Abstract
Electrical stimulation of the greater occipital nerve (GON) has recently shown promise as an effective non-pharmacological prophylactic therapy for drug-resistant chronic primary headaches, but the neurobiological mechanisms underlying its anticephalgic action are not elucidated. Considering that the spinal trigeminal nucleus (STN) is a key segmental structure playing a prominent role in pathophysiology of headaches, in the present study we evaluated the effects of GON electrical stimulation on ongoing and evoked firing of the dura-sensitive STN neurons. The experiments were carried out on urethane/chloralose-anesthetized, paralyzed and artificially ventilated male Wistar rats. Extracellular recordings were made from 11 neurons within the caudal part of the STN that received convergent input from the ipsilateral facial cutaneous receptive fields, dura mater and GON. In each experiment, five various combinations of the GON stimulation frequency (50, 75, 100 Hz) and intensity (1, 3, 6 V) were tested successively in 10 min interval. At all parameter sets, preconditioning GON stimulation (250 ms train of pulses applied before each recording) produced suppression of both the ongoing activity of the STN neurons and their responses to electrical stimulation of the dura mater. The inhibitory effect depended mostly on the GON stimulation intensity, being maximally pronounced when a stimulus of 6 V was applied. Thus, the GON stimulation-induced inhibition of trigeminovascular nociceptive processing at the level of STN has been demonstrated for the first time. The data obtained can contribute to a deeper understanding of neurophysiological mechanisms underlying the therapeutic efficacy of GON stimulation in primary headaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years neurophysiologists and physicians have shown increasing interest in the use of non-pharmacological interventions to manage different types of drug-resistant primary and secondary headaches. Attention is focused mainly on the invasive and non-invasive methods of electrical stimulation of various central and peripheral nervous structures, as evidenced by numerous studies and reviews published in the field (Schwedt 2009; Broggi et al. 2010; Jenkins and Tepper 2011a, b; Magis and Schoenen 2012; Hoffmann and Magis 2013; Jürgens and Leone 2013; Martelletti et al. 2013; Rasskazoff and Slavin 2013; Lambru and Matharu 2014).
On the basis of information accumulated to date, the electrical stimulation of the greater occipital nerve (GON), in regards to its efficacy, safety and the extent of invasion, seems to be the favored method of anticephalgic electro-neuro stimulation (Lambru and Matharu 2012; Palmisani et al. 2013; Mammis et al. 2015). Indeed, clinical studies that have employed different designs reported the successful use of GON electrical stimulation for the treatment of such chronic, medically intractable nosologies as occipital neuralgia (Johnstone and Sundaraj 2006), hemicrania continua (Burns et al. 2008; Pascual 2009), SUNCT and SUNA (Lambru et al. 2014), cluster headache (Burns et al. 2009; Fontaine et al. 2011; Magis and Schoenen 2011a, b) and migraine (Saper et al. 2011; Notaro et al. 2014; Tavanaiepour and Levy 2014; Young 2014; Dodick et al. 2015), although in a number of controlled trials on the effectiveness of GON stimulation in chronic migraine the primary endpoint was not achieved (Chen et al. 2015). The efficacy of GON stimulation in medically refractory hypnic headache (Son et al. 2012), headache in patients with Chiari malformation (Vadivelu et al. 2012), medication overuse headache (Serra and Marchioretto 2012), chronic tension-type headache (Bono et al. 2015) and cervicogenic headache (Rodrigo-Royo et al. 2005; Shin et al. 2011) has also been demonstrated. The general analysis of all studies cited above indicates that, as a rule, more than half of patients receiving GON stimulation procedure had substantial reductions in frequency and/or intensity of their headache attacks, displayed quality of life improvement and a decrease in the use of abortive and/or prophylactic anticephalgic drugs.
However, in spite of the successful use of GON electrical stimulation for the treatment of headaches, the precise mechanisms underlying its therapeutic action remain unclear. The current explanations of the observed anticephalgic effect are highly speculative and mostly limited to a vague assumption of the GON stimulation-induced inhibition of the Aδ and C fiber afferent activity as well as the activation of the spinal and suprasegmental structures involved in nociceptive signal processing (Schwedt 2009; Jenkins and Tepper 2011a; Lambru and Matharu 2012; Serra and Marchioretto 2012; Lambru et al. 2014; Tavanaiepour and Levy 2014; Mammis et al. 2015). For the most part, these conclusions are based on single neuroimaging data (Matharu et al. 2004; Magis et al. 2011) and arise from application of the gate control theory (Melzack and Wall 1965) to the trigemino-cervical convergence mechanisms (Bartsch and Goadsby 2002, 2003a, b; Piovesan et al. 2003; Bartsch 2005).
The lack of a clear concept is fully understandable and mainly due to the deficiency of studies investigating the effects of the GON electrical stimulation in an animal model of headache. Previously it has been demonstrated that stimulation of the GON increases metabolic activity in the trigeminal nucleus caudalis and cervical dorsal horn of the cat (Goadsby et al. 1997) as well as heightens the trigeminocervical neuron excitability to the meningeal input in rats (Bartsch and Goadsby 2002). However, these findings contribute to the understanding of mechanisms underlying cervicogenic headache and support the existence of a structural and functional continuum between the trigeminal nucleus caudalis and C1–C3 spinal dorsal horn rather than explain the GON stimulation-induced relief of cephalalgia. Furthermore, the GON stimulation parameters and protocols applied in the studies cited do not correspond to those used in clinical practice (Lambru and Matharu 2012; Lambru et al. 2014).
In rats, previously sensitized by epidural infusion of inflammatory media based on a validated model of chronic migraine, the GON electrical stimulation was shown to increase mechanical von Frey thresholds for the head, forepaw and hind paw skin (De La Cruz et al. 2015). These data provide evidence in support of the GON stimulation-induced anti-allodynia effect, suggesting an inhibitory action of the procedure on central sensitization, but do not clarify the underlying mechanisms.
Considering all the above in combination with data on the prominent role of the spinal trigeminal nucleus (STN) in the pathophysiology of headaches (Goadsby 2005; Goadsby et al. 2009; Messlinger 2009; Tajti et al. 2011), in the present work we investigated the effects of GON electrical stimulation with parameters comparable to the ones used in the clinic on activity of the dura-sensitive spinal trigeminal neurons in a rat model of trigemino-durovascular nociception. We monitored the spike activity of the STN cells with convergent meningeal, orofacial and cervico-occipital inputs and studied the changes in their ongoing firing and responses to electrical stimulation of the dura mater under preconditioning electrical stimulation of the GON. In order to evaluate the effectiveness of various GON stimulation parameters, five different intensity–frequency combinations were tested.
Materials and methods
Experiments were performed on 11 male Wistar rats (body weight 250–315 g) that were housed in the vivarium of the Pavlov Institute of Physiology (Saint Petersburg, Russia). The animals were maintained 2–5 per cage on a 12-h light/dark schedule and supplied with food and water ad libitum. All experiments were carried out in accordance with the International Association for the Study of Pain Ethical Guidelines and the European Community Council Directive (86/609/EEC). The experimental protocol was approved by the Institutional Animal Care and Use Committees of the Pavlov Institute of Physiology and the First Saint-Petersburg Pavlov State Medical University. All efforts were made to minimize the number of animals used and their suffering.
