent overviews conclude that antidepressants and some dopamine agonists are safe and well-tolerated, efficacy results are rather uncertain or reveal partial efficacy, however. Side effects of different antidepressants (e.g. TCAs, SSRIs, SNRIs, bupropion, MAOIs) and potential interactions should be considered. Need for better studies is mentioned (Pena et al. 2016; Ryan et al. 2019; Assogna et al. 2020).
Finally it should be mentioned, that according evidence from clinical studies adherence rates are generally low in patients with depression. This is mainly because mood disorders impairing cognitive focus, energy, and motivation might affect the willingness and ability of patients to maintain the treatment. The occurrence of treatment-related side effects, such as weight gain, sexual dysfunction, nausea, headache, and sleep disturbances, is also common and may influence treatment adherence. So, psychoeducation is highly important for adherence and compliance of the pharmacotherapy.
Repetitive transcranial magnetic stimulation (rTMS)
In an early study, rTMS was as effective as fluoxetine in DPD patients (Fregni et al. 2004). A meta-analysis of 8 RCTs including 312 pts with rTMS vs sham or SSRIs found similar effects of rTMS and SSRIs, rTMS showed improvement on the unified Parkinson’s disease rating scale (UPDRS) and ADL. So, the authors concluded additional advantage regarding motor function (Xie et al. 2015). Due to sufficient evidence for rTMS practice implication according Seppi et al. 2019 is “possibly useful”.
Psychotherapy
Armento et al. (2012) reviewed 15 articles of empirical studies of CBT-based treatments for DPD. Results varied significantly, results were seen as encouraging. In a ten-session RCT of CBT for DPD that included some patients already taking antidepressants, symptom severity improved by 56% relative to 8% for the control group. CBT can be rated “likely efficacious” for the treatment of depression in PD, and the practice implication is “possibly useful”(Seppi et al. 2019).
Outlook
A recent Delphi survey of 37 experts in psychiatry, neurology, and geriatrics achieved consensus systematically screening for depression to be necessary ruling out apathy and anhedonia. Clinical scales like the GDS could help establishing the diagnosis of depression. Efficious and well-tolerated pharmacological options were SSRIs (sertraline), SNRIs (venlafaxine, duloxetine), bupropion, tianeptine, mirtazapine, vortioxetine, and dopamine agonists (pramipexole, rotigotine). TCAs and combining MAOI-B inhibitors with serotonergic drugs should not be prescribed. In severe cases ECT can be applied, CBT in some cases. The report has been characterized as management guidance (Aguera-Ortiz et al. 2021).
In existing guidelines so far no statements, algorithms and recommendations are given for diagnosis and treatment of DPD. Methodologically adequate designed RCTs and comparative studies (NIS) which offer evidence-based results are urgently needed having the impact of DPD in mind. Diagnosis of depression must be operationalized by rating scales, due to overlap of symptoms target symptoms of depression should be focused. Psychiatrists should elaborate if the depression could be classified as organic affective disorder, as major depression, as dysthymia, minor or subsyndromal depression or adjustment disorder (grief, demoralization to the diagnosis of PD) according ICD-10 or -11 due to implications for the therapy chosen. Regarding antidepressant medication plasma levels (therapeutic drug monitoring) should be measured and monitored as well as interactions with anti-Parkinson-medication. Clinical studies should last 3 to 6 months, longitudinal monitoring and adjustments in treatment are indicated until the depressive disorder completely remits. Depression onset is often before onset of PD, so preventive or early studies are necessary.
