Abstract
The complex phenomenological understanding of dystonia has transcended from the clinics to genetics, imaging and neurophysiology. One way in which electrophysiology will impact into the clinics are cases wherein a dystonic clinical presentation may not be typical or a “forme fruste” of the disorder. Indeed, the physiological imprints of dystonia are present regardless of its clinical manifestation. Underpinnings in the understanding of dystonia span from the peripheral, segmental and suprasegmental levels to the cortex, and various electrophysiological tests have been applied in the course of time to elucidate the origin of dystonia pathophysiology. While loss of inhibition remains to be the key finding in this regard, intricacies and variabilities exist, thus leading to a notion that perhaps dystonia should best be gleaned as network disorder. Interestingly, the complex process has now spanned towards the understanding in terms of networks related to the cerebellar circuitry and the neuroplasticity. What is evolving towards a better and cohesive view will be neurophysiology attributes combined with structural dynamic imaging. Such a sound approach will significantly lead to better therapeutic modalities in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since dystonia has a broad clinical spectrum, and still lacks ideal consensus criteria, the clinician faces challenges in arriving at good diagnosis. While typically characterized by sustained or intermittent muscle contractions causing abnormal, often repetitive movements, postures, or both, dystonia classification has evolved paving the way for directed diagnostic tests and prognostication (Kaňovský et al. 2015). From another challenging front, a limited understanding of the pathophysiology of dystonia has further led to a laggard development of new and effective treatments for this movement disorder. Neurophysiology may, in a way, impact into the clinics especially in cases wherein a dystonic clinical presentation may not be typical or a “forme fruste” of the disorder.
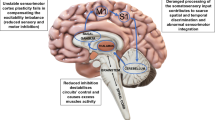
Contemporary understanding gleans dystonia as a network disorder, and this pathophysiological concept, formulated years ago (Rosales and Dressler 2010; Kaňovský and Rosales 2011) still dominates in recent literatures (Fig. 1). Evidences accrue that dystonia arises from an aberrant functional neural network, involving the sensorimotor cortex, brainstem and cerebellum (Hendrix and Vitek 2012; Alexander 1994; Bradnam and Barry 2013; Corp et al. 2019; Prudente et al. 2014; Jinnah and Albanese 2014; Shakkottai et al. 2017). Furthermore, this pathophysiologic precept became a determining factor in brain plasticity mechanisms, especially in the cerebral cortex of idiopathic dystonia (Nevrly et al. 2018; Sadnicka et al. 2020). Perhaps as shared concepts, likely overlooked in past, a resurgence has come about looking into the non-motor features of dystonia (Conte et al. 2016). While still broadening the list of phenomena that accompany dystonia, the basic neurophysiological “mirror “ (or “rebound “) of dystonia has not changed over the last two decades. Mapping the character of dystonic dyskinesia may also be understood in the context of an intersection of both concepts. Still debated as well are the muscle tone disorders, which are classified among dystonic phenotypes (Menšíková et al. 2020).
The classical (s.c. Alexander’s) model of the motor circuits (direct and indirect). The striatum receives inputs from the cortex and communicates with the efferent neurons in the globus pallidus medialis (Gpi) and in the substantia nigra pars reticularis (SNr) directly. The striatum also communicates with the synaptic connections the subthalamic nucleus (STN) indirectly via the globus pallidus lateralis (Gpe). Ventrolateral thalamus (VL) finally receives inputs from Gpi and SNr, and projects back into the cortex. Excitatory pathways are pictured in red, inhibitory pathways are pictured in blue. It is supposed that in the physiological conditions the acetylcholine, GABA and dopamine work in the neurotransmitter homeostasis
This present work aims to review the contemporary neurophysiological imprints in dystonia, and gather insights into a better understanding of its pathophysiology. Obviously, the central role of neurophysiology during functional surgery for dystonia cannot be over-emphasized, but will not be part of this present review. Likewise, non-invasive neuromodulation therapies in dystonia, employing neurophysiologic principles will not be part of this present work, but discussed elsewhere in accompanying reviews (Han-Joon and Jeon 2020; Oyama and Hattori 2020).
Abnormal sensory-motor programs in dystonia: “peripheral level” influences
The muscle spindles behave abnormally in dystonia, as has been postulated by Rosales and Dressler 10 years ago (Rosales and Dressler 2010). Interestingly, despite paucity of studies regarding structural changes in dystonia, these abnormal neurophysiologic attributes of muscle spindles have been well demonstrated (Swash and Fox 1976; Grünewald et al. 1997; Grünewald 2012; Konczak and Abbruzzese 2013; Berman and Jinnah 2015; Brugger et al. 2019). For dystonia as a complex disorder of movement planning and performance, it would seem that the involuntary muscle contraction and its dystonic character (sustained, twisting or repetitive), may also have functional underpinnings at the peripheral level (i.e. muscle spindles, see Fig. 2). A supportive clinical feature of this “abnormal muscle spindle circuitry” is the reduction/abolition of dystonic muscle contractions with cooling, with “geste antagoniste,” and its dystonic appearance/worsening with application of tonic vibration (Kaji et al. 1995a, b; Murase et al. 2000; Pohl et al. 2002; Frima et al. 2003). One other application of this understanding is the concept of of botulunim toxin (BoNT) therapy in dystonia as a form of a “proprioceptive sensory trick.” Both “geste antagoniste” to abolish dystonia and efficacy of botulinum toxins in dystonia therapy eventually dwindle over time as well (Rosales and Dressler 2016; Supnet and Rosales 2018).
Figure taken from Rosales and Dressler (2010): Schematic diagram showing the inhibitory and excitatory pathways in the spinal cord from the muscle spindles. The excitatory synapses are indicated using the V-shaped symbols while the inhibitory synapses are indicated by the small filled circles. The larger filled cricles correspond to the inhibitory interneurons, MN indicates motor neuron
Beside its peripheral proprioceptive functions, muscle spindles are also principally involved in the mutual body-space position perception. Having the kinematic properties they are able to catch the information about the movement of the given body part. The abovementioned properties were tested in the experiments with vibration-induced illusion of movement (VIIM). The impaired perception of VIIM may peripherally involve the muscle spindles, i.e., when the more stiff fibers of muscle spindles from repeated dystonic muscle contractions show “lazy “ reaction to fast stimuli/stretch. In effect, this phenomenon may lead to a delayed and (mainly) prolonged activation of the Ia group afferents during the passive stretch. Likewise, when the muscle becomes “tired” with repeated stretches, elasticity changes occur in the nuclear bag fibers and increase their sensitivity to vibration (Rosales and Dressler 2010).