Anesthesia and surgical preparation
Anesthesia was induced by administering urethane (800 mg/kg, i.p.; ICN Biomedicals, Aurora, OH, USA) and α-chloralose (60 mg/kg, i.p.; MP Biomedicals, Solon, OH, USA). The main experimental procedures were performed as described previously (Sokolov et al. 2010, 2012; Lyubashina et al. 2012). Briefly, once a surgical level of anesthesia was achieved, each animal was placed on a thermostatically controlled heating pad. A catheter was inserted into the femoral artery for continuous monitoring of blood pressure, and the femoral vein was cannulated for administration of anesthetics and myorelaxants. After the trachea was intubated, the head of the animal was fixed in a stereotaxic frame. The distal part of the left GON was exposed before its entering the subaural area, put on hook silver bipolar stimulating electrodes and kept moist with warm mineral oil (37 °C).
The muscles of the dorsal neck were separated along the midline and C1 laminectomy was performed. The medulla and the C1 spinal cord were exposed by removing the atlanto-occipital membrane and the dura mater. Thereafter a longitudinal left-side parietal craniotomy was performed. The bipolar stimulating electrodes with resistance of 50 KΩ were placed on the dura mater in close proximity to the superior sagittal sinus or visible blood vessels and the area was covered with mineral oil. The electrodes consisted of two varnish-insulated silver wires with beads (0.3 mm in diameter) at the end. The animal was paralyzed with pipecuronium bromide (i.v., 1.2 mg/kg initially, maintenance 0.6 mg/kg as required; Gedeon Richter, Budapest, Hungary) and artificially ventilated at a rate of 75–100 cycles/min (2–4 ml per cycle) using room air. Rectal temperature was monitored and kept between 37 and 38 °C. The sufficient depth of anesthesia was judged from the absence of gross (>20 % from the baseline level) blood pressure fluctuations in response to noxious stimulation; supplementary anesthetic was administered when necessary.
Dural and GON electrical stimulations
For electrical stimulation of the dura mater, single rectangular pulses of 300–700 µA (15–35 V) with a duration of 0.4–0.8 ms were used. The pulses were delivered by a computer-controlled stimulator at intensity of 1.5 times the neuronal response threshold. Stimuli applied to the GON were varied in their parameters; five intensity–frequency combinations were tested successively in each animal: (1) 1 V, 50 Hz; (2) 1 V, 100 Hz; (3) 6 V, 50 Hz; (4) 6 V, 100 Hz; (5) 3 V, 75 Hz. In all cases, the duration of current pulses was 0.4 ms.
Extracellular recordings
Neuronal activity within the left spinal trigeminal nucleus was recorded extracellularly by varnish-insulated tungsten microelectrodes (tip diameter 1 µm, impedance 1 MΩ; World Precision Instruments, Sarasota, FL, USA). The electrodes were lowered into the spinal cord at the C1 level using an electronic microdrive unit with steps of 4 µm. The signals were amplified and fed into an IBM-compatible computer A/D converter through a multifunctional acquisition card (sampling period 25 μs, hardware filters 100–5000 Hz). Online acquisition, processing and displaying of data were carried out using custom-written software. The three-level amplitude discrimination was used online to isolate the activity of single units from stimulus artifacts, adjacent cell potentials and noise.
Recordings of neuronal activity were analyzed as peristimulus time histograms in which signals gated through the amplitude discrimination were collected into successive 1 ms bins. For the analysis of dural stimulation-evoked responses, the histograms were constructed online from 20 recordings (one per 3 s) and assessed over 50 ms period after each electrical stimulus. For ongoing activity, the pseudo-stimulation was used, which consisted in using the same software as for constructing histograms of evoked responses, except that electrical stimulation was not actually applied. The histograms were created automatically from 20 recordings (one per 3 s) and the ongoing firing was estimated within 250 ms after each pseudo-stimulus. All recorded units apart from responses to the dural electrical stimulation and mechanical stimulation of their facial cutaneous receptive fields (von Frey filaments, North Coast Medical, Morgan Hill, CA, USA) were also tested for convergent cervico-occipital input by single-pulse electrical stimulation of the GON. Only neurons demonstrating all three kinds of responses were selected for further testing.
Experimental protocol and data processing
Effects of the GON stimulation on activity of spinal trigeminal neurons were evaluated in a preconditioning paradigm. Preconditioning stimuli applied to the GON consisted of 250 ms train of pulses stopped immediately before each dural electrical stimulus or, in the case that the ongoing activity was tested, before each pseudo-stimulus. Recordings of neuronal activity with simultaneous creation of peristimulus time histograms were performed just prior and under preconditioning GON stimulation with certain parameters. Various GON stimulation parameter combinations were tested successively in 10 min interval. In all experiments, only one unit was recorded in each animal. At the end of the experiment, the animals were given a lethal dose of urethane (>3 g/kg, i.v.). An electrolytic lesion through the recording electrode was made and the spinal cord was removed for routine histological verification of the lesion sites.
Using peristimulus histograms, the ongoing and electrically evoked activities of the spinal trigeminal neurons were presented as a mean number of spikes per second (spikes/s) or a mean number of spikes per stimulus (spikes/stimulus), respectively. In order to estimate the effects of GON stimulation, the ongoing firing and evoked responses were normalized and expressed as percentages of the mean value prior to the nerve stimulation.
Statistical analysis
Based on the results of the Shapiro–Wilk test of normality, the nonparametric Friedman, Wilcoxon signed rank and Mann–Whitney–Wilcoxon tests were used to assess the significance of GON stimulation-induced changes in neuronal firing. A value of p < 0.05 was considered to represent a statistical significance. The data are presented as the mean value ± SEM. For the analysis, the Origin 7.5 (OriginLab, Northampton, MA, USA) and GraphPad InStat 3.02 (GraphPad Software, La Jolla, CA, USA) software packages were employed.
Results
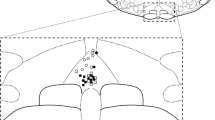
Extracellular recordings were made from 11 neurons within the caudal part of the spinal trigeminal nucleus that received convergent input from the ipsilateral facial cutaneous receptive fields, dura mater and GON. The recorded cells were located in the region of the nucleus defined by a rostrocaudal direction from 1.2 to 3.2 mm caudal to the obex and mediolaterally from 1.0 to 2.2 mm left to the middle line at the depth of 0.2–1.1 mm from the dorsal surface of the spinal cord. Six neurons were located in laminae II–III, and five cells were located in lamina IV (Fig. 1a).
Localization and neuronal properties of recording sites in the spinal trigeminal nucleus. a Locations of recording sites for all experiments at the C1 level. I–IX laminae, CCN central cervical nucleus, IBN internal basilar nucleus, LatC lateral cervical nucleus, LSp lateral spinal nucleus, Pyt pyramidal tract. b The cutaneous receptive field of all neurons studied. c–e Representative native oscillographic recordings and corresponding online-produced histograms demonstrating the ongoing activity of the spinal trigeminal neuron (c), the trigeminal neuronal response to electrical stimulation of the dura mater (d) and the neuronal response to single-pulse electrical stimulation of the greater occipital nerve (e). The histograms are produced from 20 recordings, bin = 1 ms. The arrow indicates the stimulus artifact
All the neurons had facial cutaneous mechanoreceptive fields, mostly restricted to the first division of the trigeminal nerve, including periorbital area (n = 4), dorsum (n = 2) and lateral surface of the nose (n = 5; Fig. 1b); they responded to both noxious (pinch) and non-noxious (touch) mechanical stimulation and were therefore classified as wide-dynamic range neurons. Recorded units showed a wide range of frequencies of initial ongoing activity within an interval of 4–35 spikes/s (Fig. 1c). The mean rate of ongoing firing was 15.6 ± 3.4 spikes/s (n = 11). All tested neurons showed an excitatory response to electrical stimulation of the dura mater with the mean latency of 10.0 ± 0.9 ms n = 11) mostly corresponding to activation of Aδ-fibers (Fig. 1d). The number of spikes in the response varied between 2 and 6 per one trial. At baseline, the mean rate of evoked firing was 4.0 ± 0.5 spikes/stimulus (n = 11).
