Abstract
Background
Deep brain stimulation (DBS) of the globus pallidus internus (GPi) has become an accepted treatment for severe cervical dystonia (CD). Assessment of therapeutic efficacy of DBS mostly focused on head position at rest but hardly on limitations of head and neck mobility, which represent a functionally important impairment in CD.
Objective
We aimed to determine prospectively head and neck range of motion (ROM) preoperatively and during chronic bilateral GPi DBS in a series of 11 patients with idiopathic CD or segmental dystonia with prominent CD using a computerized motion analysis.
Methods
Maximum horizontal rotation of the head in the transverse plane and lateral inclination in the frontal plane were measured preoperatively and at a median of 7 months of chronic GPi DBS, using an ultrasound-based three-dimensional measuring system combined with surface electromyography of cervical muscles.
Results
Horizontal rotation of the head increased from 78.8° ± 31.5° (mean ± SD) preoperatively to 100.7° ± 24.7° with GPi DBS (p < 0.01), thereby improvement of head rotation to the anti-dystonic side (+ 14,2° ± 12,2°) was greater than to the pro-dystonic side (+ 7,8° ± 9,2°; p < 0.05). Movement-related agonistic-antagonistic EMG modulation during head rotation was enhanced with GPi DBS in both sternocleidomastoid (modulation index (MI) 35.8% ± 26.7% preoperatively vs. 67.3% ± 16.9% with GPi DBS, p < 0.01), and splenius capitis muscles (MI 1.9% ± 24.5% preoperatively vs. 44.8% ± 11.6% with GPi DBS, p < 0.01).
Conclusion
Chronic bilateral GPi DBS significantly improves head ROM in CD, likely due to enhanced agonist–antagonist EMG activity with reduced co-contraction. Computerized motion analysis provides an objective measurement to assess the improvement of head and neck mobility in CD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dystonia is a heterogeneous movement disorder characterized by sustained or phasic muscle contractions as well as dystonic tremor, resulting in twisting and repetitive movements or abnormal fixed postures (Albanese et al. 2013; Fahn et al. 1998). Physical activity or mental stress frequently intensifies or exacerbates dystonic movements. Despite normal cognitive abilities, symptoms of dystonia frequently cause physical and social disability at all levels of functioning with a significant deterioration in health-related quality of life (Blahak et al. 2008; Camfield et al. 2002; Skogseid et al. 2007; Tsuboi et al. 2020b).
In cervical dystonia (CD), the abnormal head position at rest and in particular the impairment of voluntary head movements with a decreased range of motion has a major impact on disability and on activities of daily living (ADL). This notably concerns the general working capacity (Martikainen et al. 2010), but also car driving, housekeeping tasks and social activities (Camfield et al. 2002).
Pharmacological treatment of dystonia is often disappointing. Local treatment with botulinum toxin is still the first choice in the management of focal dystonias (Wissel et al. 2001; Poewe et al. 2016). Furthermore, botulinum toxin can improve health-related quality of life in restricted forms of dystonia (Skogseid et al. 2007), but its efficacy in more widespread forms of dystonia is confined due to dose limitations.
Bilateral deep brain stimulation (DBS) of the globus pallidus internus (GPi) as a reversible and modifiable treatment has demonstrated good efficacy in treating different subtypes of dystonia with improvement of both motor symptoms and quality of life in inherited and idiopathic segmental and generalized dystonia (including the cervical component) (Blahak et al. 2008; Kupsch et al. 2006; Moro et al. 2017), but also in CD (Kiss et al. 2007; Krauss et al. 1999; Skogseid et al. 2012; Tsuboi et al. 2020a; Volkmann et al. 2014; Walsh et al. 2013). To assess the effect of treatment on dystonia motor symptoms common rating scales like the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS), the Tsui score or the Burke–Fahn–Marsden (BFM) dystonia rating scale are usually used. The evaluation of the cervical component of dystonia in all of these rating scales, however, focuses on the head position at rest with the maximum excursion in different axes (torticollis, laterocollis, antero-/ retrocollis and lateral/sagittal shift). Just the TWSTRS contains a single item on the functionally important range of motion of the head, but using a rather arbitrary gradation (Consky and Lang 1994).
Little is known about the impact of DBS on the head and neck mobility in CD which represents a functionally important impairment. Hence, the objective of the present study was to assess active head and neck range of motion preoperatively and during chronic DBS in CD.