Based on the aforementioned physiological findings, one may suppose that non-primary focal dystonia may develop in patients with certain predisposing factors, such as abnormal fast Ia fibers afferent processing. On the other side, also the group II afferent information in dystonia is processed abnormally. The whole abnormal sensorimotor processing is more inclined to develop the abnormal motor programs, i.e., to defective ordering of motor routines and motor subroutines (Kaňovský 2002; Kaňovský and Rosales 2011). Also, the neurophysiological abnormalities, which are supposed to be the rebound of the abnormal sensorimotor processing in dystonia at higher levels of the neuroaxis (i.e. brainstem, basal ganglia, thalamus, cerebral cortex) were reported in the past, when the somatosensory evoked potentials (SEP) and paired transcranial magnetic stimulation (TMS) paradigms were used for the experimental examinations in patients suffering from dystonia (Kaňovský et al. 1998, 2003; Kaňovský et al. 1999a, b).
In regard to symptomatic treatment, BoNT effects as applied peripherally do reduce dystonic muscle contractions through chemical denervation (neurophysiologically imprinted mainly by single fiber electromyography) of extrafusal and intrafusal muscles. Believed to have a comparatively longer clinical effect, the latter BoNT blockade of the cholinergic intrafusal muscle spindle endings, potentially modify sensory-motor programs from the segmental to suprasegmental levels ((Supnet and Rosales 2018; Dressler et al. 2020).
Abnormal sensory-motor programs in dystonia: the reflex influences
The long–latency reflexes in dystonia are purportedly abnormal due to abnormal processing of afferent Ia signal mediated by the muscle spindles (i.e. they depend on the function of muscle spindles). Whether there is a one precisely located generator of long-latency reflexes has not been elucidated yet. Nevertheless, it rather seems that these reflexes are generated by the structures lying at different levels of the neuroaxis, such as the spinal cord, brainstem and cerebral cortex. Usually the defective processing of the spindle-mediated afferent Ia impulses lead to prolongation of long-latency reflexes. In short, the abnormal functioning of muscle spindles in dystonia lead to the different abnormalities in the proprioceptive circuitry. In the past, several neurophysiological modalities have been tested in dystonia to elucidate the behavior of the proprioceptive system. The physiological research then focused on the phenomenon that directed toward a defective inhibition; dystonia with abnormal muscle co-contractions. In a normally functioning motor system, the antagonist co-contractions serve to modulate the movement of a given body part. In dystonia, they are present in inappropriate muscle groups, frequently close to the agonist, and in some cases, to remote muscle groups. This phenomenon may even be present on the opposite side of the body, the muscle groups of the opposite limb. It is speculated that the character of dystonic co-contractions (and also those seen in spasticity) is a result of the abnormal synchronization of pre-synaptic inputs to antagonist motor neurons, when up to half of the synaptic impulses are shared between agonists and antagonists (Rothwell 1995; Berardelli et al. 1998; Abbruzzese et al. 2015; Oku and Furuya 2019). The presence of abnormal co-contractions can be used as a neurophysiological imprint testifying the organic character of dystonia, as against those in psychogenic dystonia (Kamble and Pal 2016).
Abnormal sensory-motor programs in dystonia: the cortical excitability influences
Abnormal behavior of muscle spindles is reflected not only in the abnormalities of the long-latency reflexes and presence of abnormal co-contraction. Neurophysiology has brought in last two decades enough evidence, that the principal cortical attributes, essential for the normal functioning of the motor planning and motor execution (i.e. the cortical excitability and intracortical inhibition), are abnormal in dystonia.
The cortical excitability of brain regions, involved in motoricity, is tested using the recording and analysis of cortical components of SEP, which are elicited by the stimulation of motor fibers of peripheral nerves. In the past, the most frequently used modalities were the recordings of the median or tibial nerve SEP, however, the application expanded to other peripheral nerves, such as the ulnar, radial, peroneal or even the trigeminal nerves. In reality, the studies focused on the analysis of cortical components of the median (P22/N30) or tibial (P37/N50) nerves. It has been found, that in dystonia these components have apparently increased amplitude, and that in the lateralized phenotypes of dystonia, it can even have a lateralized shape, which corresponds to the supposed side of the brain with the disorder (Mazzini et al. 1994; Kaňovský et al. 1997, 1998; Tinazzi et al. 1999a). These findings have been recently confirmed in the classical paradigm and also in somatosensory mismatch negativity recordings using the “odd-ball” paradigm (Macerollo et al. 2016, 2018; Chen et al. 2018). Since there is a direct evidence from intracerebral recordings that the P22/N30 SEP components are generated in the premotor cortex, the cortical disorder in dystonia can be localized into this brain region (Kaňovský et al. 2003). Other than the neurophysiological abnormalities related to the motor system and movement processing and performance, the neurophysiological abnormalities related to the somatosensory system were reported in the past. Proprioceptive abnormalities have been found in the muscles involved in dystonia, and with tandem defects in the spatial orientation. Studies indicated abnormalities in the somatosensory temporal and spatial discrimination in dystonia (Tinazzi et al. 1999b; Tinazzi et al. 2002; Bove et al. 2004; Antelmi et al. 2017).
