Abstract
Patients affected by 22q11.2 deletion syndrome (22q11DS) present a characteristic cognitive and psychiatric profile and have a genetic predisposition to develop schizophrenia. Although brain morphological alterations have been shown in the syndrome, they do not entirely account for the complex clinical picture of the patients with 22q11DS and for their high risk of psychotic symptoms. Since Friston proposed the “disconnection hypothesis” in 1998, schizophrenia is commonly considered as a disorder of brain connectivity. In this study, we review existing evidence pointing to altered brain structural and functional connectivity in 22q11DS, with a specific focus on the role of dysconnectivity in the emergence of psychotic symptoms. We show that widespread alterations of structural and functional connectivity have been described in association with 22q11DS. Moreover, alterations involving long-range association tracts as well as midline structures, such as the corpus callosum and the cingulate gyrus, have been associated with psychotic symptoms in this population. These results suggest common mechanisms for schizophrenia in syndromic and non-syndromic populations. Future directions for investigations are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chromosome 22q11.2 deletion syndrome (22q11DS) is a neurogenetic disease with a prevalence of 1/4000–1/6000 live births (Oskarsdottir 2004), caused by a microdeletion on the chromosome 22. Key clinical features of the 22q11DS phenotype include congenital heart defects, cleft palate, recurrent infections and hypocalcemia (Oskarsdottir et al. 2005). The syndrome has been of particular interest due to its association with specific cognitive difficulties and psychiatric disorders, notably an increased risk to develop schizophrenia. The cognitive picture associated with 22q11DS includes mild mental delay with specific visuo-spatial difficulties (Niklasson and Gillberg 2010; Swillen et al. 1997), as well as impaired executive functions (Antshel et al. 2008) and social skills (Campbell et al. 2015; Jansen et al. 2007; Kiley-Brabeck and Sobin 2006; Woodin et al. 2001). At the clinical level, the phenotype associated with the microdeletion is marked by a strong risk of schizophrenia, which is diagnosed in about one-third of the adults with 22q11DS (Murphy et al. 1999; Schneider et al. 2014a). With such a high genetic predisposition to develop schizophrenia, 22q11DS represents a unique neurodevelopmental model to study behavioural and neural biomarkers associated with the development of psychotic symptoms (Bassett and Chow 1999; Murphy et al. 1999).
Initial neuroimaging studies in 22q11DS contributed to the delineation of brain alterations associated with the microdeletion. During childhood, decreased white and grey matter volumes have been identified in posterior parts of the brain, particularly in the occipital and parietal lobes (Gothelf et al. 2008; Tan et al. 2009). In adults, volumetric reduction is mostly located in the frontal and temporal lobes, suggesting the presence of abnormal brain development in these regions (Gothelf et al. 2008; Tan et al. 2009). Furthermore, 22q11DS and non-syndromic schizophrenia show common anatomical impairments. Notably, in 22q11DS, associations between grey matter alterations and psychotic symptoms have been found in the anterior cingulate cortex (ACC) (Dufour et al. 2008), in the adjacent ventromedial and orbital prefrontal cortices (Jalbrzikowski et al. 2013), in the left dorsolateral prefrontal cortex (Gothelf et al. 2011), in the medial temporal lobe and in the cerebellum (Kates et al. 2011). These same structures have also been involved in non-syndromic schizophrenia [reviewed in (Keshavan et al. 2008)], suggesting common mechanisms for the emergence of psychotic symptoms in 22q11DS and in the general population. However, these studies reporting localized neuroanatomical alterations do not give any information about the way these affected regions interact together and with the rest of the brain. In fact, the idea that schizophrenia might be a dysconnectivity syndrome is an old hypothesis that dates back to Wernike in (1906) and Bleuler in (1911), and that re-emerged following new developments in magnetic resonance imaging (MRI). Indeed, modern MRI methodologies provide a way to empirically test in vivo the integrity of structural and functional large-scale brain networks.
Several methods have been employed to identify abnormal brain network organization in schizophrenia and in 22q11DS. At the structural level, metrics extracted from diffusion tensor imaging (DTI) provide valuable insights about white matter integrity, by measuring the characteristics of water diffusion. Tractography algorithms can also be used on DTI images to reconstruct white matter tracts anatomy, providing a “virtual dissection” of the brain (Hagmann et al. 2003). Functional connectivity, by contrast, is defined as the synchronized brain activity of distant brain regions and is often investigated using resting-state fMRI. This technique refers to the recording of brain activity when the subject is quietly lying in the scanner without performing any particular task. Resting-state fMRI is commonly used on young and cognitively impaired populations because it requires limited contribution from the participants. Furthermore, resting-state fMRI has shown to reflect endogenous characteristics of brain connectivity leading to the identification of large-scale functional networks that are highly reproducible across populations (Rosazza and Minati 2011). The description of the methods for investigating brain connectivity is beyond the scope of this review; however, we reported essential definitions of technical terms employed in this manuscript (Box 1, Glossary).
By using DTI and resting-state fMRI, widespread impairment of structural and functional connectivity has been described in schizophrenia. A recent meta-analysis showed that the majority of the studies using DTI in schizophrenia found altered white matter integrity in the left frontal and temporal lobes (Ellison-Wright and Bullmore 2010). This fronto-temporal dysconnectivity has been implicated in self-monitoring impairments and in the inability to distinguish between internal thoughts and external stimuli (Hubl et al. 2004). Tractography studies also reported alterations in several long association fibres, such as the superior (SLF) and inferior longitudinal fasciculi (ILF), the inferior fronto-occipital fasciculus (IFOF) and the cingulum bundle (Canu et al. 2015). Functional studies investigating brain connectivity at rest reported impairments in several regions including the default mode network (DMN), a network particularly active during rest that is responsible for self-oriented thoughts and mind wandering (Raichle 2001). Dysconnectivity in auditory/language networks, cortical-subcortical networks and in the prefrontal cortex have also been described in patients with schizophrenia (Karbasforoushan and Woodward 2012). In agreement with the alterations of the long-range white matter tracts, graph theory analysis revealed reduced measures of integration in important hubs such as prefrontal, limbic, temporal and parietal regions (Fornito et al. 2012; Griffa et al. 2015). Thus, far in 22q11DS, depending on the methods employed and the demographic characteristics of the patients’ sample, the application of the same techniques gave heterogeneous results. Findings published in 22q11DS, however, provide a better delineation of the anatomical and functional brain phenotype associated to the microdeletion, leading to a better comprehension of the pathophysiological mechanisms underlying the cognitive and clinical manifestations of 22q11DS. Furthermore, patterns of connectivity in 22q11DS could represent risk factors for the development of schizophrenia and related psychotic disorders, and may provide a framework for better understanding the mechanisms that underlie the emergence of this disorder in the general population.
