Abstract
Transcranial direct current stimulation (tDCS) has been used to reduce pain in range of chronic pain states. The aim of this review is to evaluate the effectiveness of tDCS on pain reduction and related disability in patients with non-specific chronic low back pain (CLBP). A computer-based systematic literature search was performed in five databases according to PRISMA guidelines. Randomized controlled trials (RCTs) that assessed the effects of tDCS on pain and related disability in patients with non-specific CLBP were included. Modified Jadad scale and Cochrane's risk of bias assessment were used to determine the studies’ quality and risk of bias. Meta-analyses were performed by calculating the standardized mean difference (SMD) at 95% confidence interval (CI). Nine RCTs (411 participants) were included in the systematic review according to inclusion criteria, while only five studies could be included in the meta-analysis. The primary motor cortex (M1) was the main stimulated target. The meta-analysis showed non-significant effect of multiple sessions of tDCS over M1 on pain reduction and disability post-treatment respectively, (SMD = 0.378; 95% CI = − 0.264–1.020; P = 0.249), (SMD = 0.143; 95% CI = − 0.214–0.499; P = 0.434). No significant adverse events were reported. The current results do not support the clinical use of tDCS for the reduction of pain and related disability in non-specific CLBP. However, the limited number of available evidence limits our conclusions on the effectiveness of these approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Low back pain (LBP) is the major cause of years lived with disability worldwide (Roth et al. 2017). The pathophysiology of non-specific chronic low back pain (CLBP) is not fully understood, but different causes have been proposed. Unlike acute low back pain, a peripheral cause is often absent in non-specific CLBP, and central mechanisms have been hypothesised to explain the development and maintenance of pain (Latremoliere and Woolf 2009; Woolf 2011). Indeed, existing data have recognized a broad range of changes in both structure and function of different brains areas (Apkarian et al. 2009; Tracey and Bushnell 2009; Baliki et al. 2011). Accordingly, imaging studies in patients with CLBP reported reductions in cortical grey matter density in the dorsolateral prefrontal cortex (DLPFC), the right posterior thalamus and the middle cingulate cortex (Apkarian et al. 2004; Ivo et al. 2013). Moreover, CLBP patients have significantly lower increases in blood flow in the periaqueductal grey (PAG) than controls when exposed to equally painful stimuli, suggesting a decreased pain descending inhibition (Giesecke et al. 2006).
Though inconsistently, other brain imaging studies support the hypothesis of decreased activation of motor cortex (M1), anterior cingulate cortex, prefrontal cortex (PFC) and nucleus accumbens in chronic pain (Baliki et al. 2006, 2011; Apkarian et al. 2009; Tracey and Bushnell 2009; Burns et al. 2016; Konno and Sekiguchi 2018).
A variety of conservative and pharmacological strategies for CLBP management showed a significant effect in reducing pain and related disability (Chou et al. 2017a, b). However, these strategies are associated with small to moderate, primarily short-term effects on CLBP, thus suggesting the need for improvement (Chou et al. 2017a, b). This is not surprising, indeed, because by definition, the underlying pathology of non-specific CLBP is “unidentified”, suggesting that different causes could concur to symptoms. Then, clinicians should apply a precision-medicine-like approach by selecting an appropriate intervention for each individual patient, supposed that analgesic effects and acceptability of for each treatment are known. With this aim, Thompson et al. 2020 have recently proposed a protocol to perform a network meta-analysis (assessing multiple competing interventions by synthesizing data across a network of different treatments) to determine the relative efficacy and acceptability of primary care treatments for non-specific CLBP (Thompson et al. 2020). In this context, there is growing interest to treat chronic pain by means of invasive and non-invasive brain stimulation (Luedtke et al. 2012b; O’Connell et al. 2018). Transcranial direct current stimulation (tDCS) is a new adjunctive intervention that can modulate cortical excitability through positively or negatively charged currents, so it can modulate a wide neural network involved in pain processing (Luedtke et al. 2012b; Antal et al. 2017; O’Connell et al. 2018). The use of tDCS has been extensively investigated in different diseases such as stroke, Parkinson's disease, mental illness, and chronic pain (da Silva et al. 2013; Shigematsu et al. 2013; Wu et al. 2013; Ngernyam and Jensen 2014; Lefaucheur et al. 2017; Ricci et al. 2019). Different systematic reviews and meta-analysis have shown that tDCS induces a significant analgesic effect (Luedtke et al. 2012b; Ngernyam and Jensen 2014; O’Connell et al. 2018). Furthermore, previous studies using anodal tDCS over M1 reported a significant pain reduction and improvement in mood and quality of life (QoL) in patients with CLBP (Mendonca et al. 2016; Hazime et al. 2017). Additionally, tDCS improves emotional appraisal of pain, descending pain inhibition and modulation of the endogenous opioid system (Garcia-Larrea and Peyron 2007; Pagano et al. 2011; DosSantos et al. 2012).
Altogether, the different changes in the brain structure and function in CLPB patients create a rational basis for the use of tDCS to improve the related symptoms of CLBP. Previous studies showed encouraging results regarding the effect of tDCS on chronic pain. However, to the best of our knowledge, the effects of tDCS on non-specific CLBP are still uncertain and only one systematic review in 2019, based on two studies exploring low back pain and tDCS, concluded a Level A recommendation against the use of M1 tDCS for LBP (Baptista et al. 2019). Therefore, the objective of this review was to assess the effectiveness of tDCS for pain reduction and related disability in patients with non-specific CLBP.
Methods
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses reporting guideline (Liberati et al. 2009).
We followed the PICOS framework to organize the inclusion criteria. Population (P): studies that recruited adult participants with CLBP affected for longer than 3 months; intervention (I): anodal or cathodal tDCS applied alone or in combination with conventional intervention; comparison (C): sham tDCS control or non-interventional control; outcomes (O): the primary outcome is related to pain intensity and the secondary outcomes include quality of life and disability; and study design (S): randomized controlled trials (RCTs) published in English language. Since the aim of this review was to investigate the available literature concerning the effect of tDCS on pain and related disability in non-specific CLBP, we decided not to pre-specify a minimal number of patients per treatment group and a minimal follow-up duration to enrol all the available literature meeting our search criteria.