The hypothesis of the defective sorting of subroutines and routines into the supposed motor program was suggested in the past as one of the possible origins of dystonia (Kaňovský 2002). If this is of any importance, there should be also some evidence of abnormality in the very early phase of motor action. Such an evidence might be found in the results of experiments dealing with slow cortical potentials and EEG desynchronization (ERD), which are usually seen as a correlate of the “motor programming” process. Bereitschaftpotential (or readiness potential, BP, RP) has been found to be abnormal in dystonia, and its abnormality has also been found lateralized depending on the side of dystonic symptomatology (Fève et al. 1994; Deuschl et al. 1995; Van der Kamp et al. 1995; Zeuner et al. 2009). Contingent negative variation (CNV), a DC-potential strongly connected with the process of motor preparation has also been found to be abnormal and lateralized in dystonia (Kaji et al. 1995a, b; Ikeda et al. 1996; Hamano et al. 1999; Lim et al. 2004). Both potentials (RP and CNV) are presumed to be generated in the cortex, however, these were also repeatedly recorded in subcortical structures (Bareš and Rektor 2001; Rektor et al. 2001a, b, c, 2005). In parallel, the ERD has been also examined in dystonia, and it has been found to be abnormal (Toro et al. 2000; Tseng et al. 2014). Based on the aforementioned electrophysiological evidences, it can be assumed that the abnormalities of RP, CNV, and ERD indicate (with a high level of probability) a disorder in the motor programming process in dystonia. In effect, there may be a corresponding poor motor performance, as reflected in the abnormalities of previously described reciprocal inhibition, long-latency reflexes, cortical excitability and intracortical inhibition.
Neurophysiologic imprints of dystonia through transcranial magnetic stimulation
TMS has been used as a non-invasive corticomotor neurophysiology tool to assess dystonia. Applied over the human primary motor cortex (M1), the modality probes into the excitability of the corticomotor pathway and intracortical neural circuit (Turco et al. 2018a, b). TMS employs various parameters that address the different aspects of cortical neurophysiology. The motor threshold in TMS is determined by the lowest single-pulse TMS intensity needed to evoke a response of a given size at rest (rest motor threshold, RMT), or during voluntary muscle contraction (active motor threshold, AMT). The motor threshold reflects the total intrinsic membrane excitability from the stimulated M1, spinal cord, neuromuscular junction and muscle (Ziemann et al. 1996a). One can infer the corticomotor excitability (CME) through a measure of the amplitude of the motor-evoked potential (MEP), following a TMS suprathreshold single-pulse stimulation (Chen 2000). CME assessments may be applied through a single TMS intensity or through a stimulus–response (S-R) curve across various ranges of TMS intensities, measuring the amplitude of MEP responses (Devanne et al. 1997). To assess the gamma-aminobutyric acid (GABA) receptor-mediated cortical inhibition within M1, a number of TMS measures can be employed. Cortical silent period (CSP) is derived when single-pulse TMS is made over the contralateral M1, while voluntarily activating a targeted muscle. Thus, GABAB receptor-mediated inhibition can be inferred, through a measure of the duration of the CSP, as derived from active electromyography (EMG) (Wassermann et al. 2008). So as to be able to infer within hemisphere intracortical inhibition or facilitation, the paradigm of paired pulse TMS (pTMS) that delivers two stimuli from one coil at inter-stimulus intervals (ISI), is usually adopted. To indicate short interval intracortical inhibition (SICI), while delivered at short intervals, MEP suppression is purportedly mediated by GABAA synapses (Kujirai et al. 1993). On the other hand, long interval intracortical inhibition (LICI), while delivered at long intervals, MEP suppression is purportedly mediated by GABAB synapses (Ziemann et al. 1996a, b). pTMS can also be applied to assess intracortical facilitation (ICF), which is likely to involve NMDA-mediated receptor activity from excitatory glutamatergic transmission (Ziemann et al. 1998). Lastly, to assess sensory-motor integration occurring within the cortical motor strip, one may apply the afferent-mediated inhibition that pairs electrical stimulation of a digital or peripheral nerve with TMS made in the contralateral M1 (Turco et al. 2018a, b). This time mediated by cholinergic (DiLazzaro et al. 2000) and GABAA transmission (Di Lazzaro et al. 2007; Turco et al. 2018a, b), the parameter of short- latency afferent inhibition (SAI) at intervals of ~ 20 ms, leads to an inhibitory modulation of the M1, stemming from thalamocortical projections or directly from the somatosensory cortex (Di Lazzaro et al. 2007; Tokimura et al. 2000; Turco et al. 2018a, b). Again, with the GABAA-mediated transmission (Turco et al. 2018a, b), the long-latency afferent inhibition (LAI) adopts ISIs of ~ 100—200 ms, indicating activity in cortico-cortical pathways (i.e. the M1 and the primary and secondary somatosensory areas). Certainly, other TMS protocols exist, but the aforementioned paradigms are the ones commonly employed.
Historically, intracortical inhibition was believed to be a phenomenon driven by the GABAergic projections to the premotor and motor cortex. Ridding in (1995) introduced in his thesis the technique of pTMS applied with short (2–7 ms) and middle (10–15 ms) inter-stimulus intervals (ISI). The seminal experiment was published in the same year, and the consensus paper, which petrified the short ISI pTMS paradigms was presented in 2008 (Berardelli et al. 2008). However, some experiments also used the ISI ranges of 3–7 ms and 10–20 ms (Kaňovský et al. 2003). Nevertheless, practically all these experiments brought an evidence, that the intracortical inhibition is abnormal in dystonia, and that this abnormality might be lateralized. It has been even shown, that the lateralization of abnormal cortical excitability (examined by the SEP recordings) and intracortical inhibition (examined by short ISI pTMS) probably share the side pattern, i.e., that both abnormalities are present at the same time and in the same cortical area (Kaňovský et al. 2003). Along with the short ISI pTMS, other TMS modalities were used to study the phenomenon of intracortical inhibition in dystonia, namely the CSP, LICI (100–400 ms) pTMS and the rather non-specific intra-hemispheric inhibition (Amadio et al. 2000; Ridding et al. 2000; Beck et al. 2009; Samargia et al. 2014; Udupa and Chen 2019). An interesting feature of intracortical inhibition in dystonia is the “surround inhibition” (Beck et al. 2009), which represents a motor cortex mechanism on how to proceed with the targeted movement in the absence of activity of the muscles; albeit, not involved in the task. Physiologically, it probably corresponds to the “gating” of cortical SEP components during the motor task (Kaňovský et al. 2003). This “surround inhibition” is abnormal (decreased) in dystonia, and in the neurophysiological laboratory, it can be examined using different paradigms of short ISI pTMS.