In this review, we aim to summarize research on structural and functional brain connectivity in 22q11DS. We start by reviewing the brain connectivity alterations described in structural and functional large-scale brain networks in children, adolescents and adults with 22q11DS, thereby looking for a common pattern of alterations that may constitute a core connectivity phenotype associated to the microdeletion. Then, we review evidence for relationships between dysconnectivity and cognitive or clinical symptoms expression, with particular emphasis on psychotic symptoms and schizophrenia.
Method
Published studies that investigated structural or resting-state connectivity in 22q11DS were selected in August 2015 using the PubMed database with the following keywords: “22q11.2 connectivity”, “22q11.2 DTI”, “22q11.2 fractional anisotropy”, “22q11.2 tractography”, “22q11.2 resting-state”. We excluded all the studies investigating brain morphology in 22q11DS, as they have already been reviewed elsewhere (Gothelf et al. 2008; Tan et al. 2009) and provide less direct information about cerebral connectivity. Moreover, we included only the studies using MRI acquisitions, namely DTI and resting-state fMRI, and excluded the EEG studies.
The PubMed search retrieved 17 published studies investigating structural connectivity in 22q11DS, using either using voxel-based DTI methodologies (Barnea-Goraly et al. 2003, 2005; da Silva et al. 2011; Deng et al. 2015; Jalbrzikowski et al. 2014; Kates et al. 2015; Kikinis et al. 2012; Perlstein et al. 2014; Radoeva et al. 2012; Simon et al. 2005, 2008; Sundram et al. 2010; Villalon-Reina et al. 2013) or tractography, with measures of fibres’ count (Kikinis et al. 2013; Ottet et al. 2013a; Padula et al. 2015) or graph theory (Ottet et al. 2013b). In addition, 4 published studies to date examined resting-state functional connectivity in 22q11DS (Debbane et al. 2012; Padula et al. 2015; Scariati et al. 2014; Schreiner et al. 2014).
The results extracted from some of these studies cannot be considered fully independent, because they used overlapping samples of participants recruited by the same lead investigator in the same University centre. By examining the authors’ list and description of participants, we identified seven different cohorts of patients: Stanford University (Barnea-Goraly et al. 2003, 2005), Brigham and Massachusetts Hospitals in Boston (Kikinis et al. 2012, 2013), Children’s Hospital of Philadelphia (Simon et al. 2005, 2008), University of California Los Angeles (Jalbrzikowski et al. 2014; Schreiner et al. 2014), State University of New York (SUNY) Upstate Medical University (Kates et al. 2015; Perlstein et al. 2014; Radoeva et al. 2012), the University of California Davis Medical Centre (Deng et al. 2015; Villalon-Reina et al. 2013) and our cohort of subjects recruited at the Geneva University (Debbane et al. 2012; Ottet et al. 2013a, b; Padula et al. 2015; Scariati et al. 2014).
Results
Structural connectivity in 22q11DS: DTI studies
The main results of the 17 studies that used structural connectivity in individuals with 22q11DS are reviewed in Table 1. The majority of the studies (n = 13) reviewed in this manuscript used voxel-based DTI measures, namely fractional anisotropy (FA) (Barnea-Goraly et al. 2003, 2005; da Silva et al. 2011; Deng et al. 2015; Jalbrzikowski et al. 2014; Kates et al. 2015; Kikinis et al. 2012; Perlstein et al. 2014; Radoeva et al. 2012; Simon et al. 2005, 2008; Sundram et al. 2010; Villalon-Reina et al. 2013), or axial (AD) and radial (RD) diffusivity (Jalbrzikowski et al. 2014; Radoeva et al. 2012; Simon et al. 2008; Villalon-Reina et al. 2013). More recent investigations used tractography techniques (Kikinis et al. 2013; Ottet et al. 2013a, b; Padula et al. 2015), with measures of fibre tracts count (Kikinis et al. 2013; Ottet et al. 2013a; Padula et al. 2015) or graph theoretical metrics (Ottet et al. 2013b).
Most of the voxel-based studies were conducted using whole-brain approaches (Barnea-Goraly et al. 2003; da Silva et al. 2011; Jalbrzikowski et al. 2014; Kikinis et al. 2012; Radoeva et al. 2012; Simon et al. 2005, 2008; Sundram et al. 2010; Villalon-Reina et al. 2013), while a few of them investigated the integrity of specific tracts of interest (Deng et al. 2015; Kates et al. 2015; Perlstein et al. 2014).
Whole-brain studies reported discordant results in 22q11DS, pointing to both reduced (Barnea-Goraly et al. 2003; da Silva et al. 2011; Kikinis et al. 2012; Radoeva et al. 2012; Simon et al. 2005; Sundram et al. 2010; Villalon-Reina et al. 2013) and increased (da Silva et al. 2011; Jalbrzikowski et al. 2014; Simon et al. 2005; Sundram et al. 2010) FA in several brain regions spanning mainly the frontal, parietal and temporal lobes (see Table 1 for detailed findings of the voxel-based studies).