Studies meeting any of the following criteria were excluded: (1) Studies including participants with chronic pain conditions such as (neuropathic pain or fibromyalgia) other than CLBP; (2) Studies used surgically implanted brain stimulators and/or repetitive transcranial magnetic stimulation; (3) studies published as conference abstracts, dissertations, or in books; and (4) studies without sufficient data to enable pooling of data.
Data search and study selection
A comprehensive systematic search of Medline, EMBASE, Scopus, Web of Science and Cochrane Central Register of Controlled Trials. We searched for articles published from the first date available to January 1st 2020. The following keywords were searched: ‘transcranial direct current stimulation,’ ‘tDCS,’ ‘Electrical Stimulation Transcranial,’ ‘low back pain,’ ‘chronic low back pain,’ ‘nonspecific chronic low back pain,’ ‘LBP,’ ‘NSLBP.’ Search strategies were developed for each database using both free-text terms and the Medical Subject Headings (MeSH). The reference lists of relevant articles were screened for potential related articles. The database search strategy used are listed in the Appendix 1. Study inclusion was decided independently by two authors (F.L. and M.D.) based on the inclusion criteria.
Data extraction
Two authors (RC and EC) independently extracted the following data: (i) demographic characteristics including sample size, age and trial design, (ii) stimulation parameters (site of stimulation, duration, intensity, and mode), (iii) the control paradigm used (placebo/sham/no intervention), and the nature of outcome measures. Then, the extracted data were entered into a predesigned data extraction table. To facilitate combination of results in a meta-analysis, it was required that pain measurements [means and standard deviations at baseline and post-intervention, change over time and standard errors, or confidence intervals (CI) for mean values or change over time] were reported. Disagreements were resolved through discussion or, if required, adjudication by a third author (S.N.). In case the original data was unclear or lacking adequate data, the researchers attempted to contact the corresponding authors to provide missing data.
Risk of bias and quality assessment
Two authors (M. A. and M. E.) analysed the methodological quality of the studies using the modified Jadad scale (Chalmers et al. 1981; Jadad et al. 1996) and the Cochrane's risk of bias assessment (Higgins 2011).
The modified Jadad scale score ranges from 1 to 8; points are awarded if study: is described as randomized, 1 point; has appropriate randomization method, 1 point; is described as subject-blinded, 1 point; is described as evaluator-blinded, 1 point; and has description of withdrawals and dropouts, 1 point; presented the inclusion/exclusion criteria, 1 point; described the adverse effects, 1 points; and described statistical analysis, 1 point. Studies with a modified Jadad score ≥ 4 were considered to be high-quality randomized controlled trials (RCTs) (Jadad et al. 1996).
The risk of bias assessment appraises a study in six domains: adequate sequence generation, allocation concealment, blinding of participants, personnel and outcome assessors, incomplete outcome data, selective outcome reporting and other sources of bias. Each domain can be rated as “yes” (low risk of bias), “no” (high risk of bias), or “unclear” (uncertain risk) (Higgins 2011).
Data synthesis and meta-analysis
Meta-analyses were carried out using the comprehensive meta-analysis, version 2.2.064 software package (Biostat, Englewood, New Jersey, USA). Standardized mean difference (SMD), 95% confidence interval (CI), and P value were calculated by the comparing the change in the included outcomes between the real tDCS and the sham tDCS using random-effect model of analysis (Muller and Cohen 1989; Borenstein et al. 2005). Heterogeneity in treatment effect was examined by calculating I2 index (Higgins 2011). The level of significant was set at P of up to 0.05 for the SMD and heterogeneity.
Results
Study selection
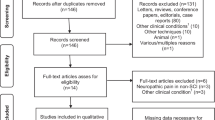
Nine studies were included (Table 1). Databases searches provided 182 publications. After adjusting for duplication, 84 had been removed. On the basis of the title and abstract, 30 articles were excluded; 13 articles were excluded because of the included participants or intervention, and 17 studies because of the study design. Of the remaining 68, 59 articles did not meet the inclusion criteria. Finally, nine trials met the inclusion criteria for review and five articles for meta-analysis. A flowchart illustrating the selection process is shown in (Fig. 1).
Study and participant characteristics
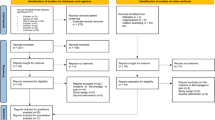
Nine RCTs met the criteria for inclusion in this systematic review (Table 1). No additional studies met the inclusion criteria upon searching the reference list of the included studies. The included studies constituted a total of 411 participants with a mean age between 30 and 63 years. Random allocation of participants was in either a parallel (n = 7) (O’Connell et al. 2013; Luedtke et al. 2015; Hazime et al. 2017; Straudi et al. 2018; Jafarzadeh et al. 2019; Mariano et al. 2019; Jiang et al. 2020) or crossover design (n = 2) (Luedtke et al. 2012a; Schabrun et al. 2014) with 168 participants receiving real tDCS. The study quality and the risk of bias of the nine included studies are summarizes in (Table 1 and Fig. 2). All the included studies were rated as high quality RCT (modified Jadad scores ≥ 4), except one study (modified Jadad scores ≤ 4) (Schabrun et al. 2014). All the nine studies were reported as randomized trials, but in three studies (Luedtke et al. 2012b; Schabrun et al. 2014; Jafarzadeh et al. 2019), the appropriate randomization method was not described. Two Studies (Luedtke et al. 2012b; Schabrun et al. 2014) did not report the randomization method. All studies, except Study (Schabrun et al. 2014), had adequate description of dropouts (Table 1).
Four of the nine studies (Luedtke et al. 2012b; Schabrun et al. 2014; Hazime et al. 2017; Jafarzadeh et al. 2019) had at least one domain rated as high risk of bias despite having a modified Jadad score ≥ 4. Only five studies had low risk of bias in all domains (O’Connell et al. 2013; Luedtke et al. 2015; Straudi et al. 2018; Mariano et al. 2019; Jiang et al. 2020). In three studies (Luedtke et al. 2012b; Schabrun et al. 2014; Hazime et al. 2017), neither the participants nor the evaluators were blinded, hence there was a high risk of bias in blinding. Although one study (Hazime et al. 2017) reported using a double blind study design, it was not truly double-blinded; low risk in subject blinding, but it was an unclear risk in evaluator-blinding because the assessors might not be blind to treatment allocation (Fig. 2).