Systematic study on the cortical neurophysiology of primary isolated dystonia and non-dystonic adults
From the clinico-genetic standpoint, monogenic forms of dystonia can be dichotomized to primary isolated dystonias (Domingo et al. 2020) and combined dystonias (Weissbach et al. 2020). Idiopathic forms of isolated dystonia (IID) are likewise encountered in the clinics, and shall constitute the subject of a neurophysiologic systematic publication cited thenceforth. McCambridge and Bradnam (2020) recently did the first meta-analysis of TMS cortical neurophysiology outcomes that compared patients with IID versus healthy individuals. Out of 78 studies, 57 studies met inclusion criteria for individuals with focal hand dystonia (FHD), cervical dystonia (CD), blepharospasm (BLP) and spasmodic dysphonia (SD). Overall, the CSP, SICI, and afferent-induced inhibition were reduced in IID compared to controls. Excerpts from data and interpretation are as follows:
-
1.
CSP: A high effect size, i.e., shorter CSP duration in IID versus controls. The reduction in GABAB-mediated inhibition, determined through shorter CSP durations were not different between the dystonia sub-types investigated (CD, FHD, SD, BLP). Within M1 reduced GABAB-mediated inhibition could be related to a widespread neural network dysregulation, eventually affecting excitation and inhibition in M1 balance.
-
2.
SICI: A moderate effect size, i.e., reduced SICI in IID compared to controls. The reduction in GABAA-mediated inhibition was determined through reduced SICI in the local hand muscle cortical representations of FHD only; but were not found in remote hand muscle representations of CD or SD sub-types. It is possible that deficits in GABAA-mediated inhibition through reduced SICI may only occur in analogous dystonic body region cortical representations of the dystonic muscles. Further evidences to date in this regard leaves much to be desired therefore, to arrive at a robust conclusion.
-
3.
Afferent-induced inhibition: A moderate effect size, i.e., reduced afferent-induced inhibition in IID compared to controls. This was hinged on a reduced afferent-induced inhibition in local and remote hand/forearm muscle cortical representations in IID compared to controls. This abnormal sensorimotor integration showing reduced afferent-induced inhibition is not unusual, given that cortical processing of sensory information is abnormal in dystonia. As has been reported in dystonia, because of the abnormal sensorimotor control, there may be impairments in proprioception, oculomotor control, spatial and temporal perception or even impaired sensation. Occurrence of a “sensory trick” (geste antagoniste) may also imply an abnormal reliance on sensorimotor networks, and thus another pathophysiologic construct for the reduction/abolition of dystonic contraction. Focusing on IID mechanisms that lead to reduced afferent-induced inhibition may be a potential way for novel therapeutic targets and drive future research regarding sensorimotor symptom alleviation.
-
4.
Other TMS findings: Perhaps driven by research methodological variations, thus to be interpreted with caution are: (a) intracortical facilitation—glutamatergic facilitation within M1, measured by ICF, revealed no differences between IID and controls, and (b) assessed through CME or motor thresholds, the net corticomotor excitability or intrinsic membrane excitability remains normal in IID. The data thus far suggest normal neurophysiological processes in a dystonic brain, based on the aforementioned glutamatergic facilitation, net corticomotor excitability, and intrinsic membrane excitability TMS studies.
Neurophysiological evidence of cerebellar involvement in dystonia
The role of cerebellum in the pathophysiology of dystonia has been noted almost 60 years ago, and it is not surprising that it was Irving Cooper (1965) who hypothetized about the disruption of the pathological cerebello-thalamo-cortical circuits during stereotactic surgery performed for the treatment of dystonia. However, it was not until 1969 that beyond the cerebellar cortex, the deep cerebellar nuclei were alluded to as “key players” in the process (Heimburger 1969). Later and recent structural imaging studies, point also to cerebellar lesions that may cause the focal dystonia (LeDoux and Brady 2003; Jinnah and Hess 2006; Draganski et al. 2003; Delmaire et al. 2007; Obermann et al. 2007; Corp et al. 2019). Neurophysiological evidence of purely cerebellar abnormalities in patients suffering from focal dystonia (“eyeblink conditioning”) were for the first time published by Teo et al. (2009). The neurophysiological correlates of cerebellar dystonia have been summarized several years later when the hypothesis of the cerebellar mechanism leading to dystonia was presented in that work (Shakkottai 2014). Nowadays, the important role of cerebellum, mainly the cerebellar cortex, in the development of dystonia and its accompanying features, including tremor associated with dystonia (TAWD) have been incorporated in the pathophysiological constructs, despite some evolving debates derived from neurophysiological experiments in humans (Katschnig-Winter et al. 2014; Sadnicka et al. 2012; 2014a, b; 2015; Filip et al. 2017; Bareš and Filip 2018; Hvizdošová et al. 2020, see also Fig. 3). In short, the neurophysiological evidence of direct cerebellar input to the development of dystonia and the abnormal functioning of cerebellum might include abnormal eyeblink conditioning, abnormal visuospatial processing and reduced cerebellar influence on motor cortex excitability (Teo et al. 2009; Filip et al. 2017; Popa et al. 2018).
Figure illustrates decreased functional connectivity of the a left cerebellar lobule VI and b left cerebellar crus I in cervical dystonia patients in comparison with healthy volunteers (P < 0.05 family-wise error-corrected at the cluster level, threshold T = 3.27) using functional magnetic resonance imaging according to Filip et al. (2017). Panel A shows reduced connectivity with the right dorsolateral prefrontal cortex in cervical dystonia (CD) versus healthy volunteers (HV; group comparison CD < HV) and reduced connectivity with the angular gyrus and bilateral basal ganglia in CD patients versus HV during the time estimation task (Interaction Group x Condition). Panel B shows decreased connectivity with the left cerebellar lobule VIIb and VIII and the left middle temporal gyrus (group comparison CD < HV). Right is right according to neurological convention. For more details, see Filip et al. (2017)
Brain plasticity, neurophysiology and neuroimaging
Compatible with the view that dystonia is a network disorder, the majority of patients in routine clinical imaging studies lack overt lesions. However, experimental imaging methods involving positron emission tomography (PET) or MRI have revealed subtle quantitative abnormalities for many types of dystonia. MRI-based studies have involved multiple imaging techniques, including voxel-based morphometry (VBM), diffusion tensor imaging (DTI), functional MRI (fMRI), designed either as task-related fMRI or as resting state fMRI (rsMRI). These imaging studies have been reviewed several times (Neychev et al. 2011; Léhericy et al. 2013; Ramdhani and Simonyan 2013; Zheng et al. 2012). A number of conclusions emerged: (a) Functional abnormalities can be detected in multiple brain regions including the basal ganglia, cerebellum, cerebral Cortex, and others; (b) Connectivity between these regions is often abnormal; (c) The regions affected across these studies are similar, but not always consistent; (d) It is challenging to discriminate regions that cause dystonia from regions that may show secondary changes (Jinnah et al. 2017), and (e) There is marked discrepancy between the observed functional differences and the lack of consistent structural abnormalities (Gracien et al. 2019).