In terms of fibre bundles, alterations have been shown in several long association tracts including the superior (SLF) and inferior (ILF) longitudinal fasciculus, the inferior fronto-occipital fasciculus (IFOF), the uncinate fasciculus, the arcuate fasciculus and the cingulum bundle. Some of the results are consistent and show reduced FA in the ILF (Barnea-Goraly et al. 2003; Sundram et al. 2010; Villalon-Reina et al. 2013), the uncinate fasciculus (Radoeva et al. 2012; Sundram et al. 2010) and the fronto-parietal course of the arcuate fasciculus (Sundram et al. 2010). More discordant results have been found in the SLF with Barnea-Goraly et al. (2003) and Villalon-Reina et al. (2013) indicating reduced FA and Jalbrzikowski et al. (2014) and Simon et al. (2008) reporting increased FA. These studies present, however, some methodological differences. Although both Barnea-Goraly et al. (2003) and Jalbrzikowski et al. (2014) used a sample comprising children, adolescents and young adults, in Jalbrzikowski et al. (2014), the analysis was corrected for the age and the gender of the participants, while in Barnea-Goraly et al. (2003) these confounding effects have not been taken into account. Similarly, Sundram et al. (2010) and Villalon-Reina et al. (2013) conducted their analyses in a sample of children and adolescents, but (Villalon-Reina et al. 2013) included only female subjects and corrected the analysis for age and total brain volume. Simon et al. (2008) also found increased FA in the IFOF, while the opposite result was reported by Sundram et al. (2010), when correcting for the IQ. Inconsistent results about reduced (Jalbrzikowski et al. 2014; Sundram et al. 2010) and increased (da Silva et al. 2011; Simon et al. 2005) FA in the cingulum bundle have also been reported. However, a recent study that included a large sample of adolescents and adults and that specifically investigated the integrity of this white matter tract (Kates et al. 2015) reported decreased FA and AD in the anterior portion of the cingulum bundle and a concomitant increase in FA and decrease in RD in its more posterior aspect. Impairments in the fornix, another white matter tract involved in the limbic system, have been reported in 22q11DS by two other studies that specifically investigated the integrity of this tract (Deng et al. 2015; Perlstein et al. 2014). Finally, alterations in the corpus callosum (Jalbrzikowski et al. 2014; Simon et al. 2005; Sundram et al. 2010) and in the internal capsule including the corona radiata (Jalbrzikowski et al. 2014; Perlstein et al. 2014; Sundram et al. 2010; Villalon-Reina et al. 2013) have been observed in patients with 22q11DS.
By contrast to FA results, studies reporting AD and RD show a more consistent decrease of these measures in patients with 22q11DS. In particular, reduced AD indicating axonal damage (Budde et al. 2009; Song et al. 2003) has been reported in the SLF, ILF, IFOF and the cingulum bundle (Jalbrzikowski et al. 2014; Kates et al. 2015; Radoeva et al. 2012; Simon et al. 2008).
Although some of the studies using voxel-based measures reported inconsistent findings, robust evidence points to a prefential alteration of long-range fibres connecting the frontal lobe to the temporal, parietal and occipital lobes, as well as limbic tracts, such as the cingulum bundle and the fornix.
Consistent with voxel-based findings, three studies using tractography published by our group, confirmed the altered connectivity in fronto-temporal (Ottet et al. 2013a, b), parietal (Ottet et al. 2013b) and limbic regions (Ottet et al. 2013a; Padula et al. 2015). Using a whole-brain analysis, we showed a 10 % reduction in the number of total reconstructed streamlines in 22q11DS (Ottet et al. 2013a). More specifically, reduced number of virtual fibres was still observed when this decrease was accounted for in left fronto-temporal (−34 %) and parieto-limbic (−10 %) connections as well as in the left occipital lobe (−18 %) and in bilateral limbic structures (−6 %) (Ottet et al. 2013a). By contrast, the right frontal and parietal lobes as well as the right parieto-occipital connections showed a relative increase in the number of fibres in the patients group. In line with these findings of reduced limbic connectivity, in a following study (Padula et al. 2015), we reported a reduction of the fibre tracts connecting the anterior and posterior regions of the DMN, which are part of the cingulum bundle. An additional tractography study conducted on an adult sample by Kikinis and colleagues found a reduced FA and AD by investigating the integrity of the fibre tracts belonging to the ventral visual stream (IFOF and ILF) in 22q11DS (Kikinis et al. 2013).
Only one study investigated the impact of these alterations on the organization of the structural network (Ottet et al. 2013b). Using a graph theoretical analysis of the structural connectome, this study showed reduced global efficiency and increased characteristic path length in patients with 22q11DS compared to healthy controls. These results reflect a deficit of network integration in 22q11DS that is related to a reduced connectivity (decreased degree) of important brain hubs including the bilateral hippocampus, superior parietal and precentral regions, right rostral middle frontal and superior frontal cortices, the precuneus and the left thalamus. Segregation, as measured with the mean clustering coefficient, was preserved in patients with 22q11DS.
Association between altered white matter integrity and age in 22q11DS
Given the altered trajectories of clinical and cognitive development in patients with 22q11DS, it is of particular interest to examine age-related effects in altered connectivity. Indeed, patients affected by 22q11DS undergo cognitive decline (Schneider et al. 2014b; Vorstman et al. 2015) and have an increased risk to develop psychiatric symptoms (Schneider et al. 2014b) when they transition from adolescence to adulthood. Furthermore, given that neurodevelopmental processes have been strongly involved in schizophrenia development (Rapoport et al. 2005), understanding the temporal course of connectivity alterations in 22q11DS is crucial to identify potential risk markers for psychosis. Neuroimaging studies reported evidence for altered cortical volume and thickness development (Gothelf et al. 2007, 2011; Schaer et al. 2009), which may account for the behavioural alterations. However, no longitudinal studies on brain connectivity have been conduced to date in patients with 22q11DS. Nevertheless, four cross-sectional studies suggested an abnormal development of white matter with age in 22q11DS (Deng et al. 2015; Jalbrzikowski et al. 2014; Ottet et al. 2013a; Padula et al. 2015). A first analysis conducted by our group showed that while the total white matter volume increases with age in both healthy controls and patients with 22q11DS, whole-brain mean FA was positively correlated with age only in controls (Ottet et al. 2013a). By contrast, no association between age and the total number of streamlines was observed in any of the groups. Subsequently, several analyses focusing on tracts of interest revealed similar findings. In a partially overlapping sample of patients, we reported a lack of normal increase in the number of streamlines between the anterior and posterior nodes of the DMN with age in patients with 22q11DS (Padula et al. 2015). In this study, we also showed that the number of tracts connecting the anterior and posterior DMN was preserved in children and adolescents with 22q11DS, while impairments started to appear in young adults (Padula et al. 2015). In Jalbrzikowski et al. (2014), the authors found an increase in ILF FA and a reduction in ILF RD with age in control participants, but not in patients with 22q11DS. Similarly, Deng et al. (2015) reported a lack of the age-associated increase in AD and RD in the fornix in patients compared with healthy individuals (Deng et al. 2015). These findings give preliminary evidence of altered white matter maturation in 22q11DS and suggest that structural dysconnectivity, together with cortical abnormalities, may precede the development of psychosis. Indeed, a recent study showed that in non-syndromic patients at risk for schizophrenia who have prodromal psychotic symptoms, white matter abnormalities are already present before the onset of full psychosis (Chung and Cannon 2015).
