Abstract
Upper limb function was investigated in children with ADHD using objective methods. We hypothesised that children with ADHD exhibit abnormal dexterity, force application during manipulation of a novel object, and movement rhythmicity. Two groups of age- and gender-matched children were investigated: 35 typically developing children (controls, 10.5 ± 0.4 years, 32M–3F) and 29 children (11.5 ± 0.5 years, 27M–2F) with formally diagnosed ADHD according to DSM-IV-TR criteria. Participants underwent a series of screening tests and tests of upper limb function while “off” medication. Objective quantification of upper limb function involved measurement of force during a grip and lift task, maximal finger tapping task, and maximal pinch grip. Acceleration at the index finger was also measured during rest, flexion and extension, and a postural task to quantify tremor. The Movement Assessment Battery for Children-2 (MABC-2) was also administered. Significant between-group differences were observed in movement rhythmicity, manipulation of a novel object, and performance of the MABC-2 dexterity and aiming and catching components. Children with ADHD lifted a novel object using a lower grip force (P = 0.036), and held the object with a more variable grip force (P = 0.003), than controls. Rhythmicity of finger tapping (P = 0.008) and performance on the dexterity (P = 0.007) and aiming and catching (P = 0.042) components of the MABC-2 were also significantly poorer in the ADHD group than controls. Movement speed, maximum pinch grip strength, and tremor were unaffected. The results of the study show for the first time that ADHD is associated with deficits in multiple, but not all domains of upper limb function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a neurodevelopmental disorder characterised by inattention and/or hyperactivity-impulsivity (American Psychiatric Association 2013). Up to half of children who are diagnosed with ADHD also exhibit a form of movement dysfunction (e.g. Kadesjo and Gillberg 1998). Use of objective testing methods has consistently revealed deficits in fine motor function, such as poor handwriting (Schoemaker et al. 2005; Shen et al. 2012) and performance on pegboard and visuomotor tracking tasks (Meyer and Sagvolden 2006; Pitcher et al. 2003; Slaats-Willemse et al. 2005). Deficits in fine motor function have also been observed with subjective and observational methods (e.g. Fliers et al. 2009; Harvey et al. 2007; Jucaite et al. 2003; Piek et al. 1999; Pitcher et al. 2003; Rosa Neto et al. 2015; Whitmont and Clark 1996) and the deficits are equivalent to a 12–18-month delay in the development of the motor system (Rosa Neto et al. 2015). The association between ADHD and gait and balance has also been investigated but the results are less consistent with little or no change in gait or balance during quiet stance (Buderath et al. 2009; Kooistra et al. 2009; Papadopoulos et al. 2014).
The presence of movement dysfunction in children with ADHD is not surprising given that pathological and pathophysiological changes have been observed in several movement-related brain regions in this population. For example, reduced grey matter volume in the cerebellum, right putamen and globus pallidus, and in the motor, premotor, and somatosensory cortices of ADHD-diagnosed children have been observed with voxel-based morphometry (Carmona et al. 2005; Frodl and Skokauskas 2012). Data from a large prospective study on ADHD-diagnosed children also suggest a marked delay in attaining peak cortical thickness throughout most of the cerebrum, except the primary motor cortex. Early-to-normal maturation of the primary motor cortex coupled with late maturation of higher order motor control regions could disrupt motor control (Shaw et al. 2007).
Interpretation of the literature on movement in ADHD-diagnosed children is difficult for several reasons. First, many studies focus on one type of movement (e.g. finger tapping) and thus it is not possible, and inappropriate to make generalised statements about movement in this population. Second, use of primarily subjective and observational tests of movement quality, which are not designed to detect subtle changes in movement planning or execution, limit interpretation of the results. Third, inconsistent and heterogeneous samples limit comparison between studies, and many studies did not consider the effect of current or past use of psychostimulant medication on movement. This is evidenced by inclusion of ADHD-diagnosed children with and without a history of use of psychostimulant medication in the sample (e.g. Ben-Pazi et al. 2003; Harvey et al. 2007) or failure to report the medication history of all participants (e.g. Jucaite et al. 2003; Shen et al. 2012). This is important because some elements of movement (e.g. synchronised finger tapping) in drug naïve ADHD-diagnosed children differ between placebo, acute and longer term (4 weeks) administration of methylphenidate (Rubia et al. 2003). Methylphenidate and other psychostimulant medications affect dopaminergic neurotransmission and dopamine plays a key role in movement. The importance of dopamine in movement is evidenced by the clinical manifestations of Parkinson’s disease, a neurodegenerative disease characterised by loss of primarily dopaminergic neurons in the substantia nigra (Fearnley and Lees 1991).
The aim of the current study was to perform a detailed investigation of upper limb function in a uniform sample of ADHD-diagnosed children using objective methods. Upper limb function in these children was compared to a group of age- and gender-matched typically developing controls. A secondary aim was to determine if upper limb function in the ADHD group is associated with history of use of psychostimulant medication and morphology of the substantia nigra, a brain region with a high density of dopaminergic neurons (Fearnley and Lees 1991). The morphology of the substantia nigra, viewed with transcranial ultrasound, is abnormally bright and enlarged (hyperechogenic) in ADHD-diagnosed children medicated with methylphenidate (Krauel et al. 2010; Romanos et al. 2010), but the relationship between this abnormality and motor function has not been investigated. It was hypothesised that ADHD-diagnosed children exhibit abnormal movement rhythmicity, dexterity, and force application during manipulation of a novel object, but movement speed and pinch grip strength are unaffected. It was also hypothesised that greater movement dysfunction would be observed in ADHD-diagnosed children with a greater history of use of psychostimulant medication and area of substantia nigra echogenicity. Evidence that supports the hypotheses include (1) previously documented deficits in fine motor function (e.g. Meyer and Sagvolden 2006; Pitcher et al. 2003; Slaats-Willemse et al. 2005) and an abnormally enlarged area of substantia nigra echogenicity (Krauel et al. 2010; Romanos et al. 2010) in children diagnosed with ADHD, and (2) an association between illicit use of amphetamines and increased area of substantia nigra echogenicity (Todd et al. 2016) and overestimation of the grip force required to lift a novel object (Pearson-Dennett et al. 2014) in adults. Such quantification of multiple upper limb movements, within a single ADHD sample, will further understanding of the motor deficits associated with this disorder and inform on the potential use of motor parameters as a biomarker for ADHD diagnosis and/or differentiation between the subtypes of ADHD.