Interventions
Anodal tDCS was delivered at a current density of 1 mA in one study (Schabrun et al. 2014), or 2 mA for six studies (O’Connell et al. 2013; Luedtke et al. 2015; Hazime et al. 2017; Jafarzadeh et al. 2019; Jiang et al. 2020). Cathodal tDCS was administered at an intensity of 2 mA for one study (Mariano et al. 2019), while one study used anodal or cathodal at current density of 1 mA for 2 separated groups (Luedtke et al. 2012a). The target sites were (1) primary motor cortex contralateral to the side of the pain complaint (M1; corresponding to C3 on the 10–20 system for electrode placement in EEG) (O’Connell et al. 2013; Schabrun et al. 2014; Hazime et al. 2017; Straudi et al. 2018; Jiang et al. 2020) with the cathode placed over contralateral supraorbital (O’Connell et al. 2013; Schabrun et al. 2014; Straudi et al. 2018; Jiang et al. 2020) or ipsilateral supraorbital (Hazime et al. 2017); (2) left motor cortex (Luedtke et al. 2012a, 2015; Jafarzadeh et al. 2019) and the cathode placed over the contralateral supraorbital (Luedtke et al. 2015; Jafarzadeh et al. 2019) or right orbita; (3) cathodal electrode over the left dorso anterior cingulate cortex (dACC) and the anode electrode was placed over the contralateral mastoid process (Mariano et al. 2019). The single pulse transcranial magnetic stimulation applied in three studies (Luedtke et al. 2012a, 2015; Schabrun et al. 2014) to determine accurately the location of the M1. For the tDCS sham condition, all but one study reported using the same electrode montage as the active condition with the same current density, which lasted for durations of between 10 and 43 s. In seven studies active tDCS stimulation was applied for 20 min (O’Connell et al. 2013; Luedtke et al. 2015; Hazime et al. 2017; Straudi et al. 2018; Jafarzadeh et al. 2019; Mariano et al. 2019; Jiang et al. 2020), one study applied for 30 min (Schabrun et al. 2014), and for 15 min (Luedtke et al. 2012b). However, the number of sessions varied; three studies reported that tDCS applied for 1 day (Luedtke et al. 2012b; Schabrun et al. 2014; Jiang et al. 2020), two studies for 5 consecutive days (Luedtke et al. 2015; Straudi et al. 2018), one study for 6 days (Jafarzadeh et al. 2019), for 10 days (Mariano et al. 2019), one study for 12 days (Hazime et al. 2017) and the maximum days was reported in one study which used tDCS for 15 days (O’Connell et al. 2013).
Four studies applied tDCS alone without combination (Luedtke et al. 2012b; O’Connell et al. 2013; Mariano et al. 2019; Jiang et al. 2020). Moreover, two studies combined tDCS with peripheral electrical stimulation (Schabrun et al. 2014; Hazime et al. 2017), or cognitive behavioural management (4 week multidisciplinary programme of 80 h) (Luedtke et al. 2015). One study followed tDCS by 10 sessions of group exercise (Straudi et al. 2018), another study combined tDCS with postural training for 20 min, three sessions per week for two weeks (Jafarzadeh et al. 2019) (Table 1).
Outcome measures
Outcome parameters in all included trials included a numerical rating scale (NRS) (Schabrun et al. 2014; Hazime et al. 2017; Jiang et al. 2020) or visual analogue scale (VAS) (O’Connell et al. 2013; Luedtke et al. 2015; Straudi et al. 2018; Jafarzadeh et al. 2019). Moreover, pain intensity also reported through defence and veterans pain rating scale (DVPRS) in one study (Mariano et al. 2019), while another study reported pain by thermal perception and pain thresholds (Luedtke et al. 2012a). Disability related to CLBP evaluated by Roland Morris Disability Questionnaire (RMDQ) in four studies (O’Connell et al. 2013; Hazime et al. 2017; Straudi et al. 2018; Mariano et al. 2019), and one study used oswestry disability index (ODI) (Luedtke et al. 2015). Quality of life (QoL) assessed by EQ-5D in one study (Straudi et al. 2018), and SF-36 was used in another trial (Luedtke et al. 2015).
Effect of tDCS on pain intensity
Five studies applied multiple tDCS stimulations over M1 (O’Connell et al. 2013; Luedtke et al. 2015; Hazime et al. 2017; Straudi et al. 2018; Jafarzadeh et al. 2019), and just three studies (Hazime et al. 2017; Straudi et al. 2018; Jafarzadeh et al. 2019) reported significant improvement in numerical pain scales after applying real anodal tDCS and combined intervention compared to sham tDCS and no intervention (Table 1). However, the pooled analysis showed no statistically significant improvements in the numerical pain scales in favour of anodal tDCS over M1 after intervention [SMD = 0.378, 95% CI = − 0.264–1.020; P = 0.249], I2 = 80.10, P = 0.000 (Fig. 3). In addition, three studies applied single session of tDCS (Luedtke et al. 2012a; Schabrun et al. 2014; Jiang et al. 2020), and two studies (Schabrun et al. 2014; Jiang et al. 2020) reported significant improvement in numerical pain scales after applying real anodal tDCS over M1 compared to sham tDCS (Table 1). Only one study applied multiple sessions of cathodal tDCS stimulation over the left dACC (Mariano et al. 2019). However, real cathodal stimulation showed no significant change in DVPRS scores post intervention and after 6 weeks follow up (Table 1).
Effect of tDCS on disability and quality of life
Four studies used RMDQ (O’Connell et al. 2013; Hazime et al. 2017; Straudi et al. 2018) or ODI (Luedtke et al. 2015) to assess the disability reported that no significant improvement in RMDQ or ODI in favour of anodal tDCS over M1 (Table 1). In addition, one study (Mariano et al. 2019) assessed the disability by RMDQ after cathodal stimulation over left dACC and reported no significant change in favour of cathodal stimulation. However, the pooled analysis of the study applied anodal tDCS stimulation over M1 (O’Connell et al. 2013; Luedtke et al. 2015; Hazime et al. 2017; Straudi et al. 2018) showed no significant improvement in disability scales [SMD = 0.143, 95% CI = − 0.214–0.499; P = 0.434], I2 = 34.32, P = 0.206 (Fig. 4). Moreover, two studies using the EQ-5D scale (Straudi et al. 2018) and SF-36 scale (Luedtke et al. 2015) to assess QoL, although no significant improvement in QoL found in favour of real anodal tDCS stimulation over M1 (Table 1).