Functional connectivity in 22q11DS: resting-state fMRI studies
To date, four studies investigated functional brain connectivity using resting-state fMRI in 22q11DS (Debbane et al. 2012; Padula et al. 2015; Scariati et al. 2014; Schreiner et al. 2014). The first three were performed on our cohort of patients, while the last (Schreiner et al. 2014) used an independent sample. We performed two whole-brain connectivity investigations (Debbane et al. 2012; Scariati et al. 2014). In Scariati et al. (2014), we investigated the distribution of the functional connectome alterations associated with 22q11DS. We found widespread alterations of the functional network with a predominant frontal dysconnectivity and an impairment of visuo-spatial networks including occipital, parietal and right inferior temporal connectivity. In Debbané et al. (2012), we investigated the functional networks communities using an ICA in a partially overlapping sample of adolescents with the microdeletion compared to healthy controls. This study also showed the presence of altered connectivity in visuo-spatial networks as well as in sensory-motor and default mode networks.
Two seed-based studies (Padula et al. 2015; Schreiner et al. 2014) further investigated DMN connectivity in patients with 22q11DS and confirmed a specific impairment of this network. In Padula et al. (2015), we used a seed-based multimodal approach focusing on DMN connectivity in a sample of patients with the microdeletion ranging from children to adults (Padula et al. 2015). More specifically, we found decreased functional connectivity between anterior and posterior DMN nodes, mirroring the above-mentioned structural connectivity impairment, and an isolated decrease in functional connectivity between left and right inferior parietal DMN nodes. The study from Schreiner and colleagues (2014) used a seed-to-voxel and a seed-to-seed analysis centred on the posterior cingulate cortex (PCC) to identify the DMN. They also found a global decrease in functional connectivity within the DMN in 22q11DS, specifically involving the connections between the anterior ventro-medial prefrontal cortex and the PCC. Moreover, they found that the DMN was more diffuse in 22q11DS with an increased connectivity between the PCC and the right inferior frontal cortex (Schreiner et al. 2014). Only this last study tested the effect of age on functional connectivity in 22q11DS and suggested the presence of abnormal DMN development with age. In particular, patients showed an increase with age of long-range connections between the PCC and regions located outside the DMN.
Association between impaired connectivity, cognitive abilities and manifestation of psychotic symptoms in 22q11DS
Several of the studies discussed above reported an association between altered structural and functional connectivity and cognitive (Barnea-Goraly et al. 2005; Debbane et al. 2012; Jalbrzikowski et al. 2014; Kates et al. 2015; Radoeva et al. 2012; Schreiner et al. 2014; Simon et al. 2008) or psychiatric (da Silva et al. 2011; Debbane et al. 2012; Jalbrzikowski et al. 2014; Kates et al. 2015; Ottet et al. 2013a, b; Perlstein et al. 2014; Scariati et al. 2014; Sundram et al. 2010) impairments in 22q11DS.
Two studies showed that structural dysconnectivity in the parietal lobe is associated with poorer arithmetic abilities (Barnea-Goraly et al. 2005) and visuo-spatial attention (Simon et al. 2008) in patients with the syndrome. However, the direction of the FA difference was different in the 2 studies, with one reporting reduced (Barnea-Goraly et al. 2005) and the other reporting increased (Simon et al. 2008) FA in 22q11DS. Furthermore, Radoeva et al. (2012) showed an association between white matter microstructural impairment in parietal/occipital cortices, SLF, IFO and poorer executive functions (Radoeva et al. 2012). Also, resting-state functional dysconnectivity of the DMN (Schreiner et al. 2014) and reduced axonal coherence in IFO and uncinate fasciculus (Jalbrzikowski et al. 2014) were associated with dysfunctional social behaviour in patients with 22q11DS. An additional study showed that patients with 22q11DS with a low IQ have reduced connectivity in striatal structures (cortico-striatonigral-thalamocortical circuit) with respect to 22q11DS individuals with higher IQ (Meskaldji et al. 2011).
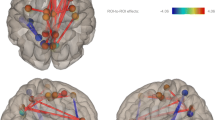
Relationships between structural and functional connectivity and psychosis have also been reported.White matter tracts alterations that have shown associations with psychotic symptoms in patients with 22q11DS are shown in Fig. 1. By comparing a group of patients with the microdeletion suffering from schizophrenia to a group of patients that does not, Da Silva Alves et al. (2011) have shown that schizophrenia is associated to increased FA in the left cingulum bundle, the anterior corona radiata and in the corpus callosum. These authors further refined their results by performing correlations between FA and the different dimensions of schizophrenia using the PANSS [positive and negative syndrome scale (Kay et al. 1987)]. They showed the presence of a negative relationship between the intensity of positive symptoms and FA in bilateral uncinate fasciculus and ILF as well as in the left IFOF, corpus callosum and superior corona radiata. Associations between positive symptoms and the microstructure of the bilateral IFOF (Jalbrzikowski et al. 2014), the uncinate (Perlstein et al. 2014) and the superior cingulum bundle (Kates et al. 2015) were confirmed on an independent sample of patients. Furthermore, positive symptoms also showed a positive correlation with FA in the anterior limb of the internal capsule,and a negative correlation at the same place in patients with 22q11DS (Perlstein et al. 2014). Finally, the graph theory study (Ottet et al. 2013b), even though performed at the cortical level, also reported congruent results by showing a correlation between the local efficiency of left middle and inferior frontal cortices and transverse temporal gyri.