Methods
Upper limb function and substantia nigra morphology were assessed in 64 children aged 6–18 years. Two groups of age- and gender-matched children were investigated: 35 typically developing children with no history of neurological and/or psychological illness (control group) and 29 children who had been formally diagnosed with ADHD, by a paediatric psychiatrist, according to criteria published in the Diagnostic and Statistical Manual of Mental Disorders 4th Edition—Text Revision (DSM-IV-TR; codes: 314.01, 314.00, and 314.01; American Psychiatric Association 2000). The DSM-IV-TR was used in the current study because data collection commenced prior to publication of the fifth edition (DSM-5). The main difference between the two editions is that more examples are provided for items listed in the DSM-5, and the DSM-5 requires the presence of symptoms prior to 12 years of age, compared to 7 years of age in the DSM-IV-TR (American Psychiatric Association 2000, 2013). An additional inclusion criterion for the ADHD group was history of use of psychostimulant medication for the treatment of ADHD. Exclusion criteria for the ADHD group included history of a previously diagnosed (by a paediatric psychiatrist or neurologist) comorbid neurological (e.g. Chiari malformation), neurodevelopmental motor disorder (e.g. development coordination disorder), and/or mental disorder that may affect movement, and/or history of neuroleptic use (due to the association between substantia nigra morphology and neuroleptic-induced parkinsonism; Berg et al. 2001). This information was obtained via an in-house questionnaire administered to the participant and their parent or guardian. Participants were recruited by community advertisement, and distribution of the advertisement to members of the Attention Disorder Association of South Australia Inc. Each participant (or the participants’ parent or guardian) received an honorarium ($50) for participating in the study. All experimental procedures were approved by the University of South Australia Human Research Ethics Committee and the Children, Youth, and Women’s Health Service Human Research Ethics Committee. Experimental procedures were conducted according to the Code of Ethics of the World Medical Association (Declaration of Helsinki) and its later amendments or comparable ethical standards. Written informed consent was obtained from all participants and their parent or guardian.
Screening
Each participant undertook a series of screening tests prior to participation, with the help of their parent and/or guardian. The screening tests involved a brief medical history questionnaire (Rossi et al. 2009), Edinburgh Handedness Inventory (to determine hand dominance; Oldfield 1971), and an in-house questionnaire on participant characteristics, medication use, and ADHD diagnosis and treatments (pharmaceutical and non-pharmaceutical). For each medication, the name, dose, onset and frequency of use, duration, and time since last use were documented. Parents were also asked to complete a questionnaire derived from questions 1–22 of the DSM-IV-TR for codes 314.01, 314.00, and 314.01 (American Psychiatric Association 2000) to document the degree of inattention and hyperactivity–impulsivity.
Experimental protocol
Children in the ADHD group were tested “off” medication. The experimental session was usually scheduled in the morning and parents/guardians were advised to delay administration of the morning dose of psychostimulant until after completion of the experimental protocol. To avoid insomnia, Australian doctors usually prescribe long-acting methylphenidate for once a day morning administration, and with short-acting formulations, the last dose of dexamphetamine or methylphenidate is prescribed for the late afternoon (e.g. 3–4 pm). Thus, delaying the morning dose of psychostimulant ensured that all children in the current study were “off” medication during experimentation.
The experiment began with measurement of height and weight. Participants then completed five tests of upper limb function and a transcranial ultrasound examination of the substantia nigra. Unilateral motor tasks were performed on the dominant side, determined by the score (laterality quotient) on the Edinburgh Handedness Inventory (Oldfield 1971). Left-handedness was defined as a laterality quotient of − 100 to − 1 and right-handedness was defined as a laterality quotient of 1–100 (Oldfield 1971). Ambidextrous children (laterality quotient = 0) performed the tasks with their preferred hand.
The first movement test assessed manual dexterity with a grip and lift task. Children sat on a chair and a novel test object was placed on a table, in front of the child. The test object (342 g) consisted of an accelerometer (± 2 g, ADXL311J, RS Components, Smithfield, Australia) and two load cells (MPL-100, Transducer Techniques, Temecula, USA) mounted orthogonally for the measurement of horizontal grip force and vertical lift force (Fig. 1a). The grip load cell was mounted between two polished brass disks, 35 mm apart. Each child was instructed to grip and lift the test object to the height indicated (~ 10 cm), and to then hold it there for 3–4 s before returning it to the table when instructed. Demonstration was provided but no practice was allowed. The task was performed with the index finger and thumb (pinch grip) and the lifting movement occurred primarily through elbow flexion. The task duration was ~ 6 s.
Experimental apparatus for quantitative assessment of fine motor function. a Test object for the grip and lift task. b Strain gauge for the maximum finger tapping task. c Accelerometer for assessment of tremor during relaxation (left panel) and flexion and extension of the index finger (right panel)
The second movement test assessed maximal pinch grip strength to enable normalisation of some parameters measured during the grip and lift task. Three brief maximal voluntary contractions (2–3 s duration) were performed with the index finger and thumb. The finger tips contacted the strain gauge (model MPL-100; Transducer Techniques, Temecula, CA, USA) on two polished brass disks positioned 35 mm apart (Fig. 1a). Children were instructed to squeeze the strain gauge as hard as possible. Maximal contractions were separated by ~ 1 min to avoid fatigue, and verbal encouragement and visual feedback of force were provided during each maximal effort.
The third movement task assessed movement speed and rhythmicity with maximum finger tapping. Children were instructed to tap their index finger on a strain gauge (model MPL-100; Transducer Techniques, Temecula, CA, USA) as fast as possible for 5 s (Fig. 1b).
The fourth movement test assessed tremor in the hand during relaxation and movement. A small accelerometer (dimensions 5 × 5 × 2 mm; ± 2 g dual axis, ADXL212, Analog Devices, Norwood, Massachusetts, USA) was attached to the index finger nail (Fig. 1c) to measure small perturbations in the vertical plane (flexion–extension) with respect to acceleration due to gravity. Tremor was measured in three conditions: at rest (resting tremor), during a postural movement (postural tremor), and during an active movement (active tremor). In the resting condition, the participants hand rested on a table. In the postural condition, the participant actively held their arms in front of their chest, parallel to the floor, with the shoulders flexed at ~ 90° and the elbows fully extended. Participants were instructed to remain as still as possible while in this position. In the active condition, the participant flexed and extended their index finger with a duty cycle of ~ 2 s. Standardized verbal and visual instructions were given prior to each condition and the duration of each condition was 30 s.
The final movement test involved the Movement Assessment Battery for Children-2 (MABC-2, Henderson et al. 2007) for comparison to studies published in the literature. The MABC-2 involves three tasks that assess manual dexterity, two tasks that assess aiming and catching, and three tasks that assess static and dynamic balance. The tasks are age-specific and participants in the current study completed tasks prescribed for age band 2 (7–10.9 years) or 3 (11–16.9 years). A specified number of trials are allowed for each task and if the child passes on the first trial, then subsequent trials are not required. The MABC-2 was administered by one experienced physiotherapist (SH) with prior experience with the MABC-2 protocol (e.g. Civetta and Hillier 2008). The physiotherapist was blinded to the study group. Raw scores for each task were converted to item standard scores and summed for component scores (manual dexterity, aiming and catching, and balance) and a total score. The component and total scores were then compared to normative data. A total score equal to or less than 56 (≤ 5th percentile) indicates significant motor difficulty. A total score between 57 and 67 (6th–15th percentile) indicates a risk of motor difficulty that requires monitoring. A total score greater than 67 (> 16th percentile) indicates no movement difficulty (Henderson et al. 2007). The structural validity of the MABC-2 has been confirmed in a large sample of typically developing children (Schulz et al. 2011) and the internal consistency and reliability are reported to be excellent for a sample of 144 children with developmental coordination disorder (Wuang et al. 2012).