Patients and methods
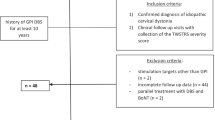
Eleven patients (mean age 59.4 ± 12.0 years; 5 women, 6 men) suffering from idiopathic CD (5/11 patients) or segmental dystonia with predominant cervical involvement (6/11 patients) were prospectively included in this study. Indication for DBS was insufficient control of dystonic symptoms by either botulinum toxin (BTX-A) injections (10/11 patients) and/or oral antidystonic medication (8/11 patients), with a wash-out phase of at least six months following the last BTX-A injection. Patients were evaluated preoperatively and at a median interval of 7 months (range 6–9 months) of chronic bilateral GPi DBS. All patients gave their written informed consent to surgery and the study, which was approved by the local ethics committee and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
In all patients, quadripolar DBS electrodes (Model 3387; Medtronic Inc., Minneapolis, MN, USA) were implanted bilaterally in the posteroventral lateral GPi using CT-guided stereotactic surgery and microelectrode recording (preliminary target coordinates related to the intercommissural line: x = 20; y = + 2, z = − 4) and connected to programmable implantable pulse generators (Medtronic Inc.) as reported in detail previously (Alam et al. 2015; Krauss et al 2004; Schrader et al. 2011; Woehrle et al. 2009). Postoperative stereotactic CT or magnetic resonance imaging demonstrated appropriate placement of the DBS electrodes in the target (with contacts 1 and 2 in the posteroventral lateral GPi). No patient suffered from perioperative complications.
To evaluate the clinical outcome, the Burke–Fahn–Marsden motor (BFM) score was assessed pre-OP and at all FU examinations. The postoperative “DBS off” assessments were performed 60 min after cessation of GPi DBS.
To assess head and neck mobility, a three-dimensional (3D) ultrasound-based real time motion analysis system (CMS 70P, Zebris, Isny, Germany) was used. The fully digitized measuring procedure is based on the travel time measurement of ultrasonic pulses that are emitted by small transmitters (markers) and received by three ultrasound microphones built into a measuring device, providing a spatial resolution < 0.5 mm. Patients were seated in a height-adjustable straight-back chair at about 1 m in front of the receiver device that was mounted on a mobile floor stand. Three of these ultrasound markers were fixed in the shape of an equilateral triangle with a side length of 25 mm on a small plastic plate of 30 × 35 mm. One of the assembled marker plates was fixed above the protuberantia mentalis at the tip of the chin and a second above the manubrium of the sternum using strong double-sided tape. In so doing, movements of the trunk could be subtracted to assess the cervical range of motion solely. In addition, surface EMGs were recorded bilaterally from the sternocleidomastoid (SCM) and the splenius capitis (SpL) muscles. EMG electrode placement was conducted following the recommendations of the SENIAM project (Hermens et al. 2000) and electromyographer guidelines (Delagi et al. 1975).
Patients then were instructed to rotate the head as far as possible in the horizontal plane successively to the left and then to the right (Figs. 1, 2), and thereafter to incline the head as far as possible to the shoulder, first on the left side and then on the right side with interim return to a neutral resting position, respectively. The described sequence of head movements was performed three times in succession, and the results for every movement direction were finally averaged. Before recordings were obtained, patients performed trials of the different movements tasks to check device integrity and, if necessary, to correct movement performance.
Raw data of a sequence of horizontal head rotation A preoperatively and B postoperatively with GPi DBS in a single patient. Depicted are the signals of a single ultrasound marker in the horizontal plane and bilateral EMGs of the SCM and SpL muscles, respectively. The vertical gray lines represent the beginning of the head rotation to one side, the gray shaded areas the period for the determination of the EMG integrals as the basis for the calculation of the respective modulation indices
The EMG signal was amplified by an active electronic circuit integrated in the EMG cable (gain = 1000), ensuring a high signal-to-noise ratio and a minimisation of cable artifacts (CMS 70P, Zebris, Isny, Germany). 3D motion data and amplified EMG signals were transferred online to a computer system via an analog–digital converter sampling at 4 kHz with 12 bits resolution. Data analyses of 3D data and signal processed EMG (band-passed filtered 10–1000 Hz, notch-filtered to eliminate DBS noise at 130 Hz, offset correction, full-wave rectification) were performed offline.
The maximum horizontal rotation of the head in the transverse plane (Fig. 2) and the maximum lateral inclination of the head in the frontal plane were calculated with macros developed by our laboratory using Microsoft Excel. In addition, EMG modulation indices (MI) between activation and relaxation of both the SCM and the SpL muscles during the respective phases of horizontal rotation of the head were calculated. Integrals of the rectified EMG of a 2 s interval, starting 1 s after the initiation of the horizontal rotation of the head (as detected by the 3D motion data, see Fig. 1), were calculated. To estimate the MI, for each muscle the respective EMG integral of the antagonistic movement (SCM: ipsilateral rotation, SpL: contralateral rotation) was subtracted from the EMG integral of the agonistic movement (SCM: contralateral rotation, SpL: ipsilateral rotation) and the result divided by the EMG integral of the agonistic movement. Finally, for both SCM and SpL the results of the left and right side were averaged.