Single-pulse electrical stimulation of the GON (0.4–2 V, 0.4 ms) elicited in all cells a short-latency response consisting of 3–5 spikes that occurred at 6–8 ms (Fig. 1e). Six neurons showed low threshold to the GON stimulation (0.4–0.8 V), whereas in five cells the threshold current intensity was between 1 and 2 V. The cells belonging to the low-threshold or higher-threshold group differed neither in the initial rate of ongoing activity (p = 0.9, U = 15, Mann–Whitney–Wilcoxon test), nor in the baseline dural stimulation-evoked firing (p = 0.54, U = 11, Mann–Whitney–Wilcoxon test). Moreover, they did not demonstrate any specific feature regarding cutaneous receptive fields and/or location within the spinal trigeminal nucleus.
Effects of GON stimulation on the ongoing neuronal activity
At all parameter sets applied, preconditioning GON stimulation caused a decrease in ongoing activity of the spinal trigeminal neurons (n = 11, p < 0.0001, Fr = 29.95, Friedman test) as compared to the initial level. However, strength of the effect depended on the stimulation protocol. To assess the individual action of each parameter combination more precisely, the firing frequency under a given GON stimulation protocol was compared in pairwise fashion to the discharge rate recorded just prior the onset of the stimuli tested. Under GON stimulated with 1 V current pulses at 50 Hz, the mean rate of the ongoing firing decreased to 79 ± 13 % (11.2 ± 2.7 versus 15.6 ± 3.4 spikes/s before the stimulation, n = 11), but the reduction was not significant (p = 0.18, Wilcoxon signed rank test; Fig. 2a). When stimulus frequency was changed to 100 Hz, the neuronal discharge rate declined to 74 ± 16 % of the pre-stimulation value, although the alteration still did not reach the level of significance (p = 0.15, Wilcoxon signed rank test).
Effects of preconditioning stimulation of the greater occipital nerve (GON) with various parameters on ongoing (a) and dural stimulation-evoked (b) activity of the spinal trigeminal neurons. Each filled column represents corresponding mean firing rate under GON stimulation with certain intensity and frequency (percentage versus level just prior the nerve stimulation). Data are shown as mean value ± SEM. Significant differences are indicated as follows: # p < 0.05, ## p < 0.01, ### p < 0.001 versus corresponding pre-stimulation level; *p < 0.05, **p < 0.01, ***p < 0.001 versus other stimulation parameter combinations; the Bonferroni corrections are omitted
The inhibitory effect of the GON stimulation substantially enhanced as the current intensity was increased to 6 V. When a stimulus of such amplitude was applied to the GON with a 50 Hz frequency, ongoing firing of the spinal trigeminal neurons fell to 29 ± 8 % (4.6 ± 1.6 versus 16.2 ± 3.4 spikes/s prior the stimulation, n = 11; Figs. 2a, 3); the decrease was maximally significant compared to the pre-stimulation level (p = 0.0009, Wilcoxon signed rank test, Bonferroni correction p = 0.0045). Increasing stimulation frequency did not lead to a greater effect. Under GON stimulated with 6 V current pulses delivered at 100 Hz, the spinal trigeminal neurons showed only moderately significant (p = 0.0098, Wilcoxon signed rank test, Bonferroni correction p = 0.049) reduction in the mean discharge rate to 48 ± 15 % of that before the stimulation (4.8 ± 2.0 versus 13.3 ± 2.1 spikes/s, n = 11).
Representative native oscillographic recordings and corresponding off-line processed histograms demonstrating inhibitory effect of preconditioning stimulation of the greater occipital nerve (GON) with 6 V intensity and 50 Hz frequency on ongoing activity of the spinal trigeminal neurons. Activity of the same neuron is shown prior (a) and under GON stimulation (b). The black line indicates the time of the GON electrical stimulation. The histograms are produced from 20 recordings each, bin = 1 ms
At lower parameters, those of 3 V intensity and 75 Hz frequency, stimulation of the GON produced maximally significant decrease in the ongoing neuronal activity to 40 ± 9 % (5.2 ± 1.6 versus 12.8 ± 1.7 spikes/s prior the stimulation, n = 11, p = 0.0009, Wilcoxon signed rank test, Bonferroni correction p = 0.0045). The Friedman test revealed a substantial difference between the actions of various parameter combinations (n = 11, p = 0.0004, Fr = 20.39). The inhibitory effects of the 6 V/50 Hz and 3 V/75 Hz stimulations of the GON were statistically comparable (p = 0.13, Wilcoxon signed rank test), but both were greater than those of the 1 V/50 Hz intensity–frequency combination (p = 0.02 and p = 0.0098, correspondingly, Wilcoxon signed rank test) and 1 V/100 Hz parameter set (p = 0.019 and p = 0.014, correspondingly, Wilcoxon signed rank test; Fig. 2a). However, the statistical analysis based on Bonferroni correction did not confirm the significant differences between the actions of the 6 V/50 Hz and 1 V/50 Hz (p = 0.1), the 6 V/50 Hz and 1 V/100 Hz (p = 0.095), as well as the 3 V/75 Hz and 1 V/100 Hz stimulations (p = 0.07). Meanwhile, the significant difference can be still found between the 3 V/75 Hz and 1 V/50 Hz parameter sets (p = 0.049).
It should be noted that the 1 V/50 Hz combination was more effective in the cells showing low (<1 V) threshold to the single-pulse stimulation of the GON as compared to the neurons demonstrating higher threshold (p = 0.004, U = 0, Mann–Whitney–Wilcoxon test). None of the GON stimulation parameter sets produced long-lasting changes in ongoing activity of the spinal trigeminal neurons. The mean rate of firing which was recorded in 10 min after each successive GON stimulation, just prior the next one, at any time point did not differ from its initial level (15.6 ± 3.4 spikes/s, n = 11, p = 0.99, Fr = 0.24, Friedman test).
Effects of GON stimulation on the responses to electrical stimulation of the dura mater
Under preconditioning activation of the GON, the dural stimulation-evoked responses of the studied spinal trigeminal neurons also declined in comparison to the initial level (n = 11, p < 0.0001, Fr = 27.45, Friedman test). Depending on the GON stimulation parameters, the suppressive effect was more or less pronounced. In all cases, there was no correlation between the sensitivity of a given neuron to single-pulse stimulation of the GON (belonging to the distinguished low-threshold or higher-threshold group) and changes in its evoked activity occurred under precondition activation of the nerve. When a stimulus of 1 V was applied to the GON with a 50 Hz frequency, the neurons showed a decrease in the mean rate of evoked firing to 88 ± 8 % of that just prior the stimulation (3.6 ± 0.5 versus 4.0 ± 0.5 spikes/stimulus, n = 11; Fig. 2b). However, the reduction was not statistically significant (p = 0.10, Wilcoxon signed rank test). At 1 V intensity and 100 Hz frequency, stimulation of the GON resulted in further decrease in the neuronal responses to 82 ± 8 % of the value preceding the onset of the stimuli, although the alteration still did not reach the level of significance (p = 0.12, Wilcoxon signed rank test).