Adverse events and side effects
It was stated that patients did not experience adverse reactions from the intervention in four studies (Luedtke et al. 2012a, 2015; Schabrun et al. 2014; Mariano et al. 2019). Five studies reported mild or minor adverse effects following intervention including skin redness (Hazime et al. 2017; Straudi et al. 2018), tingling and itching (Hazime et al. 2017; Straudi et al. 2018; Jafarzadeh et al. 2019; Jiang et al. 2020) under the site of stimulation, sleepiness (Hazime et al. 2017; Straudi et al. 2018), headache (O’Connell et al. 2013; Hazime et al. 2017; Straudi et al. 2018; Jafarzadeh et al. 2019), dizziness (O’Connell et al. 2013; Jiang et al. 2020), mood change and trouble to concentrate (Hazime et al. 2017; Straudi et al. 2018), pain (Hazime et al. 2017; Jafarzadeh et al. 2019), nausea (Hazime et al. 2017) and burning sensations (Jafarzadeh et al. 2019) but these were equally distributed across groups of active and control stimulation.
Discussion
We systematically evaluated the effectiveness of tDCS on pain and related disability in patients with non-specific CLBP. Nine RCTs that investigated the efficacy of real tDCS against sham tDCS on pain and related disability in CLBP patients, were included in this review. The meta-analysis pooled together five studies that assessed the effects of multiple sessions of tDCS applied over M1 (Table 1). Overall, the pooled analysis results showed no significant improvements in favour of tDCS in pain, disability and QoL. These results add to a growing body of meta-analytical work that failed to show any effect of real tDCS compared to sham tDCS on pain, disability and QoL. These results are in line with previous studies that demonstrated that tDCS has little or no effect on chronic pain states such as multiple sclerosis, chronic pelvic pain and fibromyalgia (Luedtke et al. 2012b; Zhu et al. 2017) as well as LBP (Baptista et al. 2019).
Only one of the selected studies (Mariano et al. 2019) explored the effects of cathodal tDCS over left dACC. Since the stimulation of different brain areas is not expected to produce similar efficacy and safety, the latter study was not pooled to the others. Among studies treating M1, three applied tDCS as the sole treatment, while five studies applied tDCS in combination with peripheral electrical stimulation or with exercise or with cognitive behavioural therapy (Table 1). It has been previously hypothesized that combining tDCS with traditional interventions (rehabilitative technique) can enhance the results of the tDCS treatment (Boggio et al. 2009; Riberto 2011; Cosentino et al. 2012) by exerting significant effects on different dimensions of cognition, including the psychological status (Alwardat et al. 2019). Indeed, four studies reported significant improvement in numerical pain scales in favour of real anodal tDCS over M1 combined to other interventions (see Table 1 for details) (Schabrun et al. 2014; Hazime et al. 2017; Straudi et al. 2018; Jafarzadeh et al. 2019) whereas only one study exploring tDCS as the sole treatment reported positive results. (Jiang et al. 2020). It is known that analgesic effects of non-invasive brain stimulation may differ after multiple sessions. The pooled analysis (Fig. 3) did not reveal any significant improvement for pain reduction in patients with CLBP post multiple sessions of anodal tDCS over M1.
There may be several possible explanations for these negative findings. First, CLBP is a complex, heterogeneous condition; different mechanisms and factors are involved in its pathogenesis. Thus, it is possible that the included participants had mechanical disorder such as undiagnosed sacroiliac joint or facet joint or discogenic pain. Moreover, visceral pain referred to lower back can also be a misleading condition, which is not related to CLBP. However, only one of the included studies (Luedtke et al. 2015) reported that participants with CLBP were carefully selected with clear inclusion/exclusion criteria by using European guidelines (Airaksinen et al. 2006). Thus, future studies are recommended in which precise selection criteria for non-specific CLBP are carefully applied. In particular, given the complexity of etiological diagnosis of CLBP, selection criteria should be clearly stated in the methods of any clinical work, including the exclusion criteria for mechanical and peripheral causes (i.e. diagnostic blocks). Second, when M1 is targeted, it is usually applied contralateral to the side of the pain complaint. However, CLBP can be either medial or lateralised, being defined as “pain localised below the costal margin and above the inferior gluteal folds, with or without sciatica” (Dionne et al. 2008). We suppose that if pain was medial rather than lateral, left M1 was targeted being left the dominant hemisphere. If the side of pain is not clearly stated, this could lead to heterogeneity in case definitions of CLBP, which limits consistency and comparative analysis between studies. A precise description of the anatomical area should be clearly stated as well as a clear statement of the targeted cerebral cortex to enhance results and make data comparable. Third, the intensity, frequency, duration and stimulation target of the tDCS may not have been sufficient to challenge and/or modulate the structurally and functionally adapted brain of patients with CLBP. Indeed, 3 included studies (Luedtke et al. 2012a; Schabrun et al. 2014; Jiang et al. 2020) carried out a single session of anodal or cathodal tDCS and showed short term efficacy. Previous meta-analyses of transcranial magnetic stimulation (TMS) and tDCS in chronic pain have reported that the analgesic effects of non-invasive brain stimulation were enhanced after multiple sessions (Cruccu et al. 2016; O’Connell et al. 2018). To ensure sufficient improvement the tDCS stimulation pattern should be standardized in future studies. Fourth, it should be pointed out that we included five studies designed to explore the efficacy of tDCS combined to other interventions, against other treatments and not specifically designed to assess the efficacy of tDCS alone. Therefore, any conclusions may be cautious as these results may be biased by any strict selection or intervention applied. Finally, pain is multidimensional and influenced by numerous factors. Thus, the follow-up evaluation in the included studies is heterogeneous.