References
Aarsland D, Marsh L, Schrag A (2009) Neuropsychiatric symptoms in Parkinson’s disease. Mov Disord 24:2175–2186
Aarsland D, Pahlhagen S, Ballard CG et al (2011) Depression in Parkinson disease - epidemiology, mechanisms, and management. Nat Rev Neurol 8:35–47
Agüera-Ortiz L, García-Ramos R, Grandas Pérez FJ et al (2021) Focus on depression in Parkinson’s Disease: A Delphi consensus of experts in psychiatry, neurology, and geriatrics. Parkinsons Dis 2021:6621991. https://doi.org/10.1155/2021/6621991
Andersen J, Aabro E, Gulmann N et al (1980) Anti-depressive treatment in Parkinson’s disease A controlled trial of the effect of nortriptyline in patients with Parkinson’s disease treated with L-DOPA. Acta Neurol Scand 62:210–219
Antonini A, Tesei S, Zecchinelli A et al (2006) Randomized study of sertraline and low-dose amitriptyline in patients with Parkinson’s disease and depression: effect on quality of life. Mov Disord 21:1119–1122
Armento M, Stanley M, Marsh L et al (2012) Cognitive behavioral therapy (CBT) for depression and anxiety in Parkinson’s disease: a clinical review. J Park Dis 2:135–151
Armstrong MJ, Okun MS (2020) Diagnosis and treatment of Parkinson disease: a review. JAMA 323:548–560. https://doi.org/10.1001/jama.2019.22360
Assogna F, Pellicano C, Savini C et al (2020) Drug choices and advancements for managing depression in Parkinson’s disease. Curr Neuropharmacol 18:277–287. https://doi.org/10.2174/1570159X17666191016094857
Barone P, Scarzella L, Marconi R et al (2006) Pramipexole versus sertraline in the treatment of depression in Parkinson’s disease: a national multicenter parallel-group randomized study. J Neurol 253:601–607
Barone P, Antonini A, Colosimo C et al (2009) The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord 24:1641–1649. https://doi.org/10.1002/mds.22643
Barone P, Poewe W, Albrecht S et al (2010) Pramipexole for the treatment of depressive symptoms in patients with Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 9:573–580
Barone P, Santangelo G, Morgante L, Onofrj M et al (2015) A randomized clinical trial to evaluate the effects of rasagiline on depressive symptoms in non-demented Parkinson’s disease patients. Eur J Neurol 22:1184–1191
Borgonovo J, Allende-Castro C, Laliena A et al (2017) Changes in neural circuitry associated with depression at pre-clinical, pre-motor and early motor phases of Parkinson’s disease. Parkinsonism Relat Disord 35:17–24. https://doi.org/10.1016/j.parkreldis.2016.11.009
Cummings JL (1992) Depression and Parkinson’s disease: a review. Am J Psychiatry 149:443–454
Dobkin RD, Menza M, Allen LA et al (2011a) Cognitive-behavioral therapy for depression in Parkinson’s disease: a randomized, controlled trial. Am J Psychiatry 168:1066–1074
Dobkin RD, Menza M, Bienfait KL et al (2011b) Depression in Parkinson’s disease: symptom improvement and residual symptoms after acute pharmacologic management. Am J Geriatr Psychiatr 19:222–229
Feigin VL, Nichols E, Alam T et al (2019) Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18:459–480. https://doi.org/10.1016/S1474-4422(18)30499-X
Fregni F, Santos CM, Myczkowski ML et al (2004) Repetitive transcranial magnetic stimulation is as effective as fluoxetine in the treatment of depression in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 75:1171–1174
Goetz CG, Tanner CM, Klawans HL (1984) Bupropion in Parkinson’s disease. Neurology 34:1092–1094
Grachev ID (2013) Dopamine transporter imaging with [123I]FP-CIT (DaTSCAN) in Parkinson’s disease with depressive symptoms: a biological marker for causal relationships? J Neurol Neurosurg Psychiatry. https://doi.org/10.1136/jnnp-2013-305380
Han JW, Ahn YD, Kim WS, Shin CM et al (2018) Psychiatric Manifestation in Patients with Parkinson’s Disease. J Korean Med Science 33:e300. https://doi.org/10.3346/jkms.2018.33.e300