The majority of the studies reported that the integrity of long-range white matter tracts connecting the frontal lobe is associated with psychotic symptoms. Results of all the studies that reported correlations between DTI measures (FA, AD, RD or number of streamlines) and positive or negative psychotic symptoms in 22q11DS are included in the figure. Precise details about the measures employed in the different studies can be found in Table 2. The tracts were reconstructed in a control subject of our cohort using the Connectome mapper (http://www.cmtk.org/mapper/) and diffusion toolkit (www.trackvis.org/dtk) and were displayed using trackvis (http://trackvis.org).
Two out of the four studies measuring resting-state connectivity in 22q11DS examined the relationship between psychosis and functional connectivity. Both were conducted on our cohort. In Debbané et al. (2012), reduced activation in the left superior frontal gyrus, considered as part of the DMN, was related to the importance of positive and negative prodromal psychotic symptoms. The second study (Scariati et al. 2014) further showed that patients with prodromal positive symptoms could be accurately distinguished from the rest of the patients using resting-state connectivity patterns. The discriminative graph contains connections originating from frontal and limbic regions (left ACC, right inferior frontal and rectus gyri) as well as superior parietal and precentral regions. Contrarily, our multimodal study (Padula et al. 2015), even if performed on a partially overlapping sample, failed to identify any relationship between psychosis and dysconnectivity in the DMN. However, this study tested only a limited sample of DMN connections, namely between anterior (medial prefrontal cortex and ACC) and posterior (PCC and precuneus) nodes that did not completely overlap with the regions identified in the previous studies.
Discussion
General considerations about brain connectivity in 22q11DS
The findings reviewed in this manuscript indicate that alterations in structural connectivity are widespread in patients with 22q11DS and involve most of the white matter tracts. However, studies using FA as measure of white matter integrity reported inconsistent results, with some of them pointing to reduced FA (Barnea-Goraly et al. 2003; da Silva et al. 2011; Kikinis et al. 2012; Radoeva et al. 2012; Simon et al. 2005; Sundram et al. 2010; Villalon-Reina et al. 2013) and some to increased FA (da Silva et al. 2011; Jalbrzikowski et al. 2014; Simon et al. 2005; Sundram et al. 2010) in convergent brain regions. The reason for this heterogeneity may be attributed to the small size of the samples and the variable age ranges, related to the low prevalence of the disease. Also, methodological differences in the DTI analysis, in terms of registration algorithms and choice of templates, can be identified through the studies (Table 1). In particular, as discussed in (Barnea-Goraly et al. 2003; Ottet 2013; Radoeva et al. 2012), registration is an important issue in patients with 22q11DS. Indeed, morphological brain alterations, such as enlarged ventricles, could impact the accuracy of registration to standard templates and affect the results. While several studies published on 22q11DS used standard templates [usually the MNI template (Mazziotta et al. 1995)], several other papers used alternative strategies. For instance, Jalbrzikowski et al. 2014 and Kikinis et al. 2012 used tract-based spatial statistics (TBSS) to create a group-based white matter “skeleton” to which individual images were non-linearly registered. A few others also performed measurements in subject space (Deng et al. 2015; Kates et al. 2015; Ottet et al. 2013a, b; Padula et al. 2015; Perlstein et al. 2014; Radoeva et al. 2012). Therefore, the different registration approaches that were adopted in the different studies may have influenced the accuracy of the results. However, it is not possible to identify a specific pattern that could explain the different directionalities in the findings. Furthermore, it should be noticed that FA summarizes the diffusion in all the three diffusion directions (x, y, z), thus being not specific for the diffusion in one single axis (Armitage and Bastin 2000). Also, the sensitivity of FA depends on the mean diffusion of the environment (Ottet 2013). Given these limitations of FA and the difficulties in the interpretation of this measure, it is important to consider complementary measures of diffusivity, such as RD and AD, which provide more specific information about the axonal damage (Budde et al. 2009; Song et al. 2003, 2005).
In line with the structural connectivity findings, resting-state studies reported altered functional connections in several networks, with most of the results pointing to impaired DMN connectivity (Debbane et al. 2012; Padula et al. 2015; Schreiner et al. 2014). Taken together, the structural and functional connectivity findings show a predominant involvement of frontal, parietal and limbic connectivity and their association with the presence of cognitive impairments (Barnea-Goraly et al. 2005; Jalbrzikowski et al. 2014; Kates et al. 2015; Radoeva et al. 2012; Schreiner et al. 2014) and psychotic symptoms (da Silva et al. 2011; Debbane et al. 2012; Jalbrzikowski et al. 2014; Kates et al. 2015; Ottet et al. 2013a, b; Perlstein et al. 2014; Scariati et al. 2014).
The studies reporting associations between altered white matter connectivity and psychosis are also heterogeneous, with different directions of FA changes being reported. On the one hand, three studies reported a negative correlation, with decreased FA or AD measures related to increased positive symptoms scores (da Silva et al. 2011; Jalbrzikowski et al. 2014; Sundram et al. 2010). On the other hand, two studies reported a positive correlation between increased FA values in the ALIC (Perlstein et al. 2014) and in the cingulum bundle (Kates et al. 2015) and positive symptoms scores in an overlapping cohort of participants. As noted by (Kates et al. 2015), similar positive correlations between FA values and positive symptoms have been reported in patients with schizophrenia. These authors also argue that this positive correlation may rely on reduced axonal pruning, which would cause a concomitant increase in the FA values and reduced efficiency of neural transmission. This hypothesis remains, however, speculative.
The majority of the studies included in the present review investigated the relationship between altered connectivity and positive psychotic symptoms, while only one study (da Silva et al. 2011) used also negative and general symptoms scores. As negative symptoms are predominant in the 22q11DS population (Schneider et al. 2014c), their association with white matter dysconnectivity should be better investigated in future studies.