Each child then underwent a transcranial ultrasound to assess the morphology of the substantia nigra. The examination was performed by one researcher (GT) who is experienced with the procedure (e.g. Todd et al. 2013, 2014). The researcher was blinded to the study group. The examination was performed on a Philips iU22 ultrasound system (manufactured June 2004, refurbished November 2011 with software level 6.0.2.144) with a 1–5 MHz (model s5–1) transducer (Philips Healthcare, Best, the Netherlands) positioned over the pre-auricular acoustic bone window. Images of the right and left side were acquired in the B-mode setting with a dynamic range and penetration depth of 60 dB and 14–16 cm, respectively. A qualitative rating of the bone window was made (1—excellent, 2—good, 3—poor, 4—very poor) and the area of echogenicity at the anatomical site of the substantia nigra was measured at its greatest extent according to international guidelines (Berg et al. 2008). The internal diameter of the third ventricle was also measured (Berg et al. 2008). Inter-rater reliability and reproducibility of the operator and procedure has been previously published (Todd et al. 2013).
Data recordings
For movement tests one to four, force and acceleration signals were sampled at 400 Hz using a 1902 Amplifier and Power 1401 Interface with specialised software (Spike2; Cambridge Electronic Design, Cambridge, UK). Signals were amplified (force: × 1000; acceleration: × 1 or × 3) and low-pass filtered (Butterworth, 100 Hz).
Data analysis
Analysis of lifetime psychostimulant use involved (1) quantifying the total lifetime consumption of each psychostimulant, (2) conversion of methylphenidate doses into dexamphetamine equivalents (performed in consultation with Mr. David Ellis, Senior Specialist Pharmacist, Pharmacy Department, Women’s and Children’s Hospital, Adelaide, Australia), and (3) calculating the total lifetime dexamphetamine and/or dexamphetamine equivalent doses. Data derived from the grip and lift task were analysed using a published procedure (Todd et al. 2010, 2014). Data were first divided into a lift (dynamic) and hold (stationary) phase to standardise measurement across lifts and groups. The lift phase ranged from lift onset (0 s) to 1.5 s and the hold phase ranged from 1.5 to 2.5 s. The following parameters were measured during the lift phase: peak force and acceleration, time-to-peak force, minimum lift force (degree of downward push before lift-off), preload duration (grip onset relative to lift onset), maximum rate of change in force (dGF/dtmax and dLF/dtmax), and the temporal relationship between grip force and lift force (cross-correlation of dGF/dt and dLF/dt). The hold phase parameters included mean grip force and the coefficient of variation of grip force (%). Analysis of the maximal pinch grip strength involved measurement of the mean grip force over a 1-s period during each maximal voluntary contraction. Analysis of the finger tapping task involved measurement of the number of taps performed in 5 s and the inter-tap interval (ms), and calculation of the coefficient of variation of the inter-tap interval (%). Analysis of the tremor task involved the removal of the DC offset (time constant 0.25 s) from the raw acceleration trace followed by fast Fourier transform (size 1024, resolution 0.4 Hz, Hanning window) to construct a power spectral density. A separate fast Fourier transform was performed for each tremor condition. Data in the 0–2.7 Hz range were ignored because it comprises voluntary finger movement. For each child, mean and peak power (index of tremor amplitude) was calculated for 3.1–6.6 Hz (pathological frequency range) and 7.0–13.3 Hz (physiological frequency range). The frequency corresponding to the peak power was also measured. Table 1 summarises the dependent variables for each test of upper limb function.
Statistical analysis
Group data are presented as mean ± standard error. Kolmogorov–Smirnov test (with group as a factor) and Levene’s test was performed on all variables to test for normality and homogeneity of variance, respectively. An independent t test was used to compare age and the total MABC-2 score between the groups. The component (manual dexterity, aiming and catching, and balance) scores on the MABC-2 were transformed by addition of a constant (0.1) and then ranked. Mann–Whitney U was then used to compare the transformed component MABC-2 scores between groups. Height and weight and the ultrasound, finger tapping, and grip and lift variables were analysed with one-way analysis of covariance (ANCOVA) with age as a covariate. Variables that were significantly non-normal in one or both groups (P < 0.05), or that had variances that significantly differed in the two groups (P < 0.05), were transformed to ranks and ANCOVA was performed on the ranked data. Tremor amplitude (peak power in the spectral analysis and the frequency at which this occurred) was log transformed and analysed with repeated measures ANCOVA with age as a covariate and state as the repeated measure (rest, postural, active) (IBM SPSS Statistics 23, Armonk, NY, USA). Post hoc discrimination was made with the Holms–Bonferroni procedure. In the ADHD group, Pearson Product Moment or Spearman Rank Order correlation was used to investigate the relationship between (1) area of substantia nigra echogenicity (largest side) and movement parameters, (2) total lifetime dexamphetamine (and/or dexamphetamine equivalent) doses and substantia nigra echogenicity and (3) total lifetime dexamphetamine (and/or dexamphetamine equivalent) doses and movement parameters (SigmaPlot 11.0; Systat Software Inc, San Jose, CA, USA). Statistical significance was set at P ≤ 0.05.
Results
Subject characteristics
Table 2 shows the mean characteristics of each group. Age, height, weight, and handedness (laterality quotient) did not significantly differ between the groups. In the control group, four children were medicated for asthma and two children had dermatological conditions (eczema and psoriasis). In the ADHD group, 26 of the children had the combined type of ADHD (code 314.01 in DSM-IV-TR) and three had the predominantly inattentive type (code 314.00 in DSM-IV-TR). The mean age at diagnosis was 7.2 ± 0.4 years and all children had a history of use of psychostimulant medication (dexamphetamine and/or methylphenidate). The mean duration of psychostimulant use was 3.1 ± 0.5 years and the mean lifetime consumption of psychostimulant medication was 14,861 ± 2970 mg. Twenty-two of the 29 children were currently medicated with psychostimulants and these children were tested “off” medication (see “Methods”). Some children had also been previously diagnosed with a comorbid psychological disorder (obsessive compulsive disorder—3, auditory processing disorder—2, sensory processing disorder—1, anxiety—1, depression—1, Irlen syndrome—1) and/or learning difficulty (dyslexia—2, dysgraphia—2) and all of the families had previously tried to reduce the severity of ADHD symptoms through diet modification (e.g. fish oil supplements and/or food elimination). Some children had also undertaken behavioural and/or cognitive therapy (7/29), occupational therapy (5/29), speech therapy (3/29), and/or physiotherapy (2/29). Eleven participants were currently taking prescribed medications for asthma (6/29), blood pressure (5/29), and eczema (1/29), and one child was taking a steroid and tricyclic antidepressant (amitriptyline).