For statistical analysis, because the Shapiro–Wilk test demonstrated that not all data were normally distributed, nonparametric Wilcoxon’s rank-sum test for paired variables and the Mann–Whitney U test for non-paired variables were applied to compare means using SPSS 16.
Results
All patients included in the study had clinical benefit from DBS, no adverse events occurred during or after the operative procedure. Patient characteristics, basic outcome parameters and stimulation settings are presented in Table 1. Stimulation mode was bilateral bipolar in 7 patients and bilateral monopolar in 3 patients; one patient had bipolar settings for the dominant and monopolar for the contralateral hemisphere. Stimulation voltage ranged between 1.8 and 6.1 V. Frequency was 130 Hz or 145 Hz, and stimulus width was 210 µs in all patients but 1 with 90 µs.
The “neck” sub-score of the BFM motor score decreased from 6.2 ± 1.3 (mean ± SD) pre-OP to 2.8 ± 1.9 post-OP, reflecting an improvement of 54.4%, and the mean total BFM motor score improved by 66.2% with chronic GPi DBS.
The total horizontal rotation of the head in the transverse plane (Fig. 3) improved from 78.8° ± 31.5° (mean ± SD) preoperatively to 100.7° ± 24.7° with chronic GPi DBS (p < 0.01). After discontinuation of GPi DBS at the postoperative follow-up, horizontal rotation of the head diminished again to 90.6° ± 24.6° (p = 0.11 compared to preoperative measurement). Preoperatively, horizontal head rotation to the anti- and pro-dystonic side was not different (40.6° ± 17.6° vs. 38.2° ± 16.0°; p = 0.54), but postoperatively the improvement of horizontal head rotation with GPi DBS to the anti-dystonic side (+ 14.2° ± 12.2°) was significantly greater than to the pro-dystonic side (+ 7.8° ± 9.2°; p < 0.05).
In parallel, the maximum lateral head and neck inclination in the frontal plane increased from 50.5° ± 23.7° preoperatively to 62.9° ± 17.8° with chronic GPi DBS (p < 0.05; Fig. 4), but deteriorated to 56.1° ± 20.3° when DBS was switched off (p = 0.28 compared to preoperative measurement).
Overall, 10/11 patients showed an improved horizontal rotation of the head with chronic GPi DBS, 7/11 by more than 20%. The impact of GPi DBS on lateral head and neck inclination was somewhat less, but still detectable in 8/11 patients and greater than 20% in 6/11 patients.
EMG modulation during horizontal head rotation in the SCM muscles was significantly enhanced with chronic GPi DBS (MI 35.8% ± 26.7% preoperatively to MI 67.3% ± 16.9% with GPi DBS, p < 0.01), but nearly returned to the preoperative level when DBS was discontinued (MI 38.4% ± 19.9%, p = 0.21 compared to preoperative measurement). For the SpL muscles, GPi DBS also caused a significant increase in EMG modulation (MI 1.9% ± 24.5% preoperatively to MI 44.8% ± 11.6% with GPi DBS, p < 0.001; Fig. 5), that in part persisted upon discontinuation of DBS (MI 20.6% ± 12.0%, p < 0.01 compared to preoperative measurement). Considering the pattern of dystonia, EMG modulation in the contralateral SCM muscle tended to be more improved with Gpi DBS during horizontal head rotation to the anti-dystonic side compared to a rotation to the pro-dystonic side (MI + 34.7% ± 29.4% vs. + 28.1% ± 25.4%, p = 0.12), but improved correspondingly in the ipsilateral SpL muscles during horizontal head rotation against and towards the dystonic side (MI + 43.2% ± 39.0% vs. + 42.4% ± 40.6%, p = 0.12).
Discussion
To our knowledge, this is the first study that prospectively assesses head and neck range of motion in patients with CD treated with GPi DBS. Both the maximum active horizontal rotation of the head in the transverse plane and the maximum active lateral head and neck inclination in the frontal plane were significantly increased with chronic DBS as compared to the preoperative status. Furthermore, the modulation of the SCM and SpL neck muscle EMG activity during horizontal rotation of the head significantly improved during chronic GPi DBS.