Despite the inconsistency in the results, most of the findings have shown alterations in long-range and midline white matter tracts in patients with 22q11DS. In the following paragraph, we discuss how these alterations may be implicated in the cognitive and clinical symptomatology.
Abnormalities of brain connectivity related to cognitive alterations and psychotic symptoms in 22q11DS
All the studies reporting an association between white matter dysconnectivity and psychotic symptoms (listed in Table 2) have shown an involvement of one or more long-range white matter bundle (cingulum bundle, IFOF, ILF, corona radiata, internal capsule), many of which connecting the frontal lobe. Furthermore, altered connectivity in midline structures (cingulum bundle and corpus callosum) has also been related to psychotic symptoms (da Silva et al. 2011; Kates et al. 2015; Sundram et al. 2010).
Long-range connections sustain the interaction between distant brain regions and ensure brain functional integration (van den Heuvel and Sporns 2013). Functional brain integration is essential for the execution of cognitive functions, and disruption in brain hubs has been reported in several psychiatric disorders (Crossley et al. 2014). In 22q11DS, long-range dysconnectivity may reflect impaired functional integration and communication between distant brain areas. In a previous study (Ottet et al. 2013b), we indeed found reduced efficiency of the brain network in affected patients, related to decreased connectivity of several important brain hubs. As such, reduced long-range connections and impaired efficiency may account for the difficulties observed in patients with 22q11DS, such as deficits in social skills (Campbell et al. 2015; Jansen et al. 2007; Kiley-Brabeck and Sobin 2006; Woodin et al. 2001) and altered executive functions [reviewed in (Antshel et al. 2008)]. Specifically, decreased working memory as well as altered flexibility, planning and inhibition capacities have been reported in 22q11DS (Campbell et al. 2010). A few studies reviewed in the present work confirm the link between long-range and frontal dysconnectivity and cognitive difficulties. Specifically, it has been shown that alterations in long-range white matter tracts connecting the frontal lobe (i.e. the IFO and SLF) are concomitant with impairments in working memory, executive functions (Radoeva et al. 2012) and social competences (Jalbrzikowski et al. 2014; Radoeva et al. 2012; Schreiner et al. 2014) in patients with 22q11DS. Other studies report a relationship between long-range dysconnectivity and the expression of psychotic symptoms in this population, either positive (da Silva et al. 2011; Debbane et al. 2012; Jalbrzikowski et al. 2014; Ottet et al. 2013a, b; Perlstein et al. 2014) or negative (da Silva et al. 2011; Debbane et al. 2012). Moreover, three studies, in particular, reported altered fronto-temporal connectivity in 22q11DS (da Silva et al. 2011; Ottet et al. 2013a, b). Fronto-temporal dysconnectivity has largely been implicated in the pathogenesis of schizophrenia in the general population (Samartzis et al. 2014) and has been related to the presence of and hallucinations (Ottet et al. 2013a, b) in 22q11DS, further suggesting common mechanisms in syndromic and non-syndromic schizophrenia.
Alterations in midline brain regions have been shown in previous studies investigating brain morphology in 22q11DS. For instance, increased area (Antshel et al. 2005; Machado et al. 2007; Shashi et al. 2004) and volume (Machado et al. 2007; Shashi et al. 2012) of the corpus callosum have been reported and have been related with higher cognitive scores (Shashi et al. 2012), thus suggesting that increased corpus callosum volume may be a compensatory mechanism. Shashi et al. (2012) proposed that increased corpus callosum volume could be driven by either an increased number of axons, an increase in their myelination or an enlargement of the axons’ diameter. However, the first two mechanisms are not compatible with the observation of reduced FA in the corpus callosum (da Silva et al. 2011; Sundram et al. 2010), thus suggesting that the more valid hypothesis is the axons enlargement. Future investigations in animal models are needed to confirm whether common mechanisms are present that explain both the increased corpus callosum volume and the reduction of FA.
Midline structures dysconnectivity has been related with psychotic symptoms in 22q11DS (Debbane et al. 2012; Kates et al. 2015; Ottet et al. 2013a; Scariati et al. 2014). For instance, we showed that functional ACC dysconnectivity strongly contributes in identifying patients with 22q11DS that present prodromal positive psychotic symptoms (Scariati et al. 2014). In the general population, ACC dysconnectivity has been involved in several network theories about schizophrenia. This is probably due to the fact that this region is involved in several functions [reviewed in (Bush et al. 2000)] and is part of several networks, including the saliency network and the DMN, where it plays a role in self-related processing (Murray et al. 2012); (Menon and Uddin 2010; Rosazza and Minati 2011). Recently, it has been proposed that a dysconnectivity between the DMN, the central executive (that includes the dorsolateral prefrontal and dorsal parietal cortices) and the salience networks may be involved in several psychopathologies (Menon 2011; Menon and Uddin 2010), including schizophrenia (Nekovarova et al. 2014). According to this theory, the salience network is dysfunctional and improperly manages the switch between resting and active mental activity, leading to incorrect DMN deactivation when the subject is engaged in a cognitive task. A deficient DMN deactivation has indeed been described in schizophrenic populations, as well as their unaffected relatives [reviewed in (Nekovarova et al. 2014)]. Other complementary models propose that self-monitoring deficits play a role in the development of psychosis (Frith 1992). These deficits correspond to a difficulty in recognizing self-generated from externally generated thoughts or actions and are thought to underlie key symptoms such as alien control or hallucinations. Interestingly, self-monitoring deficits have been related to abnormal functions of medial limbic structures, including the ACC (Allen et al. 2008). Although none of the reviewed studies directly investigated triple network structural and resting-state connectivity in 22q11DS, several studies reported impaired DMN connections (Debbane et al. 2012; Padula et al. 2015; Schreiner et al. 2014). Furthermore, two EEG studies reported impairment in class C and D microstates (Tomescu et al. 2014; Tomescu et al. 2015). These maps have been shown to correspond to salience and executive networks, respectively, in a normative sample of subjects (Britz et al. 2010). More specifically, the time coverage and the occurrence of class D microstate were decreased in youths with the microdeletion, while the opposite was observed for class C microstate. This result has been replicated in a sample of adults with chronic schizophrenia, strongly suggesting common mechanisms in syndromic and non-syndromic psychosis (Tomescu et al. 2015). Finally, it has been shown that patients with the microdeletion also present self-monitoring difficulties (Debbane et al. 2008; 2010) and that ACC dysfunction during a self-referential processing task was correlated with the intensity of positive symptoms in a sample of adolescents with 22q11DS (Schneider et al. 2012).