The lack of tDCS effect on pain reduction in CLBP can be also explained by the neurophysiological hypothesis. The most frequently described working mechanism for tDCS is top down pain inhibition (Medeiros et al. 2012; Konno and Sekiguchi 2018). This is identified as central pain modulation, contributing in pain relief through altered cortical activity that leads to a descending cascade of events (Ossipov et al. 2010). Although there is strong evidence supporting the reliable cortical and subcortical neurophysiological reaction to tDCS, there is not a precise area localized as the cortical origin of the descending corticothalamic pathway. Eight over nine of the included studies applied tDCS to M1 cortex despite there is lack of clear evidence that this area is modified either in function or in morphology in chronic back pain and, accordingly, a previous systematic review and meta-analysis demonstrated that the evidence for M1 changes in chronic pain is conflicting (Chang et al. 2018). In fact, the underlying mechanism of M1 stimulation in pain modulation is poorly understood. An animal study demonstrated that repetitive motor cortex stimulation can attenuate the mechanical allodynia in neuropathic pain, inducing the activation of protein kinase M zeta, a regulator of synaptic plasticity, in the ACC (An et al. 1998). Thus, M1-tDCS may act indirectly altering synaptic plasticity in the ACC, an area involved in pain perception and emotional modulation. A previous study reported that anodal tDCS applied over M1 increased the functional coupling of the M1 with the thalamus (Polanía et al. 2012). Moreover, Roche and colleagues supported this hypothesis in healthy participants (Roche et al. 2012). They observed that the modulation of the H reflex in the quadriceps muscle indicated that the influence of tDCS descended as far down as the leg through the spinal pathway (Roche et al. 2012). However, the studies using experimental pain or pain thresholds do not support these remote effects leading to pain reduction (Antal et al. 2008; Boggio et al. 2008; Csifcsak et al. 2009; Bachmann et al. 2010; Grundmann et al. 2011). In fact, all the included studies in this review reported conflicting data on the improvement in pain reduction. Furthermore, one of the included studies applied anodal tDCS over M1 and reported no significant improvement in the perception of noxious thermal, electrical stimuli and thermal pain thresholds in favour of real tDCS (Luedtke et al. 2012a). Indeed, it is surprising that most of the studies target M1 for pain modulation, which is not one cortex directly excited during pain processing. In fact, the term “pain matrix” was coined to describe different brain areas involved in pain processing connecting the major three systems, which are usually affected by pain signals: the lateral and the medial system as the two main afferent pain pathways, and the descending system involved in pain modulation. Cortices mainly involved in pain processing are represented by PFC, amygdala and medial insula as concerned the cognitive and emotional appraisal of pain, and by the sensory cortex S2 and lateral insula concerning the discriminatory sensory component of pain (Henry et al. 2011; Fabbro and Crescentini 2014). It was shown that greater functional connections between the dorsal medial PFC-amygdala-accumbens circuit contribute to risk of chronic pain in subacute back pain patients (Vachon-Presseau et al. 2016). Accordingly, disorders of the brain network have been proposed as one of the possible causes of LBP chronicity (Mano et al. 2018). A combination of sensory and affective dimensions of pain predict DLPFC grey matter changes in a brain-imaging study (Apkarian et al. 2004), and the extent of density changes displays a strict correlation with pain intensity and unpleasantness (Schmidt-Wilcke et al. 2006). Indeed, the altered function of both anterior cingulate cortex and PFC in chronic pain patients is not surprising, as these structures are involved in the descending modulation of pain (Bushnell et al. 2013). The cortical projections to the PAG, the primary control centre for descending pain modulation and pain relief, originate principally from the PFC (An et al. 1998). Upon PFC and ACC activation, PAG releases opioids that act to alleviate pain (Konno and Sekiguchi 2018). Thus, decreased activation in these brain regions may be associated with decreased function of the descending inhibitory system (Konno and Sekiguchi 2018).
Given this rational basis, tDCS could be applied to the PFC, an area directly involved in pain cognitive interpretation. It has been demonstrated that tDCS of the left DLPFC in healthy subjects induces increased perfusion in brain regions that are anatomically connected to the DLPFC, such as the insular cortex, cingulate cortex and the PAG (Stagg et al. 2013). Indeed, several lines of evidence suggest that anodal tDCS to the DLPFC improves symptoms in a range of situations, including working memory, mood, and pain perception (Ivo et al. 2013). Of Interest, placebo analgesia in chronic back pain can be predicted, by studying the neuronal interactions between prefrontal regions and pain processing regions (bilateral insula) (Hashmi et al. 2012).
We did not detect any meaningful significant results in favour of real tDCS on disability and QoL. Taking into account the relation between pain, disability and QoL, and given that cortical stimulation produced significant analgesic effects in some chronic pain states (Mori et al. 2010; Luedtke et al. 2012b) we could expect that reduced pain may improve QoL and decrease disability. The current meta-analysis does not support this analgesic effect in CLBP, as well as the improvement in QoL and disability in CLBP patients. However, no serious side-effects and/or adverse events were reported in the included studies.
Study limitations and recommendations
The main limitation of the current study is the small sample size and the small number of studies included, which made the sensitivity analyses difficult. Moreover, the heterogeneity of the studies included limited the pooled analysis. A further limitation is that we only considered the effects of tDCS on VAS and NRS pain intensity scores. VAS and NRS are the most frequently outcome measure used for clinical studies and therefore allow us to make the largest comparison possible across the included studies. The most frequently stimulated site in the included studies is M1. We recommend future studies to focus on central pain mechanisms in non-specific CLBP, and to explore the effect of tDCS on sites different from M1, such as DLPFC. The included studies used heterogeneous stimulations parameters (intensity, frequency, and duration). This prevents us to estimate and recommend the ideal tDCS parameters protocol for CLBP. Further studies are required in order to suggest the optimal parameters to be used. Five studies (O’Connell et al. 2013; Schabrun et al. 2014; Hazime et al. 2017; Straudi et al. 2018; Jiang et al. 2020) used the international 10/20 EEG System to apply the tDCS electrode over the M1 and three studies (Luedtke et al. 2012a, 2015; Schabrun et al. 2014) applied single pulse TMS to determine accurately the location of the M1. Therefore, it is difficult to assess whether the included studies targeted the M1 accurately. In addition, the size of electrodes used also differed, and it is unclear whether these different sizes may influence the effectiveness of tDCS. We suggest future research using a standard protocol to determine the brain target before the stimulation such as single pulse TMS, as well as, standard electrodes size. Further, more studies are needed to understand the utility of combining tDCS with traditional interventions in order to choose the appropriate intervention with regards to the patient's needs. Despite these limitations, the modified Jadad scale and the Cochrane's risk of bias assessment showed that the studies quality were high and the risk of bias was small.