The efficacy of bilateral GPi DBS in CD has been well documented in several case series (Kiss et al. 2007; Krauss et al. 1999; Moro et al. 2017; Skogseid et al. 2012; Walsh et al. 2013), and has been confirmed in a randomized sham-controlled trial (Volkmann et al. 2014), with improvement of motor symptoms, pain, disability and quality of life. However, the commonly used rating scales for motor symptoms in dystonia primarily assess head position with severity of dystonia at rest. Only the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) contains in its first section a single item on the cervical range of motion (I: severity score, “E”, gradation 0–4 points), to roughly evaluate the ability to move the head past midline. The sole study on GPi DBS for CD that reported detailed results of the TWSTRS only found a slight but not significant decrease of the score for the cervical range of motion with GPi DBS, most likely due to low mean scores already preoperatively (Volkmann et al. 2014). On the other hand, a recent study indicated that the range of neck mobility could even be a prognostic factor for outcome of GPi DBS (Huh and Chung 2019).
So far, no quantitative data are available regarding the therapeutic impact of GPi DBS on voluntary head and neck movements. At least for cervical botulinum toxin type A injections, in a kinematic study by Gregori et al. significant improvements of the angular amplitude of voluntary neck movements could be demonstrated (Gregori et al. 2008).
Considering the individual pattern of CD, the range of motion of horizontal head rotation to the anti-dystonic side showed a significantly greater improvement with GPi DBS compared to the rotation to the pro-dystonic side in our study. Furthermore, the EMG modulation of both the SCM and the SpL muscles—as a measure for the extent of contraction vs. relaxation during agonistic and antagonistic movements—increased significantly with GPi DBS.
These findings underline the importance of an impaired motor control for the limitation in head and neck movements in dystonia: the active range of motion is usually more impaired when patients move their head towards the anti-dystonic side, caused by both pathological co-contraction of agonistic and antagonistic muscles during rotational head movements (Kaji et al. 1995), and insufficient dystonic muscle relaxation with a longer overlap of agonist–antagonist muscle activity (Boccagni et al. 2008; Prodoehl et al. 2006). Several studies demonstrated the involvement of voluntary movements of the head (Boccagni et al. 2008; Shaikh et al. 2015) and the extremities (Currà et al. 2000) in dystonia, suggesting a generalized disturbance of movement execution caused by impairment of basal ganglia circuits projecting to non-primary motor areas (Carbon et al. 2004). The concept of a neural integrator dysfunction more recently has been supported by electrophysiological recordings from the GPi indicating bi-hemispheric asymmetry (Sedov et al. 2019, 2020).
Besides abnormal co-contraction of agonistic and antagonistic muscles, electromyographic studies proved prolonged muscle activation and lack of selectivity with overflow to adjacent muscles (Rothwell et al. 1983). Hence, loss of inhibition has been emphasized as an important pathophysiological substrate in dystonia (Quartarone and Hallett 2013). Alterations of inhibitory circuits have been shown at all levels of the motor nervous system: the spinal cord, the brainstem and the sensory-motor cortex (Hallett 2011). A key feature in this context is a change in surround inhibition, which is necessary for a specific voluntary movement (Kassavetis et al. 2018). In dystonia, surround inhibition is reduced, leading to difficulties in focusing movements and to an overflow to muscles not involved in a motor task (Beck et al. 2008). This loss of inhibition has been attributed mainly to basal ganglia dysfunction in terms of an imbalance between the direct and indirect pathway (Quartarone and Hallett 2013).
GPi DBS might at least partially correct this imbalance, suppressing motor overflow and alleviating loss of (surround) inhibition, as supported by the electrophysiological evidence of an increase in short-latency intracortical inhibition (Ruge et al. 2011a) and spinal reciprocal inhibition (Tisch et al. 2006) following GPi DBS for dystonia. Furthermore, the clinical benefit of GPi DBS in dystonia was found to be related to the normalization of cortical plasticity as tested by paired associative stimulation and pairing GPi DBS with cortical stimulation (Ni et al. 2018; Ruge et al. 2011b).
We do not only provide a quantitative clinical demonstration of an increase in the range of motion of the head during rotation and lateral inclination, but furthermore an electrophysiologic correlate of the impact of GPi DBS with improved pattern of agonist–antagonist activation of different neck muscles.
After cessation of DBS for 60 min, the cervical range of motion of both horizontal rotation and lateral inclination of the head deteriorated again, although not reaching preoperative levels. Only the EMG modulation of the SCM, but not the SpL muscles decreased to almost preoperative levels. It has been shown previously that upon withdrawal of DBS in the first two years after its initiation dystonic signs return sequentially within several hours, with a rapid worsening of phasic and a slower worsening of tonic dystonic symptoms (Grips et al. 2007). Differential clinical effects when withdrawing DBS might reflect different pathophysiological mechanisms in dystonia. On the other hand, abnormal plasticity within basal ganglia circuits has been hypothesized to be another key feature in the pathophysiology of dystonia (Erro et al. 2018; Quartarone and Hallett 2013). Given the gradual clinical benefit following pallidal DBS in dystonia (Yianni et al. 2003), that contrasts the usually immediate effects in Parkinson's disease, a process of progressive plasticity and neural reorganization as the basis for the efficacy of GPi DBS in dystonia has been suggested (Quartarone and Hallett 2013; Kroneberg et al. 2018). In consequence, re-occurrence of dystonic symptoms — including the influence of dystonia on voluntary movements like horizontal head rotation — is incomplete after 60 min of DBS withdrawal and thus hardly comparable to the preoperative assessment.