Increasing stimulus intensity produced an enhancement of the GON stimulation-induced inhibitory effect. Under 6 V current pulses delivered to the GON at 50 Hz, the evoked activity of the spinal trigeminal neurons significantly fell to 53 ± 10 % of that just prior the stimulation (2.8 ± 0.6 versus 4.6 ± 0.6 spikes/stimulus, n = 11, p = 0.002, Wilcoxon signed rank test, Bonferroni correction p = 0.01; Figs. 2b, 4). When the GON stimulation frequency was doubled to 100 Hz, the neurons demonstrated equal decrease in the mean rate of the evoked discharges to 53 ± 10 % (2.9 ± 0.8 versus 5.0 ± 0.8 spikes/stimulus before the onset of the stimuli, n = 11, p = 0.003, Wilcoxon signed rank test, Bonferroni correction p = 0.015).
Representative native oscillographic recordings and corresponding off-line processed histograms showing inhibitory effects of preconditioning stimulation of the greater occipital nerve (GON) with 6 V intensity and 50 Hz frequency on responses of the spinal trigeminal neurons to electrical stimulation of the dura mater. Evoked activity of the same neuron is shown prior (a) and under GON stimulation (b). The black line marks the GON electrical stimulation (only the last electrical GON stimulus is shown). In each histogram, the arrow indicates the time of a single electrical stimulus applied to the dura mater. The histograms are produced from 20 recordings each, bin = 1 ms
When a stimulus of 3 V was applied to the GON with a 75 Hz frequency, the reduction in the neuronal responses was less prominent (to 68 ± 8 %; 3.4 ± 0.8 versus 4.7 ± 0.9 spikes/stimulus just prior the stimulation, n = 11). Nevertheless, the alteration was maximally significant compared to the level preceding the onset of the stimulation (p = 0.0009, Wilcoxon signed rank test, Bonferroni correction p = 0.045).
The Friedman test revealed a significant difference between the actions of various parameter combinations (n = 11, p = 0.001, Fr = 18.4). Thus the suppression of the evoked firing of the spinal trigeminal neurons was significantly greater under the 6 V/50 Hz and 6 V/100 Hz stimulations of the GON as compared to the 1 V/50 Hz combination (p = 0.0049 and p = 0.0049, correspondingly, Wilcoxon signed rank test, Bonferroni corrections p = 0.025 and p = 0.025) and 1 V/100 Hz parameter set (p = 0.003 and p = 0.007, correspondingly, Wilcoxon signed rank test, Bonferroni corrections p = 0.015 and p = 0.035; Fig. 2b). In turn, the inhibitory effect of the 3 V/75 Hz intensity–frequency combination was more profound than that of the 1 V/50 Hz parameter (p = 0.04, Wilcoxon signed rank test), though the difference did not reach statistically significance after Bonferroni correction (p = 0.2).
None of the GON stimulation parameter sets produced long-lasting changes in the evoked neuronal activity, which in 10 min after each successive GON stimulation, just prior the next one, did not differ from its initial level (4.0 ± 0.5 spikes/stimulus, n = 11, p = 0.46, Fr = 3.61, Friedman test).
Discussion
This is the first study to demonstrate that preconditioning high-frequency suprathreshold electrical stimulation of the greater occipital nerve produces suppression of both the ongoing activity of the convergent spinal trigeminal neurons and their responses to electrical stimulation of the dura mater. The inhibitory effect depended on the stimulation parameters, being maximally pronounced when a stimulus of 6 V was applied to the GON. This finding is in agreement with the clinical data, which indicated a greater efficiency of suprathreshold GON stimulation (Slotty et al. 2015).
The presented work was performed using an electrophysiological model of trigemino-durovascular nociception (Akerman et al. 2013). This model is based on current knowledge about structural organization and functions of the trigeminovascular system and its role in the pathogenesis of primary headaches. The thin Aδ and C trigeminal afferent inputs from the dura mater and large cerebral vessels are known to be the main sources of pain in headaches (Goadsby et al. 2009; Messlinger 2009). According to the clinical observations made in the 1940s, electrical or mechanical stimulation of intracranial vessels like the dural sinuses and the middle meningeal artery inflicts a migraine-like headache that occurs mostly within the distribution of the first division of the trigeminal nerve (Penfield and McNaughton 1940; Ray and Wolff 1940).
It was observed in rats and cats that electrical stimulation of the superior sagittal sinus or the trigeminal ganglion led to both disturbances of cerebral blood flow and changes in neuropeptides’ plasma levels similar to those noted in migraine or cluster headache attacks (Edvinsson and Uddman 2005). Moreover, such stimulation increased the levels of c-fos (Hoskin et al. 1999; Mitsikostas and Sanchez del Rio 2001; Schuh-Hofer et al. 2006) and spike activity of the spinal trigeminal neurons (Storer et al. 2001, 2003; Akerman et al. 2007; Sokolov et al. 2010, 2012, 2015; Lyubashina et al. 2012), which are shown to be intimately involved in the modulation of nociceptive transmission from intracranial tissues (Schurks and Diener 2008; Goadsby et al. 2009). Thus, the dural electrical stimulation proves to be a valid method of activating the trigeminovascular system and mimicking nociceptive processes occurring during headache.
The model of trigemino-durovascular nociception is thought to be very informative in regards to studying the pharmacodynamics of drugs with clinically proven anticephalgic activity (Jakubowski et al. 2005, 2007; Sokolov et al. 2008, 2010; Storer and Goadsby 2013) as well as to searching for new medications to treat headache (Akerman et al. 2007; Charbit et al. 2009; Lambert et al. 2009; Andreou et al. 2015; Sokolov et al. 2015). However, apart from evaluation of various pharmacological substances’ effects, the model allows uncovering the mechanisms underlying action of non-pharmacological primary headache treatments, specifically different types of electrical neurostimulation. Indeed, in our previous study we have shown that continuous electrical stimulation of the cervical vagus nerve altered activity of the spinal trigeminal neurons, producing predominantly inhibitory effect on their dural stimulation-evoked as well as ongoing firings (Lyubashina et al. 2012); these findings directly contribute to the current knowledge of neurophysiological processes involved in providing therapeutic efficacy of the vagus nerve stimulation (VNS) in drug-resistant chronic headaches.
In the present study, preconditioning electrical stimulation of the greater occipital nerve resulted in similar, while much more pronounced and unequivocal, changes in trigeminal neuronal activity. This observation correlates well with the preferential use of the GON electrical stimulation, as evidently more effective type of electrotherapy, for non-pharmacological treatment of intractable headaches (Magis and Schoenen 2011a; Jürgens and Leone 2013; Rasskazoff and Slavin 2013; Lambru and Matharu 2014) and allows us to look on VNS and GON stimulations as being comparable headache treatments sharing common anticephalgic action mechanisms. The last assumption needs explanation from the neurophysiological point of view.
The caudal portion of the STN, in other words, the trigeminal nucleus caudalis, extends to the level of C2–C3, forming a very broad overlapping zone with the ipsilateral dorsal horn of the upper cervical spinal cord segments, that is within the C1–C2 segments the STN grey matter is immediately adjacent to the grey matter of the spinal dorsal horn. This fact determines the morphological and functional unity of these structures suggesting that they may be considered as a single neuroanatomical formation, known as the “trigeminocervical complex” (Bartsch and Goadsby 2002, 2003a, b; Storer et al. 2003; Schurks and Diener 2008; Goadsby et al. 2009). Evidently, the effects of GON stimulation on activity of the STN dura-sensitive neurons are enabled due to the convergence of the greater occipital nerve and trigeminal afferents on such cells (Bartsch and Goadsby 2002, 2003a; Bartsch 2005).