Conclusion
The results of this systematic review and meta-analysis do not provide evidence that tDCS is effective in reducing non-specific CLBP, as well as, related disability and QoL. This is the first meta-analysis to investigate the effectiveness of tDCS on CLBP and the results are not consistent with existing studies of tDCS in other chronic pain conditions. Our results are insufficient to support the use of tDCS for CLBP, however tDCS was generally a safe and easy-to-use option. However, given the limitations of the present analysis, our results should be considered necessarily tentative. Well-designed studies with more sensitive outcomes and different stimulation sites are required in the future.
References
Airaksinen O, Brox JI, Cedraschi C et al (2006) Chapter 4: European guidelines for the management of chronic nonspecific low back pain. Eur Spine J 15(Suppl 2):S192–S300. https://doi.org/10.1007/s00586-006-1072-1
Alwardat M, Schirinzi T, Di Lazzaro G et al (2019) Association between physical activity and dementia’s risk factors in patients with Parkinson’s disease. J Neural Transm 126:319–325. https://doi.org/10.1007/s00702-019-01979-0
An X, Bandler R, Öngür D, Price JL (1998) Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. J Comp Neurol 401:455–479. https://doi.org/10.1002/(SICI)1096-9861(19981130)401:4<455:AID-CNE3>3.0.CO;2-6
Antal A, Alekseichuk I, Bikson M et al (2017) Low intensity transcranial electric stimulation: safety, ethical, legal regulatory and application guidelines. Clin Neurophysiol 128:1774–1809
Antal A, Brepohl N, Poreisz C et al (2008) Transcranial direct current stimulation over somatosensory cortex decreases experimentally Induced acute pain perception. Clin J Pain 24:56–63. https://doi.org/10.1097/AJP.0b013e318157233b
Apkarian AV, Baliki MN, Geha PY (2009) Towards a theory of chronic pain. Prog Neurobiol 87:81–97
Apkarian AV, Sosa Y, Sonty S et al (2004) Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci 24:10410–10415. https://doi.org/10.1523/JNEUROSCI.2541-04.2004
Bachmann CG, Muschinsky S, Nitsche MA et al (2010) Transcranial direct current stimulation of the motor cortex induces distinct changes in thermal and mechanical sensory percepts. Clin Neurophysiol 121:2083–2089. https://doi.org/10.1016/j.clinph.2010.05.005
Baliki MN, Chialvo DR, Geha PY et al (2006) Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci 26:12165–12173. https://doi.org/10.1523/JNEUROSCI.3576-06.2006
Baliki MN, Schnitzer TJ, Bauer WR, Apkarian AV (2011) Brain morphological signatures for chronic pain. PLoS ONE. https://doi.org/10.1371/journal.pone.0026010
Baptista AF, Fernandes AMBL, Sá KN et al (2019) Latin American and Caribbean consensus on noninvasive central nervous system neuromodulation for chronic pain management (LAC 2-NIN-CP). Pain Rep 4:e692. https://doi.org/10.1097/PR9.0000000000000692
Boggio PS, Amancio EJ, Correa CF et al (2009) Transcranial DC stimulation coupled with TENS for the treatment of chronic pain: a preliminary study. Clin J Pain 25:691–695. https://doi.org/10.1097/AJP.0b013e3181af1414
Boggio PS, Zaghi S, Lopes M, Fregni F (2008) Modulatory effects of anodal transcranial direct current stimulation on perception and pain thresholds in healthy volunteers. Eur J Neurol 15:1124–1130. https://doi.org/10.1111/j.1468-1331.2008.02270.x
Borenstein M, Hedges L, Higgins J, Rothstein H (2005) Software comprehensive meta-analysis (version 2). NJ Biostat, Englewood
Burns E, Chipchase LS, Schabrun SM (2016) Primary sensory and motor cortex function in response to acute muscle pain: a systematic review and meta-analysis. Eur J Pain (United Kingdom) 20:1203–1213
Bushnell MC, Čeko M, Low LA (2013) Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 14:502–511
Chalmers TC, Smith H, Blackburn B et al (1981) A method for assessing the quality of a randomized control trial. Control Clin Trials 2:31–49. https://doi.org/10.1016/0197-2456(81)90056-8
Chang WJ, O’Connell NE, Beckenkamp PR et al (2018) Altered primary motor cortex structure, organization, and function in chronic pain: a systematic review and meta-analysis. J Pain 19:341–359
Chou R, Deyo R, Friedly J et al (2017a) Nonpharmacologic therapies for low back pain: a systematic review for an American College of physicians clinical practice guideline. Ann Intern Med 166:493–505
Chou R, Deyo R, Friedly J et al (2017b) Systemic pharmacologic therapies for low back pain: a systematic review for an American College of physicians clinical practice guideline. Ann Intern Med 166:480–492
Cosentino G, Fierro B, Paladino P et al (2012) Transcranial direct current stimulation preconditioning modulates the effect of high-frequency repetitive transcranial magnetic stimulation in the human motor cortex. Eur J Neurosci 35:119–124. https://doi.org/10.1111/j.1460-9568.2011.07939.x
Cruccu G, Garcia-Larrea L, Hansson P et al (2016) EAN guidelines on central neurostimulation therapy in chronic pain conditions. Eur J Neurol 23:1489–1499. https://doi.org/10.1111/ene.13103
Csifcsak G, Antal A, Hillers F et al (2009) Modulatory effects of transcranial direct current stimulation on laser-evoked potentials. Pain Med 10:122–132. https://doi.org/10.1111/j.1526-4637.2008.00508.x
da Silva MC, Conti CL, Klauss J et al (2013) Behavioral effects of transcranial direct current stimulation (tDCS) induced dorsolateral prefrontal cortex plasticity in alcohol dependence. J Physiol Paris 107:493–502. https://doi.org/10.1016/j.jphysparis.2013.07.003
Dionne CE, Dunn KM, Croft PR et al (2008) A consensus approach toward the standardization of back pain definitions for use in prevalence studies. Spine (Phila Pa 1976) 33:95–103. https://doi.org/10.1097/BRS.0b013e31815e7f94