Our results reveal a significant improvement of head and neck range of motion with chronic DBS without achieving the level of cervical range of motion in healthy persons: with GPi DBS, both the range of motion of the horizontal head rotation and the lateral head inclination reached about two-thirds of the range of motion ascertained in healthy adults (Loher et al. 2006). It should be noted, however, that we conducted the postoperative measurements at an interval of six to nine months after initiation of bilateral GPi DBS. In patients with predominant tonic dystonia, clinical improvement following DBS can extend to a longer period of time (Yianni et al. 2003). In particular secondary muscular and connective tissue changes like muscle and tendon shortenings that, if any, take months to years for recovery might well be responsible for the incomplete restitution of head mobility. Also, degenerative spine disorders which are more prevalent in patients with chronic dystonia might limit the range of motion (Loher et al. 2006).
A limitation of our study is that we did not assess the range of motion in a control group. However, extensive literature is available regarding the range of motion of head movements in healthy subjects (Chen et al. 1999; Kuhlman 1993), and some of them reported a good agreement between different measurement devices (Williams et al. 2010). In one study, the same measuring device was used with a different positioning of the ultrasound markers, demonstrating a good inter-device reliability to a goniometer-based system supporting the validity of the demonstrated data (Malmstroem et al. 2003). Furthermore, with the methodology used we were only able to analyze the EMG modulation of the superficial SCM and SpL muscles, but not the deep neck muscles that play an important role for head and neck movements.
As a further limitation, we did not study accompanying non-motor symptoms in detail. Although none of the patients suffered from a major depressive episode, fluctuating depressive symptoms or anxiety might have biased the results.
Availability of data and material (data transparency)
All original data are available from the authors upon request.
Code availability (software application or custom code)
Not applicable.
References
Alam M, Schwabe K, Lütjens G, Capelle HH, Manu M, von Wrangel C, Müller-Vahl K, Schrader C, Scheinichen D, Blahak C, Heissler HE, Krauss JK (2015) Comparative characterization of single cell activity in the globus pallidus internus of patients with dystonia or Tourette syndrome. J Neural Transm 122:687–699. https://doi.org/10.1007/s00702-014-1277-0
Albanese A, Bhatia K, Bressman SB, Delong MR, Fahn S, Fung VS, Hallett M, Jankovic J, Jinnah HA, Klein C, Lang AE, Mink JW, Teller JK (2013) Phenomenology and classification of dystonia: a consensus update. Mov Disord 28:863–873. https://doi.org/10.1002/mds.25475
Beck S, Richardson SP, Shamim EA, Dang N, Schubert M, Hallett M (2008) Short intracortical and surround inhibition are selectively reduced during movement initiation in focal hand dystonia. J Neurosci 28:10363–10369. https://doi.org/10.1523/JNEUROSCI.3564-08.2008
Blahak C, Wöhrle JC, Capelle HH, Bäzner H, Grips E, Weigel R, Kekelia K, Krauss JK (2008) Health-related quality of life in segmental dystonia is improved by bilateral pallidal stimulation. J Neurol 255:178–182. https://doi.org/10.1007/s00415-008-0614-3
Boccagni C, Carpaneto J, Micera S, Bagnato S, Galardi G (2008) Motion analysis in cervical dystonia. Neurol Sci 29:375–381. https://doi.org/10.1007/s10072-008-1033-z
Camfield L, Ben-Shlomo Y, Warner T, ESDE Collaborative Group (2002) Impact of cervical dystonia on quality of life. Mov Disord 17:838–841. https://doi.org/10.1002/mds.10127
Carbon M, Su S, Dhawan V, Raymond D, Bressman S, Eidelberg D (2004) Regional metabolism in primary torsion dystonia: effects of penetrance and genotype. Neurology 62:1384–1390. https://doi.org/10.1212/01.WNL.0000120541.97467.FE