It is the convergence of meningeal and cervical nociceptive inputs on the STN neurons is thought to be a cause for the neck pain being both a trigger and a complication of headache attacks or an accompanying symptom (Bogduk 2001; Bartsch 2005; Goadsby 2005; Schurks and Diener 2008; Watson and Drummond 2012; Ashina et al. 2015). The so-called myofascial trigger points (mechanical stimulation of which can provoke an episode of cephalalgia, whereas local anesthetic infiltration prevents or stops the attack) are frequently revealed in the cervico-occipital region of patients with migraine, tension-type and cluster headaches, confirming thus the existence of the cervico-trigeminal convergence as well as explaining the successful use of botulinum toxin in the preventive therapy of primary headaches (Mauskop 2004; Calandre et al. 2006, 2008; García-Leiva et al. 2007; Alonso-Blanco et al. 2012; Robertson and Garza 2012).
It has been reported that painful stimulation of the GON in healthy volunteers induced ipsilateral headache (Piovesan et al. 2001, 2003). In rats, electrical stimulation of the GON, as well as chemical activation of its cutaneous or muscle afferents, have been found not only to facilitate spike activity and c-fos expression in the spinal trigeminal neurons, but also substantially increase their excitability to dural stimulation (Bartsch and Goadsby 2002; Le Doare et al. 2006; Panfil et al. 2006). In turn, chemical activation of the dural afferents significantly facilitated trigeminal neuronal responses to electrical stimulation of the GON and to mechanical stimulation of the deep paraspinal muscles (Bartsch and Goadsby 2003a). These findings confirm the existence of anatomical basis for the development of cervicogenic cephalalgias (Bogduk 2001; Chou and Lenrow 2002; Biondi 2005), tension-type headaches (Ashina et al. 2005; Fernández-de-las-Peñas et al. 2008; Watson and Drummond 2012), as well as for the spreading of migraine pain to occipital and cervical areas (Goadsby 2005), suggesting thus an important role of the trigemino-cervical convergence in the pathogenesis of primary headaches.
In the light of the above, the use of the GON blockade with local anesthetics or corticosteroids as a therapy for drug-resistant cluster headache and migraine seems to be quite reasonable (Ambrosini et al. 2005; Ashkenazi and Levin 2007; Saracco et al. 2010). In turn, the opposite procedure—electrical stimulation of the GON—despite its potentiality to induce headache attacks, has been shown to be effective in the treatment of various types of cephalalgia (Magis and Schoenen 2011a, b; Lambru et al. 2014; Tavanaiepour and Levy 2014; Dodick et al. 2015). The dual clinical data suggest that trigemino-cervical convergence mechanisms can be inhibitory or excitatory, and thus can inhibit, induce or potentiate pain. The type of convergence has been proposed to depend on underlying disease and nociceptive status of the patient rather than on intensity of the GON stimulation (Piovesan et al. 2007). For example, the electrical stimulation of the GON can relieve pain in migraine attacks, but is able to induce headache during interictal periods.
Such a point of view seems quite suitable for explanation of the results obtained in the present study. Indeed, in our experiments two afferent inflows induced by electrical stimulations of trigeminal and cervico-occipital sensory fibers converged onto single neurons of the STN. Theoretically, the interaction between these afferent inputs might equally be synergistic (resulting in increased neuronal responses to dural stimulation) or antagonistic (when the dural stimulation-evoked firing is suppressed by stimulation of the GON).
In our experiments, the second type of the interaction was only revealed, indicating the predominance of inhibitory cervico-trigeminal convergence mechanisms. In contrast to this, the study done by Bartsch and Goadsby (2002) showed that supramaximal electrical stimulation of the GON enhanced afferent dural input in 8 of 20 tested trigeminal neurons, while in 4 neurons an initial decrease in excitability to dural electrical stimulation was observed within the first 10 min after GON stimulation. This discrepancy could probably be explained by the difference in the chosen parameters and protocol of the nerve stimulation. Indeed, in the work cited above a single session of continuous GON stimulation (2 ms, 5–30 V) was applied at 0.5 Hz for between 20 s and 5 min, whereas in the present study we used short-time (250 ms) high-frequency (50–100 Hz) trains of suprathreshold pulses (1–6 V) with a duration of 0.4 ms, which were repeatedly delivered to the GON (20 trains, one per 3 s). In our experiments, GON stimulation in the chosen mode effectively inhibited not only the dural electrical stimulation-evoked responses, but also the ongoing activity of trigeminal neurons, indicating thus the GON stimulation-induced decrease in the STN general neuronal excitability.
Since all neurons tested in this study received convergent excitatory inputs from both the occipital and the dural afferents, it seems quite possible that preconditioning high-frequency electrical stimulation of the GON could induce in a given convergent neuron a progressively increasing refractory state in which the neuronal susceptibility to all subsequent stimuli, including those from the dura mater, was depressed. A consequence of this might be the attenuation of the dural stimulation-evoked responses as well as the ongoing activity of the STN neurons we observed under preconditioning GON stimulation. However, it has been demonstrated previously that stimulation of the GON, although with other stimulus parameters, resulted in a sensitization of the spinal trigeminal neurons, as manifested by steady increase in their excitability (Bartsch and Goadsby 2002). Thus, the same type of the nerve stimulation, depending on its mode, frequency and intensity, is able to cause opposite changes in the STN neuronal activity.
It cannot be ruled out that the long-term application of high-frequency GON stimulation of an optimal intensity in headache patients may result in the STN neurons becoming insensitive (habituated) to intracranial painful stimuli. It is pertinent to note here that both habituation and sensitization can occur in the trigeminal system (Condes-Lara et al. 1981; Goadsby 2005; Serrao et al. 2010), and an imbalance between these processes, or more simply, the deficiency of habituation, plays a key role in the pathogenesis and chronification of headaches (Perrotta et al. 2008; Coppola et al. 2013; Kalita et al. 2014). Interestingly, severe cutaneous allodynia is associated with substantially decreased response to treatment with occipital transcutaneous electrical stimulation in patients with chronic migraine and chronic tension-type headache (Bono et al. 2015), suggesting thus that the higher and more steady the level of trigeminal neurons’ sensitization, the more problematic the attempt to reduce their excitability by the occipital nerve stimulation.
Apart from the speculations on the development of refractoriness in the STN cells, the GON stimulation-induced reduction in the neuronal activity may be explained by the involvement of yet unidentified central and/or peripheral inhibitory mechanisms (Bartsch and Goadsby 2011). Second-order neurons of the trigemino-cervical complex are known to provide substantial inputs to various supraspinal sites, including the brainstem structures of the endogenous antinociceptive systems (Noseda et al. 2008; Liu et al. 2009; Edvinsson 2011), which, in turn, are involved in the descending modulatory control of trigeminovascular nociception (Schurks and Diener 2008; Goadsby et al. 2009; Abdallah et al. 2013). It is entirely possible that the GON stimulation-evoked discharges of the spinal trigeminal neurons can contribute to the activation of subcortical pain-modulating centees, promoting thus the descending inhibition of the meningeal afferent signal processing in the STN (Bartsch 2005). However, the development of such inhibition seems to require some time.
It has been suggested that anticephalgic action of GON stimulation is realized via slow neuromodulatory processes at the level of upper brain stem or diencephalic centers (Magis et al. 2007). As seen on positron emission tomography scan, the occipital nerve stimulation in patients with cluster headache (Magis et al. 2011) and chronic migraine (Matharu et al. 2004) appears to stimulate normalization of metabolism and regional blood flow changes in several suprasegmental areas of the pain neuromatrix. The authors noted, however, that it took at least 1 month to see the improvements, whereas in our experiments the neuronal effect of GON stimulation became apparent almost immediately.