Quantitative tests of upper limb function
Data from eight children in the ADHD group and nine children in the control group were excluded from one or more of the analyses due to incorrect performance of one or more of the movement tasks. This was usually due to use of an additional finger/s during the object grip and lift task (which cannot be repeated due to motor learning) and/or an inability to sit still or perform consistent movements during assessment of tremor.
The covariate, age, was significantly related to maximum pinch grip strength (i.e. force during the largest maximal voluntary contraction; F 1,44 = 8.763, P = 0.005). However, there was no significant difference in pinch grip strength between the groups after accounting for age (age-adjusted control: 41.8 ± 2.8 N, age-adjusted ADHD: 39.2 ± 2.5 N).
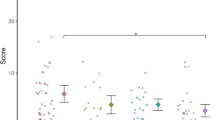
Figure 2a, b shows raw data for the object grip and lift task. Contact between the fingers and the object is evidenced by the initial increase in grip force. Movement of the object off the table is indicated by the increase in lift force and acceleration. Grip and lift force continued to increase during the lift phase and then attained a plateau while the object was held stationary above the table. At this time, grip force was larger than the lift force, indicating a safety margin to prevent slipping. The raw traces suggest that the child with ADHD performed the task with a lower grip force than the typically developing child. The covariate, age, was significantly related to the variability of grip force (coefficient of variation) during the hold phase (F 1,44 = 18.24, P < 0.001), but not to other variables measured during the object grip and lift task. The groups significantly differed in grip force (expressed as a percentage of maximal voluntary contraction; %MVC) in the lift phase (F 1,44 = 4.673, P = 0.036) and hold phase (F 1,44 = 4.077, P = 0.050) of the task. Children in the ADHD group applied less grip force than children in the control group (lift phase: see Fig. 2a–c, hold phase: control = 18.5 ± 1.8% MVC, ADHD = 13.6 ± 1.1% MVC). The groups also significantly differed in the maximum rate of change in grip force (dGF/dt, F 1,44 = 4.215, P = 0.046) and coefficient of variation of grip force (F 1,44 = 9.774, P = 0.003). Children in the ADHD group applied grip force at a slower rate than children in the control group (Fig. 2d) and their grip force varied more while holding the object stationary (control 5.1 ± 0.8%, ADHD 7.1 ± 0.9%). No other significant between-group differences were observed in performance of the grip and lift task.
Single-subject and group (n = 21 ADHD, n = 26 control) data showing parameters measured during the object grip and lift task. a Raw traces from one typically developing control child aged 9.3 years. b Raw traces from one ADHD-diagnosed child aged 10.7 years. c Group data showing peak grip force during the lift phase (expressed as a percentage of force during the largest maximal voluntary pinch grip contraction; %MVC). d Group data showing the maximum rate of change in grip force (dGF/dt) during the lift phase. In c and d, the boundary of each box indicates the 25th and 75th percentile, the whiskers (error bars) indicate the 10th and 90th percentiles, and the solid and dashed lines within each box indicate the median and mean values, respectively. *Significant difference between the control and ADHD groups (ANCOVA with age as covariate, P < 0.05)
Figure 3a, b shows raw data for the maximal finger tapping task. The raw traces suggest that the inter-tap interval was more variable in the child with ADHD than in the typically developing child. The covariate, age, was significantly related to movement speed (number of taps performed in 5 s; F 1,46 = 12.115, P = 0.001), but not rhythmicity of movement (coefficient of variation of the inter-tap interval). Movement speed did not significantly differ between the groups (i.e. number of taps in 5 s; Fig. 3c), but a significant difference was present for movement rhythmicity (F 1,46 = 7.661, P = 0.008; Fig. 3d). The coefficient of variation of the inter-tap interval was greater in the ADHD group (age-adjusted 13.3 ± 1.4%) than in the control group (age-adjusted 9.7 ± 1.3%).
Single subject and group (n = 23 ADHD, n = 26 control) data for the maximal tapping task. a Raw force trace from one typically developing child aged 13.3 years. b Raw force trace from one child aged 13.2 years with ADHD. c Group data showing the number of taps in 5 s. d Group data showing the coefficient of variation (CV) for the inter-tap interval expressed as a percentage. In c and d, the boundary of each box indicates the 25th and 75th percentile, the whiskers (error bars) indicate the 10th and 90th percentiles, and the solid and dashed lines within each box indicate the median and mean values, respectively. *Significant difference between the control and ADHD groups (P = 0.008)
Figure 4a, b shows tremor data for one typically developing child and one child with ADHD. Raw acceleration traces during relaxation are shown (a) along with the accompanying fast Fourier transform (b). There was a large peak in the fast Fourier transform in the physiological frequency range (7.0–13.3 Hz) and the peak did not differ between the two representative children. For the tremor tasks, the covariate, age, was not significantly related to tremor amplitude (peak power in the spectral analysis) in the physiological range (7.0–13.3 Hz), but it was significantly related to tremor amplitude in the pathological range (3.1–6.6 Hz, F 1,44 = 4.409, P = 0.042). Tremor amplitude significantly differed between conditions in both the physiological frequency range (7.0–13.3 Hz, F 2,88 = 104.902, P < 0.001) and pathological frequency range (3.1–6.6 Hz, F 2,88 = 145.027, P < 0.001). Figure 4c shows that the tremor amplitude was smallest during the relaxation condition and largest during the active condition (all pairwise: P < 0.001). There was also a significant condition-by-age interaction on amplitude of tremor in the physiological (F 2,88 = 4.191, P = 0.018) and pathological (F 2,88 = 9.078, P < 0.001) frequency range. However, no significant effect of group or condition-by-group interaction was observed. The covariate, age, was not significantly related to the frequency at which the peak tremor occurred in the physiological (7.0–13.3 Hz) range and the peak frequency did not significantly differ between conditions and no significant effect of group or condition-by-group interaction was observed.
Single subject and group (n = 23 ADHD, n = 26 control) data for the tremor tasks. a Raw acceleration traces during relaxation for one typically developing child (aged 14.0 years, left) and one child with ADHD (aged 14.8 years, right). b Result of the fast Fourier transform for the traces presented in a. c Group data showing tremor amplitude (peak power in the spectral analysis) during relaxation, the postural task, and flexion and extension of the index finger. The boundary of each box indicates the 25th and 75th percentile, the whiskers (error bars) indicate the 10th and 90th percentiles, and the solid and dashed lines within each box indicate the median and mean values, respectively
Movement ABC-2
Table 3 shows group data for the component and total scores on the MABC-2. The groups significantly differed in the component score for dexterity (P = 0.007) and aiming and catching (P = 0.042) but not for balance (P = 0.395). The score for dexterity and aiming and catching was lower (poorer) in the ADHD group than in the control group. The total MABC-2 score was also significantly lower (poorer) in the ADHD group than in the control group (P = 0.004). Two participants in the ADHD group exhibited a total MABC-2 score below the 5th percentile.