Chen J, Solinger AB, Poncet JF, Lantz CA (1999) Meta-analysis of normative cervical motion. Spine 15:1571–1578. https://doi.org/10.1097/00007632-199908010-00011
Consky ES, Lang AE (1994) Clinical assessments of patients with cervical dystonia. In: Jankovic J, Hallett M (eds) Therapy with botulinum toxin. Marcel Dekker, New York, pp 211–237
Currá A, Berardelli A, Agostino R, Giovannelli M, Koch G, Manfredi M (2000) Movement cueing and motor execution in patients with dystonia: a kinematic study. Mov Disord 15:103–112. https://doi.org/10.1002/1531-8257(200001)15:1%3c103::aid-mds1016%3e3.0.co;2-3
Delagi EF, Perotto A, Iazetti J, Morrison D (1975) Anatomic guide for the electromyographer. Charles C. Thomas, Springfield
Erro R, Rocchi L, Antelmi E, Liguori R, Tinazzi M, Berardelli A, Rothwell J, Bhatia KP (2018) High frequency somatosensory stimulation in dystonia: evidence for defective inhibitory plasticity. Mov Disord 33:1902–1909. https://doi.org/10.1002/mds.27470
Fahn S, Bressman SB, Marsden CD (1998) Classification of dystonia. Adv Neurol 78:1–10
Gregori B, Agostino R, Bologna M, Dinapoli L, Colosimo C, Accornero N, Berardelli A (2008) Fast voluntary neck movements in patients with cervical dystonia: a kinematic study before and after therapy with botulinum toxin type A. Clin Neurophysiol 119:273–280. https://doi.org/10.1016/j.clinph.2007.10.007
Grips E, Blahak C, Capelle HH, Bäzner H, Weigel R, Sedlaczek O, Krauss JK, Wöhrle JC (2007) Patterns of reoccurrence of segmental dystonia after discontinuation of deep brain stimulation. J Neurol Neurosurg Psychiatry 78:318–320. https://doi.org/10.1136/jnnp.2006.089409
Hallett M (2011) Neurophysiology of dystonia: the role of inhibition. Neurobiol Dis 42:177–184. https://doi.org/10.1016/j.nbd.2010.08.025
Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G (2000) Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 10:361–374. https://doi.org/10.1016/S1050-6411(00)00027-4
Huh R, Chung M (2019) Range of voluntary neck motility predicts outcome of pallidal DBS for cervical dystonia. Acta Neurochir 161:2491–2498. https://doi.org/10.1007/s00701-019-04076-z
Kaji R, Ikeda A, Ikeda T, Kubori T, Mezaki T, Kohara N, Kanda M, Nagamine T, Honda M, Rothwell JC, Shibasaki H, Kimura J (1995) Physiological study of cervical dystonia. Task-specific abnormality incontingent negative variation. Brain 118:511–522. https://doi.org/10.1093/brain/118.2.511
Kassavetis P, Sadnicka A, Saifee TA, Pareés I, Kojovic M, Bhatia KP, Rothwell JC, Edwards MJ (2018) Reappraising the role of motor surround inhibition in dystonia. J Neurol Sci 390:178–183. https://doi.org/10.1016/j.jns.2018.04.015
Kiss ZHT, Doig-Beyaert K, Eliasziw M, Tsui J, Haffenden A, Suchowersky O (2007) The Canadian Multicentre study of deep brain stimulation for cervical dystonia. Brain 130:2879–2886. https://doi.org/10.1093/brain/awm229
Krauss JK, Pohle T, Weber S, Ozdoba C, Burgunder JM (1999) Bilateral stimulation of globus pallidus internus for treatment of cervical dystonia. Lancet 354:837–838. https://doi.org/10.1016/S0140-6736(99)80022-1
Krauss JK, Yianni J, Loher TJ, Aziz TZ (2004) Deep brain stimulation for dystonia. J Clin Neurophysiol 21:18–30. https://doi.org/10.1097/00004691-200401000-00004
Kroneberg D, Plettig P, Schneider GH, Kühn AA (2018) Motor cortical plasticity relates to symptom severity and clinical benefit from deep brain stimulation in cervical dystonia. Neuromodulation 21:735–740. https://doi.org/10.1111/ner.12690
Kuhlman KA (1993) Cervical range of motion in the elderly. Arch Phys Med Rehabil 74:1071–1079. https://doi.org/10.1016/0003-9993(93)90064-H
Kupsch A, Benecke R, Müller J, Trottenberg T, Schneider GH, Poewe W, Eisner W, Wolters A, Müller JU, Deuschl G, Pinsker MO, Skogseid IM, Roeste GK, Vollmer-Haase J, Brentrup A, Krause M, Tronnier V, Schnitzler A, Voges J, Nikkhah G, Vesper J, Naumann M, Volkmann J (2006) Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med 355:1978–1990. https://doi.org/10.1056/NEJMoa063618
Loher TJ, Bärlocher CB, Krauss JK (2006) Dystonic movement disorders and spinal degenerative disease. Stereotact Funct Neurosurg 84:1–11. https://doi.org/10.1159/000092681