In the light of the above, the results of the present study may be explained by the gate control theory (Melzack and Wall 1965), which asserts the existence of negative feedback between activity of large-caliber non-nociceptive afferents and transmission of pain signals at the segmental level (Schwedt 2009; Lambru and Matharu 2012). According to this theory, electrical stimulation of thick Aβ fibers in the GON can cause inhibition of nociceptive stimuli delivered by Aδ trigeminal afferents, with a consequent attenuation of the STN evoked neuronal activity. Such mechanism can work if at the same time thin nociceptive fibers are inactivated, which has indeed been shown to occur at stimulation frequencies of more than 10 Hz (Weidner et al. 2003) due to a progressive inactivation of sodium channels (De Col et al. 2008). The suppression of trigeminal Aδ input may be realized directly within the STN via, for example, facilitation of GABA/glycine-ergic inhibitory interneurons and the following pre- and/or post-synaptic suppression of nociceptive signal transmission in the first synapse of the trigeminal pathway (Sokolov et al. 2014). However, further studies are needed to confirm or refute this assumption.
Magis and colleagues, with reference to Bartsch and Goadsby (2003a) and Le Doaré and co-authors (2006), wrote in 2011: “It was speculated that ONS might exert its action by decreasing excitability of second order nociceptors in trigeminal nucleus caudalis on which converge cervical, somatic trigeminal and visceral trigeminovascular afferents.” We suppose that the results of the present study provide convincing evidence in support of this hypothesis and at this point it can be suggested with certainty that one of the main components of neural mechanism underlying anticephalgic action of GON electrical stimulation is the suppression of nociceptive processing at the level of STN.
References
Abdallah K, Artola A, Monconduit L, Dallel R, Luccarini P (2013) Bilateral descending hypothalamic projections to the spinal trigeminal nucleus caudalis in rats. PLoS One 8(8):e73022
Akerman S, Holland PR, Goadsby PJ (2007) Cannabinoid (CB1) receptor activation inhibits trigeminovascular neurons. JPET 320:64–71
Akerman S, Holland PR, Hoffmann J (2013) Pearls and pitfalls in experimental in vivo models of migraine: dural trigeminovascular nociception. Cephalalgia 33:577–592
Alonso-Blanco C, de-la-Llave-Rincón AI, Fernández-de-las-Peñas C (2012) Muscle trigger point therapy in tension-type headache. Expert Rev Neurother 12:315–322
Ambrosini A, Vandenheede M, Rossi P, Aloj F, Sauli E, Pierelli F, Schoenen J (2005) Suboccipital injection with a mixture of rapid- and long-acting steroids in cluster headache: a double-blind placebo-controlled study. Pain 118:92–96
Andreou AP, Holland PR, Lasalandra MP, Goadsby PJ (2015) Modulation of nociceptive dural input to the trigeminocervical complex through GluK1 kainate receptors. Pain 156:439–450
Ashina S, Babenko L, Jensen R, Ashina M, Magerl W, Bendtsen L (2005) Increased muscular and cutaneous pain sensitivity in cephalic region in patients with chronic tension-type headache. Eur J Neurol 12:543–549
Ashina S, Bendtsen L, Lyngberg AC, Lipton RB, Hajiyeva N, Jensen R (2015) Prevalence of neck pain in migraine and tension-type headache: a population study. Cephalalgia 35:211–219
Ashkenazi A, Levin M (2007) Greater occipital nerve block for migraine and other headaches: is it useful? Curr Pain Headache Rep 11:231–235
Bartsch T (2005) Migraine and the neck: new insights from basic data. Curr Pain Headache Rep 9:191–196
Bartsch T, Goadsby PJ (2002) Stimulation of the greater occipital nerve induces increased central excitability of dural afferent input. Brain 125:1496–1509
Bartsch T, Goadsby PJ (2003a) Increased responses in trigeminocervical nociceptive neurons to cervical input after stimulation of the dura mater. Brain 126:1801–1813
Bartsch T, Goadsby PJ (2003b) The trigeminocervical complex and migraine: current concepts and synthesis. Curr Pain Headache Rep 7:371–376
Bartsch T, Goadsby PJ (2011) Central mechanisms of peripheral nerve stimulation in headache disorders. Prog Neurol Surg 24:16–26
Biondi DM (2005) Cervicogenic headache: a review of diagnostic and treatment strategies. JAOA 105:16–22
Bogduk N (2001) Cervicogenic headache: anatomic basis and pathophysiologic mechanisms. Curr Pain Headache Rep 5:382–386
Bono F, Salvino D, Mazza M, Curcio M, Trimboli M, Vescio B, Quattrone A (2015) The influence of ictal cutaneous allodynia on the response to occipital transcutaneous electrical stimulation in chronic migraine and chronic tension-type headache: a randomized, sham-controlled study. Cephalalgia 35:389–398
Broggi G, Messina G, Marras C, Dones I, Franzini A (2010) Neuromodulation for refractory headaches. Neurol Sci 31:87–92
Burns B, Watkins L, Goadsby PJ (2008) Treatment of hemicrania continua by occipital nerve stimulation with a bion device: long-term follow-up of a crossover study. Lancet Neurol 7:1001–1012
Burns B, Watkins L, Goadsby PJ (2009) Treatment of intractable chronic cluster headache by occipital nerve stimulation in 14 patients. Neurology 72:341–345
Calandre EP, Hidalgo J, Garcia-Leiva JM, Rico-Villademoros F (2006) Trigger points evaluation in migraine patients: an indication of peripheral sensitization linked to migraine predisposition? Eur J Neurol 13:244–249
Calandre EP, Hidalgo J, Garcia-Leiva JM, Rico-Villademoros F, Delgado-Rodriguez A (2008) Myofascial trigger points in cluster headache patients: a case series. Head Face Med 4:32
Charbit AR, Akerman S, Goadsby PJ (2009) Comparison of the effects of central and peripheral dopamine receptor activation on evoked firing in the trigeminocervical complex. J Pharmacol Exp Ther 331:752–763
Chen YF, Bramley G, Unwin G, Hanu-Cernat D, Dretzke J, Moore D, Bayliss S, Cummins C, Lilford R (2015) Occipital nerve stimulation for chronic migraine—a systematic review and meta-analysis. PLoS One 10(3):e0116786
Chou LH, Lenrow DA (2002) Cervicogenic headache. Pain Physician 5:215–225
Condes-Lara M, Calvo JM, Fernandez-Guardiola A (1981) Habituation to bearable experimental pain elicited by tooth pulp electrical stimulation. Pain 11:185–200
Coppola G, Di Lorenzo C, Schoenen J, Pierelli F (2013) Habituation and sensitization in primary headaches. J Headache Pain 14:65
De Col R, Messlinger K, Carr RW (2008) Conduction velocity is regulated by sodium channel inactivation in unmyelinated axons innervating the rat cranial meninges. J Physiol 586:1089–1103
De La Cruz P, Gee L, Walling I, Morris B, Chen N, Kumar V, Feustel P, Shin DS, Pilitsis JG (2015) Treatment of allodynia by occipital nerve stimulation in chronic migraine rodent. Neurosurgery 77(3):479–485