Huot P, Fox SH (2013) The serotonergic system in motor and non-motor manifestations of Parkinson’s disease. Exp Brain Res 230:463–476
Ishihara L, Brayne C (2006) A systematic review of depression and mental illness preceding Parkinson’s disease. Acta Neurol Scand 113:211–220
Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet 386:896–912. https://doi.org/10.1016/S0140-6736(14)61393-3
Leentjens AF (2011) The role of dopamine agonists in the treatment of depression in patients with Parkinson’s disease: a systematic review. Drugs 71:273–286
Lemke MR (2002) Effect of reboxetine on depression in Parkinson’s disease patients. J Clin Psychiatry 63:300–304
Liu J, Dong J, Wang L, Su Y, Yan P, Sun S (2013) Comparative efficacy and acceptability of antidepressants in Parkinson’s disease: a network meta-analysis. PLoS ONE 8:e76651. https://doi.org/10.1371/journal.pone.0076651
Marsh L (2013) Depression and Parkinson’s disease: Current knowledge. Curr Neurol Neurosci Rep 13:409. https://doi.org/10.1007/s11910-013-0409-5
McDonald WM, Richard ICH, DeLong MR (2003) Prevalence, etiology, and treatment of depression in Parkinson’s disease. Biol Psychiatry 54:363–375
Menza M, Dobkin RD, Marin H, Mark MH et al (2009) A controlled trial of antidepressants in patients with Parkinson disease and depression. Neurology 72:886–892
Mills KA, Greene MC, Dezube R, Goodson, et al (2018) Efficacy and tolerability of antidepressants in Parkinson’s disease: A systematic review and network meta-analysis. Int J Geriatr Psychiatry 33:642–651. https://doi.org/10.1002/gps.4834
Nazem S, Siderowf AD, Duda JE et al (2008) Suicidal and death ideation in Parkinson’s disease. Mov Disord 23:1573–1579
Paumier KL, Siderowf AD, Auinger P et al (2012) Tricyclic antidepressants delay the need for dopaminergic therapy in early Parkinson’s disease. Mov Disord 27:880–887
Peña E, Mata M, López-Manzanares L, Kurtis M et al (2016) Antidepressants in Parkinson’s disease Recommendations by the movement disorder study group of the Neurological Association of Madrid. Neurologia. https://doi.org/10.1016/j.nrl.2016.02.002
Raskin S, Durst R (2010) Bupropion as the treatment of choice in depression associated with Parkinson’s disease and it’s various treatments. Med Hypotheses 75:544–546. https://doi.org/10.1016/j.mehy.2010.07.024
Reijnders JS, Ehrt U, Weber WE et al (2008) A systematic review of prevalence studies of depression in Parkinson’s disease. Mov Disord 23:183–189
Rektorová I, Rektor I, Bares M et al (2003) Pramipexole and pergolide in the treatment of depression in Parkinson’s disease: a national multicentre prospective randomized study. Eur J Neurol 10:399–406
Richard IH, McDermott MP, Kurlan R et al (2012) A randomized, double-blind, placebo-controlled trial of antidepressants in Parkinson disease. Neurology 78:1229–1236
Richelson E (2002) The clinical relevance of antidepressant interaction with neurotransmitter transporters and receptors. Psychopharmacol Bull 36:133–150
Riedel O, Klotsche J, Spottke A et al (2010) Frequency of dementia, depression, and other neuropsychiatric symptoms in 1,449 outpatients with Parkinson’s disease. J Neurol 257:1073–1082
Riedel O, Dodel R, Deuschl G et al (2012) Depression and car-dependency in Parkinson’s disease: results from a nationwide study of 1449 outpatients. Parkinsonian Rel Disord 18:598–601
Riederer P, Bartl J, Laux G, Grünblatt E (2011) Diabetes type II: a risk factor for depression-Parkinson-Alzheimer? Neurotox Res 19:253–265. https://doi.org/10.1007/s12640-010-9203-1
Rocha FL, Murad MG, Stumpf BP, Hara C, Fuzikawa C (2013) Antidepressants for depression in Parkinson’s disease: systematic review and meta-analysis. J Psychopharmacol 27:417–423. https://doi.org/10.1177/0269881113478282