Some fMRI studies showed significant treatment‐related changes in the sensorimotor network in patients with CD receiving long‐term treatment with BoNT. fMRI studies using simple motor task showed activation changes in several brain areas, especially in SMA, cingulum, thalamus, secondary somatosensory cortex and in the central part of cerebellum close to the vermis (Opavský et al. 2011, Opavský et al. 2012, Nevrlý et al. 2018, see also Fig. 4). Several fMRI studies demonstrated changes in multiple rating state networks (e.g., Delnooz et al. 2013, 2015; Corp et al. 2019; Sarasso et al. 2020), which partly normalize with BoNT treatment, suggesting functional disruption of the motor control (Léhericy et al. 2013). Functional connectivity changes at either cortical or subcortical levels were demonstrated in these studies. The sensorimotor integration in the physiological perspective involves all parts of the motor and sensory system, including the motor circuits, in which the basal ganglia and the premotor and motor cortex are the principal components. Recently, it has been hypothesized that sensorimotor integration is a function of brain plasticity. Perhaps even along this line will modalities like contemporary and holistic neurorehabilitation practices may be set in place (Bradnam et al 2020).
Functional MRI activation map (transversal slices) in patients with cervical dystonia. Differences in activation 4 weeks after and before the first BoNT-A injection. Slices are labeled with Z/Y coordinate in standard MNI152 space. For more details, see Nevrlý et al. (2018)
Conclusion and future of clinical neurophysiology in dystonia
The complex neurophysiological examination in which all accessible and routine techniques (i.e.EMG, EP, EEG, TMS, among others) are used may help to confirm the diagnosis of dystonia in the case that phenomenology of given movement disorder is not typical. However, the discrimination between organic and psychogenic dystonia may also be a challenge as only few electrophysiological studies show differences in results from these two variants: the paired trancranial associative stimulation and the blink reflex recovery tests (Chen and Chen 2020). Combined neurophysiology and dynamic imaging studies, happening as we write, will lead to a robust understanding of dystonia, its types, its prognosis and evolution of better therapies; and these are despite the repeated imaging studies, which lead to some more controversies (Jinnah et al. 2017; Corp et al. 2019; Gracien et al. 2019).
Hinged on TMS: (a) future research directions in CD, SD and BLP should be able to assess cortical neurophysiology in local (dystonic) muscle representations, though posing a technical challenge, as it seems that some measures depend on the tested muscle cortical representation; (b) noting that voluntary movement may worsen dystonic contraction, measures of task-dependency also impact on cortical neurophysiology, and may be key areas to explore too, and (c) future dystonia interventions may potentially target deficient GABA-mediated inhibitory cortical circuits within M1. Therefrom, researches may likewise direct in future toward whether positive behavioral or clinical changes may happen in tandem.
References
Abbruzzese G, Pelosin E, Avanzino L (2015) Physiology of dystonia. In: Bhatia K, Rosales RL (eds) Kaňovský, P. Dystonia and dystonic syndromes. Springer-Verlag, Wien, pp 13–25
Alexander GE (1994) Basal ganglia-thalamocortical circuits: their role in control of movements. J Clin Neurophysiol 11:420–431
Amadio S, Panizza M, Pisano F et al (2000) Transcranial magnetic stimulation and silent period in spasmodic torticollis. Am J Phys Med Rehabil 79:361–368
Antelmi E, Erro R, Rocchi L et al (2017) Neurophysiological correlates of abnormal somatosensory temporal discrimination in dystonia. Mov Disord 32:141–148
Bareš M, Rektor I (2001) Basal ganglia involvement in sensory and cognitive processing. A depth electrode CNV study in human subjects. Clin Neurophysiol 112:2022–2030
Bareš M, Filip P (2018) Cerebellum and dystonia: The story continues. Will the patients benefit from new discoveries? Clin Neurophysiol 129:282–283
Beck S, Shamim EA, Richardson SP et al (2009) Inter-hemispheric inhibition is impaired in mirror dystonia. Eur J Neurosci 29:1634–1640
Berardelli A, Rothwell JC, Hallett M et al (1998) The pathophysiology of primary dystonia. Brain 121:1195–1212
Berardelli A, Abbruzzese G, Chen R et al (2008) Consensus paper on short-interval intracortical inhibition and other transcranial magnetic stimulation intracortical paradigms in movement disorders. Brain Stimul 1:183–191
Berman BD, Jinnah HA (2015) Dystonia: Five new things. Neurol Clin Pract 5:232–240
Bove M, Brichetto G, Abbruzzese G et al (2004) Neck proprioception and spatial orientation in cervical dystonia. Brain 127:2764–2778
Bradnam L, Barry C (2013) The role of the trigeminal sensory nuclear complex in the pathophysiology of craniocervical dystonia. J Neurosci 33:18358–18367
Bradnam LV, Meiring RM, Boyce M, McCambridge A (2020) Neurorehabilitation in dystonia: a holistic perspective. J Neural Transm. https://doi.org/10.1007/s00702-020-02265-0 ((Epub ahead of print. PMID: 33099684))
Brugger F, Peters A, Georgiev D et al (2019) Sensory trick efficacy in cervical dystonia is linked to processing of neck proprioception. Parkinsonism Relat Disord 61:50–56
Chen R (2000) Studies of human motor physiology with transcranial magnetic stimulation. Muscle Nerve 9(Suppl. 1):S26-32