Malmstroem EM, Karlberg M, Melander A, Magnusson M (2003) Zebris versus myrin: a comparative study between a three-dimensional ultrasound movement analysis and an inclinometer/compass method. Spine 28:E433-440. https://doi.org/10.1097/01.BRS.0000090840.45802.D4
Martikainen KK, Luukkaala TH, Marttila RJ (2010) Working capacity and cervical dystonia. Parkinsonism Relat Disord 16:215–217. https://doi.org/10.1016/j.parkreldis.2009.07.006
Moro E, LeReun C, Krauss JK, Albanese A, Lin JP, Walleser Autiero S, Brionne TC, Vidailhet M (2017) Efficacy of pallidal stimulation in isolated dystonia: a systematic review and meta-analysis. Eur J Neurol 24:552–560. https://doi.org/10.1111/ene.13255
Ni Z, Kim SJ, Phielipp N, Ghosh S, Udupa K, Gunraj CA, Saha U, Hodaie M, Kalia SK, Lozano AM, Lee DJ, Moro E, Fasano A, Hallett M, Lang AE, Chen R (2018) Pallidal deep brain stimulation modulates cortical excitability and plasticity. Ann Neurol 83:352–362. https://doi.org/10.1002/ana.25156
Poewe W, Burbaud P, Castelnovo G, Jost WH, Ceballos-Baumann AO, Banach M, Potulska-Chromik A, Ferreira JJ, Bihari K, Ehler E, Bares M, Dzyak LA, Belova AN, Pham E, Liu WJ, Picaut P (2016) Efficacy and safety of abobotulinumtoxin A liquid formulation in cervical dystonia: A randomized-controlled trial. Mov Disord 31:1649–1657. https://doi.org/10.1002/mds.26760
Prodoehl J, MacKinnon CD, Comella CL, Corcos DM (2006) Rate of force production and relaxation is impaired in patients with focal hand dystonia. Parkinsonism Relat Disord 12:363–371. https://doi.org/10.1016/j.parkreldis.2006.01.008
Quartarone A, Hallett M (2013) Emerging concepts in the physiological basis of dystonia. Mov Disord 28:958–967. https://doi.org/10.1002/mds.25532
Rothwell JC, Obeso JA, Day BL, Marsden CD (1983) Pathophysiology of dystonias. Adv Neurol 39:851–863
Ruge D, Cif L, Limousin P, Gonzalez V, Vasques X, Hariz MI, Coubes P, Rothwell JC (2011a) Shaping reversibility? Long-term deep brain stimulation in dystonia: the relationship between effects on electrophysiologyandclinical symptoms. Brain 134:2106–2115. https://doi.org/10.1093/brain/awr122
Ruge D, Tisch S, Hariz MI, Zrinzo L, Bhatia KP, Quinn NP, Jahanshahi M, Limousin P, Rothwell JC (2011b) Deep brain stimulation effects in dystonia: time course of electrophysiological changes in early treatment. Mov Disord 26:1913–1921. https://doi.org/10.1002/mds.23731
Schrader C, Capelle HH, Kinfe TM, Blahak C, Bäzner H, Lütjens G, Dressler D, Krauss JK (2011) GPi-DBS may induce a hypokinetic gait disorder with freezing of gait in patients with dystonia. Neurology 77:483–488. https://doi.org/10.1212/WNL.0b013e318227b19e
Sedov A, Usova S, Semenova U, Gamaleya A, Tomskiy A, Crawford JD, Corneil B, Jinnah HA, Shaikh AG (2019) The role of pallidum in the neural integrator model of cervical dystonia. Neurobiol Dis 125:45–54. https://doi.org/10.1016/j.nbd.2019.01.011
Sedov A, Usova S, Semenova U, Gamaleya A, Tomskiy A, Beylergil SB, Jinnah HA, Shaikh AG (2020) Pallidal activity in cervical dystonia with and without head tremor. Cerebellum 19:409–418. https://doi.org/10.1007/s12311-020-01119-5
Shaikh AG, Wong A, Zee DS, Jinnah HA (2015) Why are voluntary head movements in cervical dystonia slow? Parkinsonism Relat Disord 21:561–566. https://doi.org/10.1016/j.parkreldis.2015.03.005
Skogseid IM, Malt UF, Røislien J, Kerty E (2007) Determinants and status of quality of life after long-term botulinum toxin therapy for cervical dystonia. Eur J Neurol 14:1129–1137. https://doi.org/10.1111/j.1468-1331.2007.01922.x
Skogseid IM, Ramm-Pettersen J, Volkmann J, Kerty E, Dietrichs E, Røste GK (2012) Good long-term efficacy of pallidal stimulation in cervical dystonia: a prospective, observer-blinded study. Eur J Neurol 19:610–615. https://doi.org/10.1111/j.1468-1331.2011.03591.x
Tisch S, Limousin P, Rothwell JC, Asselman P, Zrinzo L, Jahanshahi M, Bhatia KP, Hariz MI (2006) Changes in forearm reciprocal inhibition following pallidal stimulation for dystonia. Neurology 66:1091–1093. https://doi.org/10.1212/01.wnl.0000204649.36458.8f