Transcranial ultrasound
The pre-auricular acoustic bone window was subjectively rated as good to excellent in 92% of children and poor in the remaining 8%. No children were excluded due to an insufficient bone window for insonation. The mean maximum rating of the bone window (right or left side) was 1.1 ± 0.1 for the control group and 1.4 ± 0.1 for the ADHD group. All children exhibited a normal diameter of the third ventricle. The covariate, age, was not significantly related to the diameter of the third ventricle and no between-group difference was observed.
Figure 5a, b shows raw single subject images of the substantia nigra and associated landmarks. The area of substantia nigra echogenicity is larger in the ADHD-diagnosed child than in the typically developing child. The covariate, age, was significantly related to the area of substantia nigra echogenicity (F 1,61 = 4.670, P = 0.035). Figure 5c shows that the mean area of substantia nigra echogenicity (largest side) was significantly larger in the ADHD group (raw 0.189 ± 0.013 cm2, age-adjusted 0.186 ± 0.010 cm2) than in the control group (raw 0.149 ± 0.007 cm2, age-adjusted 0.152 ± 0.009 cm2; F 1,61 = 6.067, P = 0.017).
Echomorphology of the substantia nigra and mesencephalic brainstem. a Raw image from one typically developing control child aged 13.2 years. b Raw image from one ADHD-diagnosed child aged 14.0 years. The outline of the substantia nigra (solid line) ipsilateral to the probe (the side at which the planimetric measurement is done) is shown along with the mesencephalic brainstem (dashed line). The calibration bar represents 0.5 cm. c Group data (n = 29 ADHD, n = 35 control) showing the area of substantia nigra echogenicity. Data represent the largest area across the right and left side. The boundary of each box indicates the 25th and 75th percentile and the whiskers (error bars) indicate the 10th and 90th percentiles. The solid and dashed lines within each box indicate the median and mean values, respectively. *Significant difference between the control and ADHD groups (ANCOVA with age as covariate: P = 0.017)
Correlations
In the ADHD group, the area of substantia nigra echogenicity (on the largest side) did not significantly correlate with age, movement parameters, or total lifetime consumption of psychostimulant medication. There was, however, a significant correlation between the total lifetime consumption of psychostimulant medication and measures of upper limb function, but not gross motor function. Figure 6 shows that there was a significant positive correlation between total lifetime consumption of psychostimulant medication and the peak frequency of tremor in the physiological range (Fig. 6a, postural: r = 0.417, P = 0.048; active: r = 0.546, P = 0.007), maximal pinch grip strength (largest force, Fig. 6b, r = 0.483, P = 0.026), and the coupling of grip force and lift force during the grip and lift task (maximum cross-correlation coefficient, Fig. 6c, r = 0.448, P = 0.041). A significant negative correlation was also observed between total lifetime consumption of psychostimulant medication and the time-to-peak lift force (Fig. 6d, r = − 0.466, P = 0.033) and acceleration (r = − 0.540, P = 0.021) in the grip and lift task.
Correlation between psychostimulant use and hand function parameters in the ADHD group. a Linear relationship between the log of the total lifetime consumption of psychostimulant medication and the peak frequency of tremor in the physiological range (7–13 Hz) during the postural task (filled circles, solid line; r = 0.417, P = 0.048) and flexion and extension of the index finger (open circles, dashed line; r = 0.546, P = 0.007). b Linear relationship between the log of the total lifetime consumption of psychostimulant medication and pinch grip strength (largest force, r = 0.483, P = 0.026). c Linear relationship between the log of the total lifetime consumption of psychostimulant medication and the coupling of grip force and lift force during the grip and lift task (maximum cross-correlation coefficient, r = 0.448, P = 0.041). d Linear relationship between the log of the total lifetime consumption of psychostimulant medication and time-to-peak lift force (filled circles, solid line; r = − 0.466, P = 0.033) and acceleration (open circles, dashed line; r = − 0.540, P = 0.021) during the grip and lift task
Discussion
The results of the study confirm that ADHD is associated with deficits in fine motor function and show for the first time that the deficits affect multiple, but not all, domains of upper limb function. ADHD is associated with abnormal manipulation of a novel object, movement rhythmicity, and performance on the dexterity and aiming and catching components of the MABC-2, but pinch grip strength, movement speed, and tremor are unaffected. Lifetime consumption of psychostimulant medication is associated with performance on some of the movement tasks but it does not appear to contribute to the movement deficits.
Children with ADHD use a different strategy to manipulate novel objects than typically developing controls. This was evidenced by a significant between-group difference in three parameters of the grip and lift task. The ability to grip and lift a novel object requires estimation of the grip force required to complete the task, prior to contacting the object. The estimation process is thought to involve an internal model based on the physical characteristics of the object, prior experience, sensory feedback, and the individual’s safety margin to prevent the object from slipping (Flanagan et al. 2006; Johansson 1998). Our results suggest that this internal model is altered in children with ADHD. Children in the ADHD group applied less grip force, in both the lift and hold phase of the task, than children in the control group. This suggests that children with ADHD underestimate the grip force required to lift and hold a novel object. The lower grip force indicates greater risk of the object slipping from the hand. A strategy to compensate for a high slip risk is to apply grip force at a slower rate or to adjust grip force while holding the object stationary. Both of these compensatory strategies were observed in the ADHD group. Children in the ADHD group applied grip force at a significantly slower rate than children in the control group and their grip force varied significantly more while holding the object stationary.
Two previous studies have used the grip and lift task to assess fine motor function in this population (Jucaite et al. 2003; Pereira et al. 2000). Neither study reported data for the first trial which is the only time point that enables assessment of motor planning, separate from the effects of motor learning. Both of these studies also had several methodological features that limit interpretation of the results. First, neither study measured maximum pinch grip strength, which makes between-subject comparison of grip force during the grip and lift task difficult. For example, two children may use the same raw grip force to lift the object (e.g. 10 N) but the effort required to generate this force can differ greatly between children if one child is stronger than the other. Grip strength increases by more than 240% from 6 to 19 years of age (McQuiddy et al. 2015) which is why it is prudent to normalise grip force to maximum pinch grip strength, particularly for cohorts containing children and adolescents. In the current study, there was no significant between-group difference in muscle strength and the between-group difference in performance of the grip and lift task was not related to strength because grip force was expressed as a percentage of maximum pinch grip strength. The Jucaite et al. (2003) study also involved children performing the task in an uncommon body position (standing with the feet close together and the arm held straight out at the level of the shoulder) and neither study reported history of psychostimulant use in the sample (Jucaite et al. 2003; Pereira et al. 2000). The latter is important because our results show, for the first time, that the temporal characteristics of the grip and lift task correlate with history of use of psychostimulant medication in ADHD children. There was a significant positive correlation between the total lifetime consumption of psychostimulant medication and the coupling of grip force and lift force during the grip and lift task. There was also a significant negative correlation between total lifetime consumption of psychostimulant medication and the time-to-peak lift force and acceleration. Collectively, the results suggest that greater cumulative use of psychostimulant medication is associated with more rapid elevation of a novel object and better coupling of grip force to changes in the effect of gravity on the object.