Chen KS, Chen R (2020) Principles of electrophysiological assessments for movement disorders. J Mov Disord 13:27–38
Chen R, Corwell B, Hallett M (1999) Modulation of motor cortex excitability by median nerve and digit stimulation. Exp Brain Res 129:77–86
Chen JC, Macerollo A, Sadnicka A et al (2018) Cervical dystonia: Normal auditory mismatch negativity and abnormal somatosensory mismatch negativity. Clin Neurophysiol 129:1947–1954
Conte A, Berardelli I, Ferrazzano G et al (2016) Non-motor symptoms in patients with adult-onset focal dystonia: Sensory and psychiatric disturbances. Parkinsonism Relat Disord 22(Suppl 1):S111–S114
Cooper I (1965) Effect of thalamic lesions upon torticollis. N Engl J Med 270:567–572
Corp DT, Joutsa J, Darby RR et al (2019) Network localization of cervical dystonia based on causal brain lesions. Brain 142:1660–1674
Delmaire C, Vidailhet M, Elbaz A et al (2007) Structural abnormalities in the cerebellum and sensorimotor circuit in writer´s cramp. Neurology 69:378–380
Delnooz C, Pasman JW, Beckmann CF, a, (2013) Task-free functional MRI in cervical dystonia reveals multi-network changes that partially normalize with botulinum toxin. PLoS ONE 8:e62877
Delnooz C, Pasman JW, Beckmann CF et al (2015) Altered striatal and pallidal connectivity in cervical dystonia. Brain Struct Funct 220:513–523
Deuschl G, Toro C, Matsumoto J et al (1995) Movement-related cortical potentials in writer’s cramp. Ann Neurol 38:862–868
Devanne H, Lavoie BA, Capaday C (1997) Input-output properties and gain changes in the human corticospinal pathway. Exp Brain Res 114:329–338
Di Lazzaro V, Oliviero A, Profice P et al (2000) Muscarinic receptor blockade has differential effects on the excitability of intracortical circuits in the human motor cortex. Exp Brain Res 135:455–461
Di Lazzaro V, Pilato F, Dileone M et al (2007) Segregating two inhibitory circuits in human motor cortex at the level of GABAA receptor subtypes: A TMS study. Clin Neurophysiol 118:2207–2214
Domingo A, Yadav R, Ozelius LJ (2020) Isolated dystonia: clinical and genetic updates. J Neural Transm. https://doi.org/10.1007/s00702-020-02268-x
Draganski B, Thun-Hohenstein C, Bogdahn U et al (2003) “Motor circuit” gray matter changes in idiopathic cervical dystonia. Neurology 61:1228–1231
Dressler D, Saberi FA, Rosales RL (2020) Botulinum toxin therapy of dystonia. J Neural Transm. https://doi.org/10.1007/s00702-020-02266-z
Fève A, Bathien N, Rondot P (1994) Abnormal movement related potentials in patients with lesions of basal ganglia and anterior thalamus. J Neurol Neurosurg Psychiatry 57:100–104
Filip P, Gallea C, Lehéricy S et al (2017) Disruption in cerebellar and basal ganglia networks during a visuospatial task in cervical dystonia. Mov Disord 32:757–768
Frima N, Rome SM, Grünewald RA (2003) The effect of fatigue on abnormal vibration induced illusion of movement in idiopathic focal dystonia. J Neurol Neurosurg Psychiatry 74:1154–1156
Gracien R-M, Petrov F, Hok P et al (2019) Multimodal quantitative MRI reveals no evidence for tissue pathology in idiopathic cervical dystonia. Front Neurol 10:914
Grünewald RA, Yoneda Y, Shipman JM et al (1997) Idiopathic focal dystonia: a disorder of muscle spindle afferent processing? Brain 120:2179–2185
Hamano T, Kaji R, Katayama M et al (1999) Abnormal contingent negative variation in writer’s cramp. Clin Neurophysiol 110:508–515
Han-Joon K, Jeon B (2020) Arching deep brain stimulation in dystonia types. J Neural Transm. https://doi.org/10.1007/s00702-020-02272-1
Heimburger RF (1969) The role of the cerebellar nuclei in dyskinetic disorders. Confin Neurol 31:57–69
Hendrix CM, Vitek JL (2012) Toward a network model of dystonia. Ann NY Acad Sci 1265:46–55
Hvizdošová L, Nevrlý M, Otruba P et al (2020) The prevalence of dystonic tremor and tremor associated with dystonia in patients with cervical dystonia. Sci Rep 10:1436
Ikeda A, Shibasaki H, Kaji R et al (1996) Abnormal sensorimotor integration in writer’s cramp: study of contingent negative variation. Mov Disord 6:683–690
Jinnah HA, Albanese A (2014) The new classification system for the dystonias: why was it needed and how was it developed? Mov Disord Clin Pract 1:280–284
Jinnah HA, Hess EJ (2006) A new twist on the anatomy of dystonia: the basal ganglia and the cerebellum? Neurology 67:1740–1741
Jinnah HA, Neychev V, Hess EJ (2017) The anatomical basis for dystonia: the motor network model. Tremor Other Hyperkinet Mov (N Y) 7:506
Kaji R, Ikeda A, Ikeda T et al (1995a) Physiological study of cervical dystonia. Task- specific abnormality in contingent negative variation. Brain 118:511–522
Kaji R, Rothwell JC, Katayama M et al (1995b) Tonic vibration reflex and muscle afferent block in writer’s cramp. Ann Neurol 38:155–162
Kamble NL, Pal PK (2016) Electrophysiological evaluation of psychogenic movement disorders. Parkinsonism Relat Disord 22(Suppl 1):S153–S158
Kaňovský P (2002) Dystonia: a disorder of motor programming or motor execution? Mov Disord 17:1143–1147
Kaňovský P, Rosales RL (2011) Debunking the pathophysiological puzzle of dystonia– with special reference to botulinum toxin therapy. Parkinsonism Relat Disord 17(Suppl1):S11–S14
Kaňovský P, Streitová H, Dufek J et al (1997) Lateralization of the P22/N30 component of somatosensory evoked potentials of the median nerve in patients with cervical dystonia. Mov Disord 12:553–560