Limitations
Taken together, existing studies in 22q11DS report a complex pattern of connectivity alterations, among which altered midline and long-range connections are mostly associated with psychotic symptoms. However, this review comes with some limitations. First of all, several studies have been published by the same groups, so they do not present independent results and likely show congruent findings since they share part of the sample. Therefore, we tried to highlight results reported by independent samples throughout this review whenever possible. Furthermore, given the low prevalence of the 22q11DS, published studies usually include a limited sample of patients with wide or variable age ranges. Studies based on DTI also share some limitations. First of all, exclusion of DTI scans because of excessive motion often relied, except for three studies (Kates et al. 2015; Perlstein et al. 2014; Radoeva et al. 2012), on visual inspection. For this reason, we cannot completely exclude the possibility that residual motion may have affected the results. Second, DTI acquisitions do not allow an accurate reconstruction of fibres that cross, kiss or fan (Bammer et al. 2003). In addition, the number of resting-state fMRI studies is limited in this population, and two studies presented a focus on DMN connectivity, which can provide a bias towards alterations found in this network. Studies targeting other networks of interest will be necessary to confirm whether the DMN is particularly affected in 22q11DS. Furthermore, studies investigating functional connectivity during cognitive tasks may reveal more precise alterations in specific domains.
Conclusion and perspectives
In conclusion, current literature in 22q11DS shows a predominant impairment of long-range connectivity, involving frontal and midline structures. Relationships between these alterations and cognitive deficits or psychotic symptoms have been described. However, future studies on independent samples are needed to confirm these results. Furthermore, new methodologies may be used to overcome the limitations of current literature and to obtain refined brain connectivity measurements. For instance, new DSI sequences that acquire more diffusion directions in a shorter amount of time are today available. This kind of acquisition would provide finer white matter tracts reconstruction while keeping a scanning duration suited to paediatric populations (Cahoon 2011). Further methodological developments will also permit the study of resting-state connectivity dynamics as well as the relationships between structural and functional connectivity alterations. Similarly, functional connectivity studies using task-related fMRI should be used to test current hypotheses about the mechanisms that underlie schizophrenia.
Finally, given the low prevalence of the 22q11DS, collaborations between different groups will be necessary to obtain large samples of patients with smaller age ranges. International collaborative work has been started by the International 22q11.2 Brain Behaviour Consortium (22q-IBBC) that already lead to the publication of results on genetic (Guo et al. 2011; Herman et al. 2012; Mlynarski et al. 2015), cognitive and clinical (Bassett et al. 2011; Schneider et al. 2014a; Vorstman et al. 2015) data including up to 1400 participants with 22q11DS. However, such collaborations will pose particular challenges for neuroimaging studies since the inclusion of different scanning sites and sequences into the same study may increase noise and introduce a systematic bias in the analyses (Focke et al. 2011; Takao et al. 2012). Several research groups are also continuing to acquire longitudinal data with the aim of describing the trajectories of brain structural and functional connectivity development. This on-going work will allow testing whether part of the described connectivity alterations precede psychosis onset and have a predictive value for the development of schizophrenia.
References
Radoeva PD et al (2012) Atlas-based white matter analysis in individuals with velo-cardio-facial syndrome (22q11.2 deletion syndrome) and unaffected siblings Behavioral and Brain Functions 8
Mlynarski Elisabeth E et al (2015) Copy-number variation of the glucose transporter gene SLC2A3 and congenital heart defects in the 22q11.2 deletion syndrome The American Journal of Human Genetics. 96:753–764. doi:10.1016/j.ajhg.2015.03.007
Allen P, Larøi F, McGuire PK, Aleman A (2008) The hallucinating brain: a review of structural and functional neuroimaging studies of hallucinations. Neurosci Biobehav Rev 32:175–191. doi:10.1016/j.neubiorev.2007.07.012
Antshel KM, Conchelos J, Lanzetta G, Fremont W, Kates WR (2005) Behavior and corpus callosum morphology relationships in velocardiofacial syndrome (22q11.2 deletion syndrome). Psychiatry Res 138:235–245. doi:10.1016/j.pscychresns.2005.02.003
Antshel KM, Fremont W, Kates WR (2008) The neurocognitive phenotype in velo-cardio-facial syndrome: a developmental perspective. Dev Disabil Res Rev 14:43–51. doi:10.1002/ddrr.7
Armitage PA, Bastin ME (2000) Selecting an appropriate anisotropy index for displaying diffusion tensor imaging data with improved contrast and sensitivity. Magn Reson Med 44:117–121. doi:10.1002/1522-2594(200007)44:1<117:AID-MRM17>3.0.CO;2-D
Bammer R, Acar B, Moseley ME (2003) In vivo MR tractography using diffusion imaging. Eur J Radiol 45:223–234. doi:10.1016/S0720-048X(02)00311-X
Barnea-Goraly N, Menon V, Krasnow B, Ko A, Reiss A, Eliez S (2003) Investigation of white matter structure in velocardiofacial syndrome: a diffusion tensor imaging study. Am J Psychiatry 160:1863–1869