Identifying the mechanism for the disturbed manipulation of novel objects in ADHD-diagnosed children, and the effect of psychostimulants on this process, is difficult and beyond the scope of the current study. The difficulty is due to the number of central and peripheral nervous system structures that are involved in the performance of the tasks, and their associated, and widely distributed, neuronal networks. For example, functional neuroimaging studies suggest that performance of precision grip tasks involves the primary motor cortex and somatosensory cortex, premotor areas, and the cingulate motor area, lateral prefrontal cortex, and areas in the parietal cortex (e.g. Ehrsson et al. 2001; Kuhtz-Buschbeck et al. 2001). Precision grip tasks also engage spinal circuitry, and involve feedback from cutaneous, muscle, and joint afferents, but the effect of ADHD on the excitability and function of these spinal and afferent circuits has not been investigated.
Movement rhythmicity, but not movement speed, was also impaired in the ADHD group during the maximal finger tapping task. The impaired movement rhythmicity was evidenced by a significantly greater coefficient of variation of the inter-tap interval in the ADHD group (age-adjusted 13.3 ± 1.4%) than in the control group (age-adjusted 9.7 ± 1.3%). The result supports our hypothesis and suggests that ADHD is associated with an inability to sustain a self-chosen, fast, regular pattern of motor output. The result is consistent with previous observations of deficits in movement rhythmicity, but not movement speed, during finger tapping tasks (Ben-Pazi et al. 2003; Meyer and Sagvolden 2006; Rubia et al. 1999, 2003). The pathophysiology that underlies the abnormal movement rhythmicity is not fully understood, but may involve changes in movement-timing related circuitry in the cerebellum and basal ganglia (Kasparek et al. 2015). The pathophysiology is unlikely to be associated with cumulative psychostimulant use because there was no correlation between total lifetime psychostimulant use and movement rhythmicity.
Our study is the first to objectively quantify tremor in children diagnosed with ADHD. Tremor was measured on the index finger with the use of an accelerometer and a spectral analysis of the resultant acceleration trace was performed. This method yields sensitive measures of both the amplitude and peak frequency of tremor and is routinely used in the investigation of tremor in movement disorders (e.g. essential tremor and Parkinson’s disease, for review see Hallett 1998). The results of the current study suggest that tremor is normal in children with ADHD. A significant positive correlation was observed between total lifetime consumption of psychostimulant medication and the peak frequency of tremor in the physiological range during the postural task and active movements of the index finger. However, the largest peak frequency of tremor observed in the current study (10.5 Hz) still fell well within the normal physiological range (8–12 Hz, for review see Hallett 1998; McAuley and Marsden 2000). ‘Tremor’ is listed as a potential side effect of dexamphetamine but this usually refers to the amplitude of tremor, not the frequency at which the tremor occurs. Only four participants in the current study were being treated with dexamphetamine and the amplitude of tremor in these participants did not differ from those treated with methylphenidate.
The movement deficits observed with the objective methods were detectible with the MABC-2. The MABC-2 component score for dexterity and aiming and catching were significantly lower (poorer) in the ADHD group than in the control group. This result supports previous MABC findings in this population (Jucaite et al. 2003; Kooistra et al. 2009; Piek et al. 1999; Pitcher et al. 2003). The motor deficits experienced by children with ADHD are likely to impact on real-world function ranging from writing in the classroom to participation in physical activities in the playground. The MABC-2 also includes an assessment of gross motor function. However, no between-group differences in the balance component were observed in the current study (see also Jucaite et al. 2003; Pitcher et al. 2003; c.f. Kooistra et al. 2009; Piek et al. 1999).
It was hypothesised that movement dysfunction would be associated with abnormal substantia nigra morphology in the ADHD group. The sonographic appearance of the substantia nigra is known to be abnormally bright and enlarged in ADHD children compared to typically developing controls (Krauel et al. 2010; Romanos et al. 2010), and this was confirmed in the current study. However, there was no correlation between the area of substantia nigra echogenicity and performance on the motor tasks. The mechanisms that underlie an abnormally large area of substantia nigra echogenicity are not well understood, but in adults, the abnormality is thought to reflect abnormal iron accumulation (Berg et al. 1999, 2002; Zecca et al. 2005), decreased neuromelanin (Zecca et al. 2005), and activation of microglia (Berg et al. 2010). Healthy older adults with this abnormality are 17 times more likely to develop Parkinson’s disease over a 3-year period (Berg et al. 2011). However, it is unclear what the long-term ramifications are for children with substantia nigra hyperechogenicity.
There are three limitations of the current study. First, the experimental design did not include tests of neuropsychological function. However, neuropsychological performance is unlikely to have had a major effect on the results of the current study because all of the children in the ADHD group were in the appropriate school level for their age and movement deficits were only observed in certain movement domains. Second, the ADHD sample did not permit comparison of movement between the subtypes of ADHD. Twenty six of the 29 children in the ADHD group had the combined type of ADHD and thus dividing the sample into subtypes was not possible. Future studies should use a larger sample to explore this issue further. The third limitation relates to the association between lifetime use of psychostimulant medication and upper limb function. All children were tested “off” medication in the current study but the research question is better suited to a longitudinal experimental design with administration of the movement tests before and 2–3 years after commencement of psychostimulant medication. A final consideration is the study exclusion criterion and sample size. A strict exclusion criterion was used in the current study to limit the incidence of comorbid disorders in the sample of children diagnosed with ADHD. The strict exclusion criterion was to enable, as far as possible, investigation of the effect of ADHD on movement and substantia nigra morphology, separate to that of other comorbid disorders. The sample size used in the current study was larger than most previous studies involving objective quantification of upper limb function in children diagnosed with ADHD (e.g. Schoemaker et al. 2005; Shen et al. 2012; Slaats-Willemse et al. 2005). However, use of a larger sample size may have revealed further subtle, statistically significant, deficits in upper limb function.
In conclusion, quantification of upper limb function, with sensitive and objective methods, reveals deficits in fine motor function in children with ADHD. The results demonstrate for the first time that deficits affect multiple, but not all, domains of upper limb function and that ADHD is associated with abnormal planning for manipulation of novel objects, but pinch grip strength, movement speed, and tremor are unaffected. Lifetime use of psychostimulant medication is associated with performance of some upper limb movements, but it does not appear to cause the motor deficits. Further objective quantification of multiple movement domains is required to progress understanding of the deficits associated with this disorder and inform on the potential suitability of motor biomarkers and interventions for ADHD.
References
American Psychiatric Association (2000) Diagnostic and Statistical Manual of Mental Disorders—text revision, 4th edn. American Psychiatric Association, Washington, D.C.