Tsuboi T, Wong JK, Almeida L, Hess CW, Wagle Shukla A, Foote KD, Okun MS, Ramirez-Zamora A (2020a) A pooled meta-analysis of GPi and STN deep brain stimulation outcomes for cervical dystonia. J Neurol 267:1278–1290. https://doi.org/10.1007/s00415-020-09703-9
Tsuboi T, Wong JK, Okun MS, Ramirez-Zamora A (2020b) Quality of life outcomes after deep brain stimulation in dystonia: a systematic review. Parkinsonism Relat Disord 70:82–93. https://doi.org/10.1016/j.parkreldis.2019.11.016
Volkmann J, Mueller J, Deuschl G, Kühn AA, Krauss JK, Poewe W, Timmermann L, Falk D, Kupsch A, Kivi A, Schneider GH, Schnitzler A, Südmeyer M, Voges J, Wolters A, Wittstock M, Müller JU, Hering S, Eisner W, Vesper J, Prokop T, Pinsker M, Schrader C, Kloss M, Kiening K, Boetzel K, Mehrkens J, Skogseid IM, Ramm-Pettersen J, Kemmler G, Bhatia KP, Vitek JL, Benecke R, DBS Study Group for Dystonia (2014) Pallidal neurostimulation in patients with medication-refractory cervical dystonia: a randomised, sham-controlled trial. Lancet Neurol 13:875–884. https://doi.org/10.1016/S1474-4422(14)70143-7
Walsh RA, Sidiropoulos C, Lozano AM, Hodaie M, Poon YY, Fallis M, Moro E (2013) Bilateral pallidal stimulation in cervical dystonia: blinded evidence of benefit beyond 5 years. Brain 136:761–769. https://doi.org/10.1093/brain/awt009
Williams MA, McCarthy CJ, Chorti A, Cooke MW, Gates S (2010) A systematic review of reliability and validity studies of methods for measuring active and passive cervical range of motion. J Manip Physiol Ther 33:138–155. https://doi.org/10.1016/j.jmpt.2009.12.009
Wissel J, Kanovsky P, Ruzicka E, Bares M, Hortova H, Streitova H, Jech R, Roth J, Brenneis C, Müller J, Schnider P, Auff E, Richardson A, Poewe W (2001) Efficacy and safety of a standardised 500 unit dose of Dysport (clostridium botulinum toxin type A haemaglutinin complex) in a heterogeneous cervical dystonia population: results of a prospective, multicentre, randomised, double-blind, placebo-controlled, parallel group study. J Neurol 248:1073–1078. https://doi.org/10.1007/s004150170028
Woehrle JC, Blahak C, Kekelia K, Capelle HH, Baezner H, Grips E, Weigel R, Krauss JK (2009) Chronic deep brain stimulation for segmental dystonia. Stereotact Funct Neurosurg 87:379–384. https://doi.org/10.1159/000249819
Yianni J, Bain PG, Gregory RP, Nandi D, Joint C, Scott RB, Stein JF, Aziz TZ (2003) Post-operative progress of dystonia patients following globus pallidus internus deep brain stimulation. Eur J Neurol 10:239–247. https://doi.org/10.1046/j.1468-1331.2003.00592.x
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
CB: conception and design of the work; data acquisition; analysis and interpretation of data; draft of manuscript. MEW: data acquisition; revision of manuscript. AS: data acquisition; revision of manuscript. HB: conception and design of the work; revision of manuscript. JKK: conception and design of the work; draft and revision of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
J. K. Krauss is a consultant to Medtronic and Boston Scientific, he received honoraria for speaking from AbbVie. C. Blahak received travel grants from Ipsen, A. Saryyeva received travel grants from Medtronic. The other authors report no financial disclosures.
Ethiacl approval
Ethics approval/consent to participate/consent for publication.
Informed consent
All patients gave their written informed consent to surgery, the study and the publication of results. The study was approves by the local ethic committee (Faculty of Medicine Mannheim, University of Heidelberg).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Blahak, C., Wolf, M.E., Saryyeva, A. et al. Improvement of head and neck range of motion induced by chronic pallidal deep brain stimulation for cervical dystonia. J Neural Transm 128, 1205–1213 (2021). https://doi.org/10.1007/s00702-021-02365-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-021-02365-5