Dodick DW, Silberstein SD, Reed KL, Deer TR, Slavin KV, Huh B, Sharan AD, Narouze S, Mogilner AY, Trentman TL, Ordia J, Vaisman J, Goldstein J, Mekhail N (2015) Safety and efficacy of peripheral nerve stimulation of the occipital nerves for the management of chronic migraine: long-term results from a randomized, multicenter, double-blinded, controlled study. Cephalalgia 35:344–358
Edvinsson L (2011) Tracing neural connections to pain pathways with relevance to primary headaches. Cephalalgia 31:737–747
Edvinsson L, Uddman R (2005) Neurobiology in primary headaches. Brain Res 48:438–456
Fernández-de-las-Peñas C, Falla D, Arendt-Nielsen L, Farina D (2008) Cervical muscle co-activation in isometric contractions is enhanced in chronic tension-type headache patients. Cephalalgia 28:744–751
Fontaine D, Christophe Sol J, Raoul S, Fabre N, Geraud G, Magne C, Sakarovitch C, Lanteri-Minet M (2011) Treatment of refractory chronic cluster headache by chronic occipital nerve stimulation. Cephalalgia 31:1101–1105
García-Leiva JM, Hidalgo J, Rico-Villademoros F, Moreno V, Calandre EP (2007) Effectiveness of ropivacaine trigger points inactivation in the prophylactic management of patients with severe migraine. Pain Med 8:65–70
Goadsby PJ (2005) Migraine, allodynia, sensitisation and all of that…. Eur Neurol 53:10–16
Goadsby PJ, Knight YE, Hoskin KL (1997) Stimulation of the greater occipital nerve increases metabolic activity in the trigeminal nucleus caudalis and cervical dorsal horn of the cat. Pain 73:23–28
Goadsby PJ, Chabrit AR, Andreou AP, Akerman S (2009) Neurobiology of migraine. Neuroscience 161:327–341
Hoffmann J, Magis D (2013) Scientific advances in headache research: an update on neurostimulation. Expert Rev Neurother 13:15–17
Hoskin KL, Bulmer DC, Goadsby PJ (1999) Fos expression in the trigeminocervical complex of the cat after stimulation of the superior sagittal sinus is reduced by L-NAME. Neurosci Lett 266:173–176
Jakubowski M, Levy D, Goor-Aryeh I, Collins B, Bajwa Z, Burstein R (2005) Terminating migraine with allodynia and ongoing central sensitization using parenteral administration of COX1/COX2 inhibitors. Headache 45:850–861
Jakubowski M, Levy D, Kainz V, Zhang XC, Kosaras B, Burstein R (2007) Sensitization of central trigeminovascular neurons: blockade by intravenous naproxen infusion. Neurosci 148:573–583
Jenkins B, Tepper SJ (2011a) Neurostimulation for primary headache disorders, part 1: pathophysiology and anatomy, history of neuromodulation in headache treatment, and review of peripheral neuromodulation in primary headaches. Headache 51:1254–1266
Jenkins B, Tepper SJ (2011b) Neurostimulation for primary headache disorders: part 2, review of central neurostimulators for primary headache, overall therapeutic efficacy, safety, cost, patient selection, and future research in headache neuromodulation. Headache 51:1408–1418
Johnstone CS, Sundaraj R (2006) Occipital nerve stimulation for the treatment of occipital neuralgia-eight case studies. Neuromodulation 9:41–47
Jürgens TP, Leone M (2013) Pearls and pitfalls: neurostimulation in headache. Cephalalgia 33:512–525
Kalita J, Bhoi SK, Misra UK (2014) Is lack of habituation of evoked potential a biological marker of migraine? Clin J Pain 30:724–729
Lambert GA, Davis JB, Appleby JM, Chizh BA, Hoskin KL, Zagami AS (2009) The effects of the TRPV1 receptor antagonist SB-705498 on trigeminovascular sensitisation and neurotransmission. Naunyn Schmiedebergs Arch Pharmacol 380:311–325
Lambru G, Matharu MS (2012) Occipital nerve stimulation in primary headache syndromes. Ther Adv Neurol Disord 5:57–67
Lambru G, Matharu MS (2014) Peripheral neurostimulation in primary headaches. Neurol Sci 35:77–81
Lambru G, Shanahan P, Watkins L, Matharu MS (2014) Occipital nerve stimulation in the treatment of medically intractable SUNCT and SUNA. Pain Physician 17:29–41
Le Doare K, Akerman S, Holland PR, Lasalandra MP, Bergerot A, Classey JD, Knight YE, Goadsby PJ (2006) Occipital afferent activation of second order neurons in the trigeminocervical complex in rat. Neurosci Lett 403:73–77
Liu Y, Broman J, Zhang M, Edvinsson L (2009) Brainstem and thalamic projections from a craniovascular sensory nervous centre in the rostral cervical spinal dorsal horn of rats. Cephalalgia 29:935–948
Lyubashina OA, Sokolov AY, Panteleev SS (2012) Vagal afferent modulation of spinal trigeminal neuronal responses to dural electrical stimulation in rats. Neurosci 222:29–37
Magis D, Schoenen J (2011a) Occipital nerve stimulation for intractable chronic cluster headache: new hope for a dreadful disease? Acta Neurol Belg 111:18–21
Magis D, Schoenen J (2011b) Peripheral nerve stimulation in chronic cluster headache. Prog Neurol Surg 24:126–132
Magis D, Schoenen J (2012) Advances and challenges in neurostimulation for headaches. Lancet Neurol 11:708–719
Magis D, Allena M, Bolla M, De Pasqua V, Remacle JM, Schoenen J (2007) Occipital nerve stimulation for drug-resistant chronic cluster headache: a prospective pilot study. Lancet Neurol 6:314–321
Magis D, Bruno MA, Fumal A, Gérardy PY, Hustinx R, Laureys S, Schoenen J (2011) Central modulation in cluster headache patients treated with occipital nerve stimulation: an FDG-PET study. BMC Neurol 11:25
Mammis A, Agarwal N, Mogilner AY (2015) Occipital nerve stimulation. Adv Tech Stand Neurosurg 42:23–32
Martelletti P, Jensen RH, Antal A, Arcioni R, Brighina F, de Tommaso M, Franzini A, Fontaine D, Heiland M, Jürgens TP, Leone M, Magis D, Paemeleire K, Palmisani S, Paulus W, May A (2013) Neuromodulation of chronic headaches: position statement from the European Headache Federation. J Headache Pain 14:86
Matharu MS, Bartsch T, Ward N, Frackowiak RS, Weiner R, Goadsby PJ (2004) Central neuromodulation in chronic migraine patients with suboccipital stimulators: a PET study. Brain 127:220–230
Mauskop A (2004) The use of botulinum toxin in the treatment of headaches. Pain Physician 7:377–387
Melzack R, Wall PD (1965) Pain mechanisms: a new theory. Science 150:971–979
Messlinger K (2009) Migraine: where and how does the pain originate? Exp Brain Res 196:179–193
Mitsikostas DD, Sanchez del Rio M (2001) Receptor systems mediating c-fos expression within trigeminal nucleus caudalis in animal models of migraine. Brain Res Rev 35:20–35
Noseda R, Monconduit L, Constandil L, Chalus M, Villanueva L (2008) Central nervous system networks involved in the processing of meningeal and cutaneous inputs from the ophthalmic branch of the trigeminal nerve in the rat. Cephalalgia 28:813–824
Notaro P, Buratti E, Meroni A, Montagna MC, Rubino FG, Voltolini A (2014) The effects of peripheral occipital nerve stimulation for the treatment of patients suffering from chronic migraine: a single center experience. Pain Physician 17:369–374