American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th edn. American Psychiatric Association, Arlington
Ben-Pazi H, Gross-Tsur V, Bergman H, Shalev RS (2003) Abnormal rhythmic motor response in children with attention-deficit-hyperactivity disorder. Dev Med Child Neurol 45:743–745. https://doi.org/10.1111/j.1469-8749.2003.tb00883.x
Berg D, Grote C, Rausch WD, Maurer M, Wesemann W, Riederer P, Becker G (1999) Iron accumulation in the substantia nigra in rats visualized by ultrasound. Ultrasound Med Biol 25:901–904. https://doi.org/10.1016/S0301-5629(99)00046-0
Berg D, Jabs B, Merschdorf U, Beckmann H, Becker G (2001) Echogenicity of substantia nigra determined by transcranial ultrasound correlates with severity of parkinsonian symptoms induced by neuroleptic therapy. Biol Psychiatry 50:463–467. https://doi.org/10.1016/S0006-3223(01)01190-8
Berg D, Roggendorf W, Schroder U, Klein R, Tatschner T, Benz P, Tucha O, Preier M, Lange KW, Reiners K, Gerlach M, Becker G (2002) Echogenicity of the substantia nigra: association with increased iron content and marker for susceptibility to nigrostriatal injury. Arch Neurol 59:999–1005. https://doi.org/10.1001/archneur.59.6.999
Berg D, Godau J, Walter U (2008) Transcranial sonography in movement disorders. Lancet Neurol 7:1044–1055. https://doi.org/10.1016/S1474-4422(08)70239-4
Berg D, Godau J, Riederer P, Gerlach M, Arzberger T (2010) Microglia activation is related to substantia nigra echogenicity. J Neural Transm 117:1287–1292. https://doi.org/10.1007/s00702-010-0504-6
Berg D, Seppi K, Behnke S, Liepelt I, Schweitzer K, Stockner H, Wollenweber F, Gaenslen A, Mahlknecht P, Spiegel J, Godau J, Huber H, Srulijes K, Kiechl S, Bentele M, Gasperi A, Schubert T, Hiry T, Probst M, Schneider V, Klenk J, Sawires M, Willeit J, Maetzler W, Fassbender K, Gasser T, Poewe W (2011) Enlarged substantia nigra hyperechogenicity and risk for Parkinson disease: a 37-month 3-center study of 1847 older persons. Arch Neurol 68:932–937. https://doi.org/10.1001/archneurol.2011.141
Buderath P, Gartner K, Frings M, Christiansen H, Schoch B, Konczak J, Gizewski ER, Hebebrand J, Timmann D (2009) Postural and gait performance in children with attention deficit/hyperactivity disorder. Gait Posture 29:249–254. https://doi.org/10.1016/j.gaitpost.2008.08.016
Carmona S, Vilarroya O, Bielsa A, Tremols V, Soliva JC, Rovira M, Tomas J, Raheb C, Gispert JD, Batlle S, Bulbena A (2005) Global and regional gray matter reductions in ADHD: a voxel-based morphometric study. Neurosci Lett 389:88–93. https://doi.org/10.1016/j.neulet.2005.07.020
Civetta LR, Hillier SL (2008) The developmental coordination disorder questionnaire and movement assessment battery for children as a diagnostic method in Australian children. Pediatr Phys Ther 20:39–46. https://doi.org/10.1097/PEP.0b013e31815ccaeb
Ehrsson HH, Fagergren E, Forssberg H (2001) Differential fronto-parietal activation depending on force used in a precision grip task: an fMRI study. J Neurophysiol 85:2613–2623
Fearnley JM, Lees AJ (1991) Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain 114:2283–2301. https://doi.org/10.1093/brain/114.5.2283
Flanagan JR, Bowman MC, Johansson RS (2006) Control strategies in object manipulation tasks. Curr Opin Neurobiol 16:650–659. https://doi.org/10.1016/j.conb.2006.10.005
Fliers EA, Franke B, Lambregts-Rommelse NN, Altink ME, Buschgens CJ, Nijhuis-van der Sanden MW, Sergeant JA, Faraone SV, Buitelaar JK (2009) Undertreatment of motor problems in children with ADHD. Child Adolesc Ment Health 15:85–90. https://doi.org/10.1111/j.1475-3588.2009.00538.x
Frodl T, Skokauskas N (2012) Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatr Scand 125:114–126. https://doi.org/10.1111/j.1600-0447.2011.01786.x
Hallett M (1998) Overview of human tremor physiology. Mov Disord 13(Suppl 3):43–48. https://doi.org/10.1002/mds.870131308
Harvey WJ, Reid G, Grizenko N, Mbekou V, Ter-Stepanian M, Joober R (2007) Fundamental movement skills and children with attention-deficit hyperactivity disorder: peer comparisons and stimulant effects. J Abnorm Child Psychol 35:871–882. https://doi.org/10.1007/s10802-007-9140-5
Henderson SE, Sugden DA, Barnett A (2007) Movement assessment battery for children, 2nd edn. Pearson, London
Johansson RS (1998) Sensory input and control of grip. Novartis Found Symp 218:45–59. https://doi.org/10.1002/9780470515563.ch4 (discussion 59–63)
Jucaite A, Fernell E, Forssberg H, Hadders-Algra M (2003) Deficient coordination of associated postural adjustments during a lifting task in children with neurodevelopmental disorders. Dev Med Child Neurol 45:731–742. https://doi.org/10.1111/j.1469-8749.2003.tb00882.x
Kadesjo B, Gillberg C (1998) Attention deficits and clumsiness in Swedish 7-year-old children. Dev Med Child Neurol 40:796–804. https://doi.org/10.1111/j.1469-8749.1998.tb12356.x
Kasparek T, Theiner P, Filova A (2015) Neurobiology of ADHD from childhood to adulthood: findings of imaging methods. J Atten Disord 19:931–943. https://doi.org/10.1177/1087054713505322
Kooistra L, Ramage B, Crawford S, Cantell M, Wormsbecker S, Gibbard B, Kaplan BJ (2009) Can attention deficit hyperactivity disorder and fetal alcohol spectrum disorder be differentiated by motor and balance deficits? Hum Mov Sci 28:529–542. https://doi.org/10.1016/j.humov.2009.01.007
Krauel K, Feldhaus HC, Simon A, Rehe C, Glaser M, Flechtner HH, Heinze HJ, Niehaus L (2010) Increased echogenicity of the substantia nigra in children and adolescents with attention-deficit/hyperactivity disorder. Biol Psychiatry 68:352–358. https://doi.org/10.1016/j.biopsych.2010.01.013
Kuhtz-Buschbeck JP, Ehrsson HH, Forssberg H (2001) Human brain activity in the control of fine static precision grip forces: an fMRI study. Eur J Neurosci 14:382–390. https://doi.org/10.1046/j.0953-816x.2001.01639.x
McAuley JH, Marsden CD (2000) Physiological and pathological tremors and rhythmic central motor control. Brain 123:1545–1567. https://doi.org/10.1093/brain/123.8.1545
McQuiddy VA, Scheerer CR, Lavalley R, McGrath T, Lin L (2015) Normative values for grip and pinch strength for 6- to 19-year-olds. Arch Phys Med Rehabil 96:1627–1633. https://doi.org/10.1016/j.apmr.2015.03.018