DosSantos MF, Love TM, Martikainen IK et al (2012) Immediate effects of tDCS on the μ-opioid system of a chronic pain patient. Front Psychiatry. https://doi.org/10.3389/fpsyt.2012.00093
Fabbro F, Crescentini C (2014) Facing the experience of pain: a neuropsychological perspective. Phys Life Rev 11:540–552
Garcia-Larrea L, Peyron R (2007) Motor cortex stimulation for neuropathic pain: from phenomenology to mechanisms. Neuroimage. https://doi.org/10.1016/j.neuroimage.2007.05.062
Giesecke T, Gracely RH, Clauw DJ et al (2006) Zentrale schmerzverarbeitung bei chronischem rückenschmerz: Hinweise auf verminderte schmerzinhibition. Schmerz 20:411–417. https://doi.org/10.1007/s00482-006-0473-8
Grundmann L, Rolke R, Nitsche MA et al (2011) Effects of transcranial direct current stimulation of the primary sensory cortex on somatosensory perception. Brain Stimul 4:253–260. https://doi.org/10.1016/j.brs.2010.12.002
Hashmi JA, Baria AT, Baliki MN et al (2012) Brain networks predicting placebo analgesia in a clinical trial for chronic back pain. Pain 153:2393–2402. https://doi.org/10.1016/j.pain.2012.08.008
Hazime FA, Baptista AF, de Freitas DG et al (2017) Treating low back pain with combined cerebral and peripheral electrical stimulation: a randomized, double-blind, factorial clinical trial. Eur J Pain (United Kingdom) 21:1132–1143. https://doi.org/10.1002/ejp.1037
Henry DE, Chiodo AE, Yang W (2011) Central nervous system reorganization in a variety of chronic pain states: a review. PM R 3:1116–1125. https://doi.org/10.1016/j.pmrj.2011.05.018
Higgins J (2011) Cochrane Handbook for systematic reviews of interventions version. In: Cochrane Collab. https://handbook.cochrane.org/. Accessed 1 Mar 2020
Ivo R, Nicklas A, Dargel J et al (2013) Brain structural and psychometric alterations in chronic low back pain. Eur Spine J 22:1958–1964. https://doi.org/10.1007/s00586-013-2692-x
Jadad AR, Moore RA, Carroll D et al (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17:1–12. https://doi.org/10.1016/0197-2456(95)00134-4
Jafarzadeh A, Ehsani F, Yosephi MH et al (2019) Concurrent postural training and M1 anodal transcranial direct current stimulation improve postural impairment in patients with chronic low back pain. J Clin Neurosci 68:224–234. https://doi.org/10.1016/j.jocn.2019.07.017
Jiang N, Wei J, Li G et al (2020) Effect of dry-electrode-based transcranial direct current stimulation on chronic low back pain and low back muscle activities: a double-blind sham-controlled study. Restor Neurol Neurosci 38:41–54. https://doi.org/10.3233/rnn-190922
Konno S, Sekiguchi M (2018) Association between brain and low back pain. J Orthop Sci 23:3–7. https://doi.org/10.1016/j.jos.2017.11.007
Latremoliere A, Woolf CJ (2009) Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 10:895–926
Lefaucheur JP, Antal A, Ayache SS et al (2017) Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol 128:56–92
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62:e1–e34. https://doi.org/10.1016/j.jclinepi.2009.06.006
Luedtke K, May A, Jürgens TP (2012a) No effect of a single session of transcranial direct current stimulation on experimentally induced pain in patients with chronic low back pain—an exploratory study. PLoS ONE. https://doi.org/10.1371/journal.pone.0048857
Luedtke K, Rushton A, Wright C et al (2012b) Transcranial direct current stimulation for the reduction of clinical and experimentally induced pain: a systematic review and meta-analysis. Clin J Pain 28:452–461
Luedtke K, Rushton A, Wright C et al (2015) Effectiveness of transcranial direct current stimulation preceding cognitive behavioural management for chronic low back pain: sham controlled double blinded randomised controlled trial. BMJ. https://doi.org/10.1136/bmj.h1640
Mano H, Kotecha G, Leibnitz K et al (2018) Classification and characterisation of brain network changes in chronic back pain. A multicenter study. Wellcome Open Res 3:19. https://doi.org/10.12688/wellcomeopenres.14069.2
Mariano TY, Burgess FW, Bowker M et al (2019) Transcranial direct current stimulation for affective symptoms and functioning in chronic low back pain: a pilot double-blinded, randomized, placebo-controlled trial. Pain Med (United States) 20:1166–1177. https://doi.org/10.1093/pm/pny188
Medeiros LF, de Souza ICC, Vidor LP et al (2012) Neurobiological effects of transcranial direct current stimulation: a review. Front Psychiatry 3:110. https://doi.org/10.3389/fpsyt.2012.00110
Mendonca ME, Simis M, Grecco LC et al (2016) Transcranial direct current stimulation combined with aerobic exercise to optimize analgesic responses in fibromyalgia: a randomized placebo-controlled clinical trial. Front Hum Neurosci. https://doi.org/10.3389/fnhum.2016.00068
Mori F, Codecà C, Kusayanagi H et al (2010) Effects of anodal transcranial direct current stimulation on chronic neuropathic pain in patients with multiple sclerosis. J Pain 11:436–442. https://doi.org/10.1016/j.jpain.2009.08.011
Muller K, Cohen J (1989) Statistical power analysis for the behavioral sciences. Technometrics 31:499. https://doi.org/10.2307/1270020
Ngernyam N, Jensen MP (2014) Transcranial direct current stimulation in neuropathic pain. J Pain Reli. https://doi.org/10.4172/2167-0846.s3-001
Wu D, Qian L, Zorowitz RD et al (2013) Effects on decreasing upper-limb poststroke muscle tone using transcranial direct current stimulation: a randomized sham-controlled study. Arch Phys Med Rehabil 94:1–8. https://doi.org/10.1016/j.apmr.2012.07.022
O’Connell NE, Cossar J, Marston L et al (2013) Transcranial direct current stimulation of the motor cortex in the treatment of chronic nonspecific low back pain: a randomized, double-blind exploratory study. Clin J Pain 29:26–34. https://doi.org/10.1097/AJP.0b013e318247ec09