Palmisani S, Al-Kaisy A, Arcioni R, Smith T, Negro A, Lambru G, Bandikatla V, Carson E, Martelletti P (2013) A six year retrospective review of occipital nerve stimulation practice–controversies and challenges of an emerging technique for treating refractory headache syndromes. J Headache Pain 14:67
Panfil C, Makowska A, Ellrich J (2006) Brainstem and cervical spinal cord Fos immunoreactivity evoked by nerve growth factor injection into neck muscles in mice. Cephalalgia 26:128–135
Pascual J (2009) Treatment of hemicrania continua by occipital nerve stimulation with a bion device. Curr Pain Headache Rep 13:3–4
Penfield W, McNaughton FL (1940) Dural headache and the innervation of the dura mater. Arch Neurol Psychiatry 44:43–75
Perrotta A, Serrao M, Sandrini G, Bogdanova D, Tassorelli C, Bartolo M, Coppola G, Pierelli F, Nappi G (2008) Reduced habituation of trigeminal reflexes in patients with episodic cluster headache during cluster period. Cephalalgia 28:950–959
Piovesan EJ, Kowacs PA, Tatsui CE, Lange MC, Ribas LC, Werneck LC (2001) Referred pain after painful stimulation of the greater occipital nerve in humans: evidence of convergence of cervical afferences on trigeminal nuclei. Cephalalgia 21:107–109
Piovesan EJ, Kowacs PA, Oshinsky ML (2003) Convergence of cervical and trigeminal sensory afferents. Curr Pain Headache Rep 7:377–383
Piovesan EJ, Di Stani F, Kowacs PA, Mulinari RA, Radunz VH, Utiumi M, Muranka EB, Giublin ML, Werneck LC (2007) Massaging over the greater occipital nerve reduces the intensity of migraine attacks: evidence for inhibitory trigemino-cervical convergence mechanisms. Arq Neuropsiquiatr 65:599–604
Rasskazoff SY, Slavin KV (2013) Neuromodulation for cephalgias. Surg Neurol Int 4:136–150
Ray BS, Wolff HG (1940) Experimental studies on headache. Pain sensitive structures of the head and their significance in headache. Arch Surg 41:813–856
Robertson CE, Garza I (2012) Critical analysis of the use of onabotulinumtoxinA (botulinum toxin type A) in migraine. Neuropsychiatr Dis Treat 8:35–48
Rodrigo-Royo MD, Azcona JM, Quero J, Lorente MC, Acín P, Azcona J (2005) Peripheral neurostimulation in the management of cervicogenic headache: four case reports. Neuromodulation 8:241–248
Saper JR, Dodick DW, Silberstein SD, McCarville S, Sun M, Goadsby PJ, Investigators ONSTIM (2011) Occipital nerve stimulation for the treatment of intractable chronic migraine headache: ONSTIM feasibility study. Cephalalgia 31:271–285
Saracco MG, Valfrè W, Cavallini M, Aguggia M (2010) Greater occipital nerve block in chronic migraine. Neurol Sci 31:179–180
Schuh-Hofer S, Tayefeh M, Reuter U, Dirnagl U, Arnold G (2006) Effects of parecoxib on plasma protein extravasation and c-fos expression in the rat. Headache 46:276–285
Schurks M, Diener HC (2008) Migraine, allodynia, and implications for treatment. Eur J Neurol 15:1279–1285
Schwedt TJ (2009) Neurostimulation for primary headache disorders. Curr Neurol Neurosci Rep 9:101–107
Serra G, Marchioretto F (2012) Occipital nerve stimulation for chronic migraine: a randomized trial. Pain Physician 15:245–253
Serrao M, Coppola G, Di Lorenzo C, Di Fabio R, Padua L, Sandrini G, Pierelli F (2010) Nociceptive trigeminocervical reflexes in healthy subjects. Clin Neurophysiol 121:1563–1568
Shin JH, Kim YC, Jang IK, Kim JH, Park SY, Lee SC (2011) Occipital nerve stimulation in a patient with an intractable chronic headache. Korean J Anesthesiol 60:298–301
Slotty PJ, Bara G, Kowatz L, Gendolla A, Wille C, Schu S, Vesper J (2015) Occipital nerve stimulation for chronic migraine: a randomized trial on subthreshold stimulation. Cephalalgia 35:73–78
Sokolov AY, Amelin AV, Ignatov YD, Panteleev SS (2008) Effect of GABA-positive drugs on the background and superior sagittalis sinus-electrostimulated activity of neurons in the nucleus trigeminalis caudalis of rats. Eksp Klin Farmakol 71:3–7
Sokolov AY, Lyubashina OA, Panteleev SS, Chizh BA (2010) Neurophysiological markers of central sensitisation in the trigeminal pathway and their modulation by the cyclo-oxygenase inhibitor ketorolac. Cephalalgia 30:1241–1249
Sokolov AY, Lyubashina OA, Panteleev SS (2012) Spinal trigeminal neurons demonstrate an increase in responses to dural electrical stimulation in the orofacial formalin test. J Headache Pain 13:75–82
Sokolov AY, Lyubashina OA, Amelin AV, Panteleev SS (2014) The role of gamma-aminobutyric acid in migraine pathogenesis. Neurochem J 8:89–102
Sokolov AY, Lyubashina OA, Berkovich RR, Panteleev SS (2015) Intravenous dextromethorphan/quinidine inhibits activity of dura-sensitive spinal trigeminal neurons in rats. Eur J Pain 19:1086–1094
Son BC, Yang SH, Hong JT, Lee SW (2012) Occipital nerve stimulation for medically refractory hypnic headache. Neuromodulation 15:381–386
Storer RJ, Goadsby PJ (2013) Topiramate is likely to act outside of the trigeminocervical complex. Cephalalgia 33:291–300
Storer RJ, Akerman S, Connor HE, Goadsby PJ (2001) 4991W93, a potent blocker of neurogenic plasma protein extravasation, inhibits trigeminal neurons at 5-hydroxytryptamine (5-HT1B/1D) agonist doses. Neuropharmacol 40:911–917
Storer RJ, Akerman S, Goadsby PJ (2003) Characterization of opioid receptors that modulate nociceptive neurotransmission in the trigeminocervical complex. Br J Pharmacol 138:317–324
Tajti J, Párdutz A, Vámos E, Tuka B, Kuris A, Bohár Z, Fejes A, Toldi J, Vécsei L (2011) Migraine is a neuronal disease. J Neural Transm (Vienna) 118(4):511–524
Tavanaiepour D, Levy RM (2014) Peripheral neuromodulation for treatment of chronic migraine headache. Neurosurg Clin N Am 25:11–14
Vadivelu S, Bolognese P, Milhorat TH, Mogilner AY (2012) Occipital nerve stimulation for refractory headache in the Chiari malformation population. Neurosurg 70:1430–1436
Watson DH, Drummond PD (2012) Head pain referral during examination of the neck in migraine and tension-type headache. Headache 52:1226–1235
Weidner C, Schmidt R, Schmelz M, Torebjork HE, Handwerker HO (2003) Action potential conduction in the terminal arborisation of nociceptive C-fibre afferents. J Physiol 547:931–940
Young WB (2014) Occipital nerve stimulation for chronic migraine. Curr Pain Headache Rep 18:396
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in animals were in accordance with the ethical standards of the institutions at which the studies were conducted.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
O. A. Lyubashina and A. Y. Sokolov contributed equally on this article.
Rights and permissions
About this article
Cite this article
Lyubashina, O.A., Panteleev, S.S. & Sokolov, A.Y. Inhibitory effect of high-frequency greater occipital nerve electrical stimulation on trigeminovascular nociceptive processing in rats. J Neural Transm 124, 171–183 (2017). https://doi.org/10.1007/s00702-016-1626-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-016-1626-2