Meyer A, Sagvolden T (2006) Fine motor skills in South African children with symptoms of ADHD: influence of subtype, gender, age, and hand dominance. Behav Brain Funct 2:33. https://doi.org/10.1186/1744-9081-2-33
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113
Papadopoulos N, McGinley JL, Bradshaw JL, Rinehart NJ (2014) An investigation of gait in children with Attention Deficit Hyperactivity Disorder: a case controlled study. Psychiatry Res 218:319–323. https://doi.org/10.1016/j.psychres.2014.04.037
Pearson-Dennett V, Flavel SC, Wilcox RA, Thewlis D, Vogel AP, White JM, Todd G (2014) Hand function is altered in individuals with a history of illicit stimulant use. PLoS One 9:e115771. https://doi.org/10.1371/journal.pone.0115771
Pereira HS, Eliasson AC, Forssberg H (2000) Detrimental neural control of precision grip lifts in children with ADHD. Dev Med Child Neurol 42:545–553. https://doi.org/10.1111/j.1469-8749.2000.tb00711.x
Piek JP, Pitcher TM, Hay DA (1999) Motor coordination and kinaesthesis in boys with attention deficit-hyperactivity disorder. Dev Med Child Neurol 41:159–165. https://doi.org/10.1111/j.1469-8749.1999.tb00575.x
Pitcher TM, Piek JP, Hay DA (2003) Fine and gross motor ability in males with ADHD. Dev Med Child Neurol 45:525–535. https://doi.org/10.1111/j.1469-8749.2003.tb00952.x
Romanos M, Weise D, Schliesser M, Schecklmann M, Loffler J, Warnke A, Gerlach M, Classen J, Mehler-Wex C (2010) Structural abnormality of the substantia nigra in children with attention-deficit hyperactivity disorder. J Psychiatry Neurosci 35:55–58. https://doi.org/10.1503/jpn.090044
Rosa Neto F, Goulardins JB, Rigoli D, Piek JP, Oliveira JA (2015) Motor development of children with attention deficit hyperactivity disorder. Rev Bras Psiquiatr 37:228–234. https://doi.org/10.1590/1516-4446-2014-1533
Rossi S, Hallett M, Rossini PM, Pascual-Leone A (2009) Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120:2008–2039. https://doi.org/10.1016/j.clinph.2009.08.016
Rubia K, Taylor A, Taylor E, Sergeant JA (1999) Synchronization, anticipation, and consistency in motor timing of children with dimensionally defined attention deficit hyperactivity behaviour. Percept Mot Skills 89:1237–1258. https://doi.org/10.2466/pms.1999.89.3f.1237
Rubia K, Noorloos J, Smith A, Gunning B, Sergeant J (2003) Motor timing deficits in community and clinical boys with hyperactive behavior: the effect of methylphenidate on motor timing. J Abnorm Child Psychol 31:301–313. https://doi.org/10.1023/A:1023233630774
Schoemaker MM, Ketelaars CE, van Zonneveld M, Minderaa RB, Mulder T (2005) Deficits in motor control processes involved in production of graphic movements of children with attention-deficit-hyperactivity disorder. Dev Med Child Neurol 47:390–395. https://doi.org/10.1111/j.1469-8749.2005.tb01159.x
Schulz J, Henderson SE, Sugden DA, Barnett AL (2011) Structural validity of the Movement ABC-2 test: factor structure comparisons across three age groups. Res Dev Disabil 32:1361–1369. https://doi.org/10.1016/j.ridd.2011.01.032
Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, Clasen L, Evans A, Giedd J, Rapoport JL (2007) Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci USA 104:19649–19654. https://doi.org/10.1073/pnas.0707741104
Shen IH, Lee TY, Chen CL (2012) Handwriting performance and underlying factors in children with attention deficit hyperactivity disorder. Res Dev Disabil 33:1301–1309. https://doi.org/10.1016/j.ridd.2012.02.010
Slaats-Willemse D, de Sonneville L, Swaab-Barneveld H, Buitelaar J (2005) Motor flexibility problems as a marker for genetic susceptibility to attention-deficit/hyperactivity disorder. Biol Psychiatry 58:233–238. https://doi.org/10.1016/j.biopsych.2005.03.046
Todd G, Gandevia SC, Taylor JL (2010) Change in manipulation with muscle fatigue. Eur J Neurosci 32:1686–1694. https://doi.org/10.1111/j.1460-9568.2010.07444.x
Todd G, Noyes C, Flavel SC, Della Vedova CB, Spyropoulos P, Chatterton B, Berg D, White JM (2013) Illicit stimulant use is associated with abnormal substantia nigra morphology in humans. PLoS One 8:e56438. https://doi.org/10.1371/journal.pone.0056438
Todd G, Haberfield M, Faulkner PL, Rae C, Hayes M, Wilcox RA, Taylor JL, Gandevia SC, Godau J, Berg D, Piguet O, Double KL (2014) Hand function is impaired in healthy older adults at risk of Parkinson’s disease. J Neural Transm 121:1377–1386. https://doi.org/10.1007/s00702-014-1218-y
Todd G, Pearson-Dennett V, Wilcox RA, Chau MT, Thoirs K, Thewlis D, Vogel AP, White JM (2016) Adults with a history of illicit amphetamine use exhibit abnormal substantia nigra morphology and parkinsonism. Parkinsonism Relat Disord 25:27–32. https://doi.org/10.1016/j.parkreldis.2016.02.019
Whitmont S, Clark C (1996) Kinaesthetic acuity and fine motor skills in children with attention deficit hyperactivity disorder: a preliminary report. Dev Med Child Neurol 38:1091–1098. https://doi.org/10.1111/j.1469-8749.1996.tb15072.x
Wuang YP, Su JH, Su CY (2012) Reliability and responsiveness of the Movement Assessment Battery for Children-Second Edition Test in children with developmental coordination disorder. Dev Med Child Neurol 54:160–165. https://doi.org/10.1111/j.1469-8749.2011.04177.x
Zecca L, Berg D, Arzberger T, Ruprecht P, Rausch WD, Musicco M, Tampellini D, Riederer P, Gerlach M, Becker G (2005) In vivo detection of iron and neuromelanin by transcranial sonography: a new approach for early detection of substantia nigra damage. Mov Disord 20:1278–1285. https://doi.org/10.1002/mds.20550
Acknowledgements
This work was funded by the Channel 7 Children’s Research Foundation (12631), National Health and Medical Research Council of Australia (GT held a Career Development Award ID 627003), and the University of South Australia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The ultrasound machine used in this study was funded by the Channel 7 Children’s Research Foundation.
Conflict of interest
The authors declare no other conflicts of interest.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study and/or their parent or guardian.
Rights and permissions
About this article
Cite this article
Hotham, E., Haberfield, M., Hillier, S. et al. Upper limb function in children with attention-deficit/hyperactivity disorder (ADHD). J Neural Transm 125, 713–726 (2018). https://doi.org/10.1007/s00702-017-1822-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-017-1822-8