Kaňovský P, Streitová H, Dufek J et al (1998) Change in lateralization of the P22/N30 cortical component of median nerve somatosensory evoked potentials in patients with cervical dystonia after successful treatment with botulinum toxin A. Mov Disord 13:108–117
Kaňovský P, Streitová H, Dufek J et al (1999a) Lateralization of the P22/N30 precentral cortical component of the median nerve somatosensory evoked potentials is different in patients with a tonic or tremulous form of cervical dystonia. Mov Disord 14:642–651
Kaňovský P, Bareš M, Rektor I (1999b) The selective gating of the N30 cortical component of the somatosensory evoked potentials of median nerve is different in the mesial and dorsolateral frontal cortex: evidence from intracerebral recordings. Clin Neurophysiol 114:981–991
Kaňovský P, Bareš M, Streitová H et al (2003) Abnormalities of cortical excitability and cortical inhibition in cervical dystonia Evidence from somatosensory evoked potentials and paired transcranial magnetic stimulation recordings. J Neurol 250:42–50
Kaňovský P, Bhatia K, Rosales RL (eds) (2015) Dystonia and dystonic syndromes. Springer, Wein. ISBN 978-3-7091-1516-9
Katschnig-Winter P, Schwingenschuh P, Davare M et al (2014) Motor sequence learning and motor adaptation in primary cervical dystonia. J Clin Neurosci 21:934–938
Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD (1993) Corticocortical inhibition in human motor cortex. J Physiol 471:501–519
LeDoux MS, Brady KA (2003) Secondary cervical dystonia associated with structural lesions of the central nervous system. Mov Disord 18:60–69
Léhericy S, Tijssen MA, Vidailhet M et al (2013) The anatomical basis of dystonia: current view using neuroimaging. Mov Disord 28:944–957
Lim VK, Bradshaw JL, Nicholls ME et al (2004) Abnormal sensorimotor processing in pianists with focal dystonia. Adv Neurol 94:267–273
Macerollo A, Chen JC, Parees I et al (2016) Abnormal movement-related suppression of sensory evoked potentials in upper limb dystonia. Eur J Neurol 23:562–568
Macerollo A, Brown MJN, Kilner JM et al (2018) Neurophysiological changes measured using somatosensory evoked potentials. Trends Neurosci 41:294–310
Mazzini L, Zaccala M, Balzarini C (1994) Abnormalities of somatosensory evoked potentials in spasmodic torticollis. Mov Disord 9:426–430
McCambridge AB, Bradnam LV (2020) Cortical neurophysiology of primary isolated dystonia and non-dystonic adults: A meta-analysis. Eur J Neurosci 29:10. https://doi.org/10.1111/ejn.14987 ((Epub ahead of print. PMID: 32991762.))
Menšíková K, Kaiserová M, Vaštík M et al (2020) The long-term effect of continuous subcutaneous apomorphine infusions on camptocormia in Parkinson’s disease. Parkinsonism Relat Disord 75:14–16
Murase N, Kaji R, Shimazu H et al (2000) Abnormal premovement gating of somatosensory input in writer’s cramp. Brain 123:1813–1829
Nevrlý M, Hluštík P, Hok P et al (2018) Changes in sensorimotor network activation after botulinum toxin type A injections in patients with cervical dystonia: a functional MRI study. Exp Brain Res 236:2627–2637
Neychev VK, Gross R, Lehericy S et al (2011) The functional neuroanatomy of dystonia. Neurobiol Dis 42:185–201
Obermann M, Yaldizli O, De Greiff A et al (2007) Morphometric changes in sensorimotor structures in focal dystonia. Mov Disord 22:1117–1123
Oku T, Furuya S (2019) Neuromuscular incoordination in musician’s dystonia. Parkinsonism Relat Disord 65:97–104
Opavský R, Hluštík P, Otruba P et al (2011) Sensorimotor network in cervical dystonia and the effect of botulinum toxin treatment: a functional MRI study. J Neurol Sci 306:71–75
Opavský R, Hluštík P, Otruba P et al (2012) Somatosensory cortical activation in cervical dystonia and its modulation with botulinum toxin: an fMRI study. Int J Neurosci 122:45–52
Oyama G, Hattori N (2020). New Modalities and Directions for Dystonia Care. J Neural Transm (accepted)
Pohl C, Happe J, Klockgether T (2002) Cooling improves the writing performance of patients with writer’s cramp. Mov Disord 17:1341–1344
Popa T, Hubsch C, James P et al (2018) Abnormal cerebellar processing of the neck proprioceptive information drives dysfunctions in cervical dystonia. Sci Rep 8:2263
Prudente CN, Hess EJ, Jinnah HA (2014) Dystonia as a network disorder: what is the role of the cerebellum? Neuroscience 260:23–35
Ramdhani RA, Simonyan K (2013). Primary dystonia: conceptualizing the disorder through a structural brain imaging lens. Tremor Other Hyperkinet Mov (N Y) 3:tre-03–152–3638–4
Rektor I, Kaňovský P, Bareš M et al (2001a) Event-related potentials, CNV, readiness potential, and movement accompanying potential recorded from posterior thalamus in human subjects. A SEEG study. Neurophysiol Clin 31:253–261
Rektor I, Bareš M, Kaňovský P et al (2001b) Intracerebral recording of readiness potential induced by a complex motor task. Mov Disord 16:698–704
Rektor I, Bareš M, Kubová D (2001c) Movement-related potentials in the basal ganglia: a SEEG readiness potential study. Clin Neurophysiol 112:2146–2153
Rektor I, Bareš M, Brázdil M et al (2005) Cognitive- and movement-related potentials recorded in the human basal ganglia. Mov Disord 20:562–568
Ridding MC, Sheean G, Rothwell JC et al (1995) Changes in the balance between motor cortical excitation and inhibition in focal, task specific dystonia. J Neurol Neurosurg Psychiatry 59:493–583
Ridding MC, Brouwer B, Nordstrom MA (2000) Reduced interhemispheric inhibition in musicians. Exp Brain Res 133:249–253