Ryan M, Eatmon CV, Slevin JT (2019) Drug treatment strategies for depression in Parkinson disease. Expert Opin Pharmacother 20:1351–1363. https://doi.org/10.1080/14656566.2019.1612877
Seppi K, Chaudhuri KR, Coelho M, Fox SH et al (2019) Update on treatments for the non-motor symptoms of Parkinson’s disease –an evidence-based medicine review. Mov Disord 34:180–198
Shabnam GN, Th C, Kho D et al (2003) Therapies for depression in Parkinson’s disease. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD003465
Simon DK, Tanner CM, Brundin P (2020) Parkinson Disease epidemiology, pathology, genetics, and pathophysiology. Clin Geriatr Med 36:1–12. https://doi.org/10.1016/j.cger.2019.08.002
Skapinakis P, Bakola E, Salanti G et al (2010) Efficacy and acceptability of selective serotonin reuptake inhibitors for the treatment of depression in Parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials. BMC Neurol 10:49
Skorvanek M, Gdovinova Z, Rosenberger J, Saeedian RG et al (2015) The associations between fatigue, apathy, and depression in Parkinson’s disease. Acta Neurol Scand 131:80–87. https://doi.org/10.1111/ane.12282
Steur EN, Ballering LA (1997) Moclobemide and selegeline in the treatment of depression in Parkinson’s disease. J Neurol Neurosurg Psychiatry 63:547
Takahashi M, Tabu H, Ozaki A, Hamano T, Takeshima T, REBORN study group (2019) Antidepressants for depression, apathy, and gait instability in Parkinson’s disease: a multicenter randomized study. Intern Med 58:361–368. https://doi.org/10.2169/internalmedicine.1359-18
Tesei S, Antonini A, Canesi M et al (2000) Tolerability of paroxetine in Parkinson’s disease: a prospective study. Mov Disord 15:986–989
Troeung L, Egan SJ, Gasson N (2013) A meta-analysis of randomised placebo-controlled treatment trials for depression and anxiety in Parkinson’s disease. PLoS ONE 8:e79510. https://doi.org/10.1371/journal.pone.0079510
Tursi MF, Baes CV, Camacho FR, Tofoli SM, Juruena MF (2013) Effectiveness of psychoeducation for depression: a systematic review. Aust N Z J Psychiatry 47:1019–1031
Weintraub D, Mamikonyan E (2019) The neuropsychiatry of Parkinson Disease: A perfect Storm. Amer J Geriatr Psychiatry 27:998–1018. https://doi.org/10.1016/j.jagp.2019.03.002
Weintraub D, Moberg PJ, Duda JE et al (2003) Recognition and treatment of depression in Parkinson’s disease. J Geriatr Psychiatry Neurol 16:178–183
Williams JR, Hirsch ES, Anderson K, Bush AL et al (2012) A comparison of nine scales to detect depression in Parkinson disease: which scale to use? Neurology 78:998–1006
Wolters ECH, Braak H (2006) Parkinson’s disease: premotor clinico-pathological correlations. J Neural Transm Suppl. https://doi.org/10.1007/978-3-211-45295-0-47
Xie CL, Chen J, Wang XD, Pan JL et al (2015) Repetitive transcranial magnetic stimulation (rTMS) for the treatment of depression in Parkinson disease: a meta-analysis of randomized controlled clinical trials. Neurol Sci 36:1751–1761. https://doi.org/10.1007/s10072-015-2345-4
Yang S, Sajatovic M, Walter BL (2012) Psychosocial interventions for depression and anxiety in Parkinson’s disease. J Geriatr Psychiatry Neurol 25:113–121
Zhuo C, Xue R, Luo L et al (2017) Efficacy of antidepressive medication for depression in Parkinson disease: a network meta-analysis. Medicine (baltimore) 96:e6698. https://doi.org/10.1097/MD.0000000000006698
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Special issue J Neural Transm 80th birthday Peter Riederer.
Rights and permissions
About this article
Cite this article
Laux, G. Parkinson and depression: review and outlook. J Neural Transm 129, 601–608 (2022). https://doi.org/10.1007/s00702-021-02456-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-021-02456-3