O’Connell NE, Marston L, Spencer S et al (2018) Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst Rev 3:CD008208. https://doi.org/10.1002/14651858.CD008208
Ossipov MH, Dussor GO, Porreca F (2010) Central modulation of pain. J Clin Invest 120:3779–3787
Pagano RL, Assis DV, Clara JA et al (2011) Transdural motor cortex stimulation reverses neuropathic pain in rats: a profile of neuronal activation. Eur J Pain 15:268.e1–268.e14. https://doi.org/10.1016/j.ejpain.2010.08.003
Polanía R, Paulus W, Nitsche MA (2012) Modulating cortico-striatal and thalamo-cortical functional connectivity with transcranial direct current stimulation. Hum Brain Mapp 33:2499–2508. https://doi.org/10.1002/hbm.21380
Riberto M (2011) Efficacy of transcranial direct current stimulation coupled with a multidisciplinary rehabilitation program for the treatment of fibromyalgia. Open Rheumatol J 5:45–50. https://doi.org/10.2174/1874312901105010045
Ricci M, Di Lazzaro G, Pisani A et al (2019) Wearable electronics assess the effectiveness of transcranial direct current stimulation on balance and gait in Parkinson’s disease patients. Sensors (Switzerland). https://doi.org/10.3390/s19245465
Roche N, Lackmy A, Achache V et al (2012) Effects of anodal tDCS on lumbar propriospinal system in healthy subjects. Clin Neurophysiol 123:1027–1034. https://doi.org/10.1016/j.clinph.2011.09.011
Roth GA, Johnson C, Abajobir A et al (2017) Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol 70:1–25. https://doi.org/10.1016/j.jacc.2017.04.052
Schabrun SM, Jones E, Elgueta Cancino EL, Hodges PW (2014) Targeting chronic recurrent low back pain from the top-down and the bottom-up: a combined transcranial direct current stimulation and peripheral electrical stimulation intervention. Brain Stimul 7:451–459. https://doi.org/10.1016/j.brs.2014.01.058
Schmidt-Wilcke T, Leinisch E, Gänßbauer S et al (2006) Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain 125:89–97. https://doi.org/10.1016/j.pain.2006.05.004
Shigematsu T, Fujishima I, Ohno K (2013) Transcranial direct current stimulation improves swallowing function in stroke patients. Neurorehabil Neural Repair 27:363–369. https://doi.org/10.1177/1545968312474116
Stagg CJ, Lin RL, Mezue M et al (2013) Widespread modulation of cerebral perfusion induced during and after transcranial direct current stimulation applied to the left dorsolateral prefrontal cortex. J Neurosci 33:11425–11431. https://doi.org/10.1523/JNEUROSCI.3887-12.2013
Straudi S, Buja S, Baroni A et al (2018) The effects of transcranial direct current stimulation (tDCS) combined with group exercise treatment in subjects with chronic low back pain: a pilot randomized control trial. Clin Rehabil 32:1348–1356. https://doi.org/10.1177/0269215518777881
Thompson T, Dias S, Poulter D et al (2020) Efficacy and acceptability of pharmacological and non-pharmacological interventions for non-specific chronic low back pain: a protocol for a systematic review and network meta-analysis. Syst Rev 9:130. https://doi.org/10.1186/s13643-020-01398-3
Tracey I, Bushnell MC (2009) How neuroimaging studies have challenged us to rethink: is chronic pain a disease? J Pain 10:1113–1120
Vachon-Presseau E, Tétreault P, Petre B et al (2016) Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain 139:1958–1970. https://doi.org/10.1093/brain/aww100
Woolf CJ (2011) Central sensitization: implications for the diagnosis and treatment of pain. Pain 152(3 Suppl):S2–S15. https://doi.org/10.1016/j.pain.2010.09.030
Zhu CE, Yu B, Zhang W et al (2017) Effectiveness and safety of transcranial direct current stimulation in fibromyalgia: a systematic review and meta-analysis. J Rehabil Med 49:2–9
Funding
The authors declare that they did not receive any specific grant from any funding agencies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1: Database and search strategies
Appendix 1: Database and search strategies
Medline | (Transcranial Direct Current Stimulation[mh] or Transcranial Direct Current Stimulation[tiab] or tDCS[tiab] or Electrical Stimulation Transcranial[tiab]) AND (low back pain[mh] or LBP[tiab] or chronic nonspecific low back Pain[tiab] or NSLBP[tiab] or back pain[mh]) |
Web of Science | ("low back Pain" or "chronic low back pain" or "chronic nonspecific low back pain" or "LBP" or "NSLBP") AND (“Transcranial Direct Current Stimulation” or tDCS or “Electrical Stimulation Transcranial”) |
Scopus | ("low back Pain" or "chronic low back pain" or "chronic nonspecific low back pain" or "LBP" or "NSLBP") AND (“Transcranial Direct Current Stimulation” or tDCS or “Electrical Stimulation Transcranial”) |
Cochrane | #1 MeSH descriptor: [Transcranial Direct Current Stimulation] explode all trees or Transcranial Direct Current Stimulation" or tDCS #2 MeSH descriptor: [low back Pain] explode all trees or "low back Pain" or " chronic low back pain" or " chronic nonspecific low back pain " or "LBP" or "NSLBP" #1 AND #2 |
EMBASE | ('transcranial direct current stimulation'/exp OR 'transcranial direct current stimulation' OR 'transcranial direct current stimulation':ab,ti OR 'tdcs':ab,ti) AND ('low back pain'/exp OR 'chronic low back pain' OR 'chronic nonspecific low back pain' OR 'LBP' OR 'NSLBP':ab,ti) |
Rights and permissions
About this article
Cite this article
Alwardat, M., Pisani, A., Etoom, M. et al. Is transcranial direct current stimulation (tDCS) effective for chronic low back pain? A systematic review and meta-analysis. J Neural Transm 127, 1257–1270 (2020). https://doi.org/10.1007/s00702-020-02223-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-020-02223-w