Rosales RL, Dressler D (2010) On muscle spindles, dystonia and botulinum toxin. Eur J Neurol 17(Suppl 1):71–80
Rosales RL, Dressler D (2016) Botulinum toxin type A therapy in dystonia and spasticity - what are current practical applications? In: Rosales RL, Dressler D (eds) Botulinum toxin therapy manual for dystonia and spasticity. Intech Open Access Publishers, Rijeka. ISBN 978-953-51-2851-9
Rothwell JC (1995) The physiology of dystonia. Marcel Dekker, New York
Sadnicka A, Hoffland BS, Bhatia KP et al (2012) The cerebellum in dystonia—help or hindrance? Clin Neurophysiol 123:65–70
Sadnicka A, Hamada M, Bhatia KP et al (2014a) Cerebellar stimulation fails to modulate motor cortex plasticity in writing dystonia. Mov Disord 29:1304–1307
Sadnicka A, Patani B, Saifee TA et al (2014b) Normal motor adaptation in cervical dystonia: a fundamental cerebellar computation is intact. Cerebellum 13:558–567
Sadnicka A, Teo JT, Kojovic M et al (2015) All in the blink of an eye: new insight into cerebellar and brainstem function in DYT1 and DYT6 dystonia. Eur J Neurol 22:762–767
Samargia S, Schmidt R, Kimberley TJ (2014) Shortened cortical silent period in adductor spasmodic dysphonia: evidence for widespread cortical excitability. Neurosci Lett 560:12–15
Sarasso E, Agosta F, Piramide N, Bianchi F, Butera C, Gatti R, Amadio S, Del Carro U, Filippi M (2020) Sensory trick phenomenon in cervical dystonia: a functional MRI study. J Neurol 267:1103–1115
Shakkottai VG (2014) Physiologic changes associated with cerebellar dystonia. Cerebellum 13:637–644
Shakkottai VG, Batla A, Bhatia K et al (2017) Current opinions and areas of consensus on the role of the cerebellum in dystonia. Cerebellum 16:577–594
Supnet ML, Rosales RL (2018) Indirect central nervous effects of botulinum toxin. In: Dressler D, Altenmüller E, Krauss JK (eds) Treatment of Dystonia. Cambridge University Press, Cambridge. ISBN 978-1-107-13286-3
Swash M, Fox KP (1976) Normal muscle spindles in idiopathic torsion dystonia. J Neurol Sci 27:525–527
Teo JT, van de Warrenburg BP, Schneider SA et al (2009) Neurophysiological evidence for cerebellar dysfunction in primary focal dystonia. J Neurol Neurosurg Psychiatry 80:80–83
Tinazzi M, Frasson E, Polo A et al (1999a) Evidence for an abnormal cortical sensory processing in dystonia: selective enhancement of lower limb P37–N50 somatosensory evoked potential. Mov Disord 14:473–480
Tinazzi M, Frasson E, Bertolasi L et al (1999b) Temporal discrimination of somesthetic stimuli is impaired in dystonic patients. NeuroReport 10:1547–1550
Tinazzi M, Fiaschi A, Frasson E et al (2002) Deficits of temporal discrimination in dystonia are independent from the spatial distance between the loci of tactile stimulation. Mov Disord 17:333–338
Tokimura H, Di Lazzaro V, Tokimura Y et al (2000) Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol 523:503–513
Toro C, Deuschl G, Hallett M (2000) Movement-related electroencephalographic desynchronization in patients with hand cramps: evidence for motor cortical involvement in focal dystonia. Ann Neurol 2000(47):456–461
Tseng YJ, Chen RS, Hsu WY et al (2014) Reduced motor cortex deactivation in individuals who suffer from writer’s cramp. PLoS ONE 9:e97561
Turco CV, El-Sayes J, Locke MB, Chen R, Baker S, Nelson AJ (2018a) Effects of lorazepam and baclofen on short- and long-latency afferent inhibition. J Physiol 596:5267–5280
Turco CV, El-Sayes J, Savoie MJ, Fassett HJ, Locke MB, Nelson AJ (2018b) Short- and long-latency afferent inhibition; uses, mechanisms and influencing factors. Brain Stim 11:59–74
Udupa K, Chen R (2019) Motor cortical circuits in Parkinson disease and dystonia. Handb Clin Neurol 161:167–186
Van der Kamp W, Rothwell JC, Thompson PD et al (1995) The movement- related cortical potential is abnormal in patients with idiopathic torsion dystonia. Mov Disord 10:630–633
Wasserman E, Epstein CM, Ziemann U, Wassermann EM (2008) The Oxford handbook of transcranial stimulation. Oxford University Press, Oxford. ISBN 978-0-19-856892-6
Weissbach A, Saranza G, Domingo, (2020) Combined dystonias: clinical and genetic updates. J Neural Transm. https://doi.org/10.1007/s00702-020-02269-w
Zeuner KE, Peller M, Knutzen A et al (2009) Slow pre-movement cortical potentials do not reflect individual response to therapy in writer’s cramp. Clin Neurophysiol 120:1213–1219
Zheng Z, Pan P, Wang W, Shang H (2012) Neural network of primary focal dystonia by an anatomic likelihood estimation meta-analysis of gray matter abnormalities. J Neurol Sci 316:51–55
Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W (1996a) Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol 40:367–378
Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W (1996b) The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res 109:127–135
Ziemann U, Chen R, Cohen LG, Hallett M (1998) Dextromethorphan decreases the excitability of the human motor cortex. Neurology 51:1320–1324
Acknowledgements
Drs. Nevrly, Hvizdosova, Hlustik and Kanovsky were supported by the grant of AZV-MH CR No. 16-30210A. UST´s Professor Raymond Rosales had been officialy granted the academic and clinical Visiting Professor status of the Palacky University in Olomouc, Czech Republic.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaňovský, P., Rosales, R., Otruba, P. et al. Contemporary clinical neurophysiology applications in dystonia. J Neural Transm 128, 509–519 (2021). https://doi.org/10.1007/s00702-021-02310-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-021-02310-6