Barnea-Goraly N, Eliez S, Menon V, Bammer R, Reiss AL (2005) Arithmetic ability and parietal alterations: a diffusion tensor imaging study in velocardiofacial syndrome. Brain Res Cogn Brain Res 25:735–740. doi:10.1016/j.cogbrainres.2005.09.013
Basser PJ, Mattiello J, LeBihan D (1994) MR diffusion tensor spectroscopy and imaging. Biophys J 66:259–267
Bassett A, Chow E (1999) 22q11 deletion syndrome: a genetic subtype of schizophrenia. Biol Psychiatry 46:882–891
Bassett AS et al (2011) Practical guidelines for managing patients with 22q11.2 deletion syndrome. J Pediatr 159(332–339):e331. doi:10.1016/j.jpeds.2011.02.039
Biswal B, Yetkin F, Haughton V, Hyde J (1995) Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34:537–541
Bleuler E (1911) Dementia praecox or the group of schizophrenias. 9International Universities Press, New York
Britz J, Van De Ville D, Michel CM (2010) BOLD correlates of EEG topography reveal rapid resting-state network dynamics. Neuroimage 52:1162–1170. doi:10.1016/j.neuroimage.2010.02.052
Budde MD, Xie M, Cross AH, Song SK (2009) Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: a quantitative pixelwise analysis. J Neurosci 29:2805–2813. doi:10.1523/jneurosci.4605-08.2009
Bush G, Luu P, Posner MI (2000) Cognitive and emotional influences in anterior cingulate cortex. Trends Cognit Sci 4:215–222. doi:10.1016/S1364-6613(00)01483-2
Cahoon G (2011) Techniques in pediatric MRI—tips for imaging children MAGNETOM Flash
Calhoun VD, Liu J, Adalı T (2009) A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. NeuroImage S45:S163–S172. doi:10.1016/j.neuroimage.2008.10.057
Campbell L et al (2010) Executive functions and memory abilities in children with 22q11.2 deletion syndrome. J Psychiatry 44:364–371
Campbell LE, McCabe KL, Melville JL, Strutt PA, Schall U (2015) Social cognition dysfunction in adolescents with 22q11.2 deletion syndrome (velo-cardio-facial syndrome): relationship with executive functioning and social competence/functioning. J Intellect Disabil Res 59:845–859. doi:10.1111/jir.12183
Canu E, Agosta F, Filippi M (2015) A selective review of structural connectivity abnormalities of schizophrenic patients at different stages of the disease. Schizophr Res 161:19–28. doi:10.1016/j.schres.2014.05.020
Chung Y, Cannon TD (2015) Brain imaging during the transition from psychosis prodrome to schizophrenia. J Nerv Ment Dis 203:336–341. doi:10.1097/NMD.0000000000000286
Crossley NA, Mechelli A, Scott J, Carletti F, Fox PT, McGuire P, Bullmore ET (2014) The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain 137:2382–2395. doi:10.1093/brain/awu132
da Silva Alves F et al (2011) White matter abnormalities in adults with 22q11 deletion syndrome with and without schizophrenia. Schizophr Res 132:75–83. doi:10.1016/j.schres.2011.07.017
Debbane M, Van der Linden M, Glaser B, Eliez S (2008) Source monitoring for actions in adolescents with 22q11.2 deletion syndrome (22q11DS). Psychol Med 38:811–820. doi:10.1017/S003329170700222X
Debbane M, Van der Linden M, Glaser B, Eliez S (2010) Monitoring of self-generated speech in adolescents with 22q11.2 deletion syndrome. Br J Clin Psychol 49:373–386. doi:10.1348/014466509X468223
Debbane M, Lazouret M, Lagioia A, Schneider M, Van De Ville D, Eliez S (2012) Resting-state networks in adolescents with 22q11.2 deletion syndrome: associations with prodromal symptoms and executive functions. Schizophr Res 139:33–39. doi:10.1016/j.schres.2012.05.021
Deng Y et al (2015) Disrupted fornix integrity in children with chromosome 22q11.2 deletion syndrome. Psychiatry Res 232:106–114. doi:10.1016/j.pscychresns.2015.02.002
Dufour F, Schaer M, Debbané M, Farhoumand R, Glaser B, Eliez S (2008) Cingulate gyral reductions are related to low executive functioning and psychotic symptoms in 22q11.2 deletion syndrome. Neuropsychologia 46:2986–2992. doi:10.1016/j.neuropsychologia.2008.06.012
Ellison-Wright I, Bullmore E (2010) Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophrenia Research 117:1–12. doi:10.1016/j.schres.2009.12.022
Focke NK et al (2011) Multi-site voxel-based morphometry—not quite there yet. NeuroImage 56:1164–1170. doi:10.1016/j.neuroimage.2011.02.029
Fornito A, Zalesky A, Pantelis C, Bullmore ET (2012) Schizophrenia, neuroimaging and connectomics. NeuroImage 62:2296–2314. doi:10.1016/j.neuroimage.2011.12.090
Frith CD (1992) The cognitive neuropsychology of schizophrenia. L Erlbaum, Hillsdale
Gothelf D, Penniman L, Gu E, Eliez S, Reiss AL (2007) Developmental trajectories of brain structure in adolescents with 22q11.2 deletion syndrome: a longitudinal study. Schizophr Res 96:72–81. doi:10.1016/j.schres.2007.07.021
Gothelf D, Schaer M, Eliez S (2008) Genes, brain development and psychiatric phenotypes in velo-cardio-facial syndrome. Dev Disabil Res Rev 14:59–68. doi:10.1002/ddrr.9
Gothelf D, Hoeft F, Ueno T, Sugiura L, Lee AD, Thompson P, Reiss AL (2011) Developmental changes in multivariate neuroanatomical patterns that predict risk for psychosis in 22q11.2 deletion syndrome. J Psychiatr Res 45:322–331. doi:10.1016/j.jpsychires.2010.07.008
Greicius M (2008) Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol 21:424–430
Griffa A, Baumann PS, Ferrari C, Do KQ, Conus P, Thiran J-P, Hagmann P (2015) Characterizing the connectome in schizophrenia with diffusion spectrum imaging. Hum Brain Mapp 36:354–366. doi:10.1002/hbm.22633
Guo T et al (2011) Genotype and cardiovascular phenotype correlations with TBX1 in 1,022 velo-cardio- facial/DiGeorge/22q11.2 deletion syndrome patients. Hum Mutat 32:1278–1289. doi:10.1002/humu.21568
Hagmann P (2005) From diffusion MRI to brain connectomics Doctoral thesis, École Polytechnique Fédérale (EPFL). Lausanne, Switzerland
Hagmann P, Thiran JP, Jonasson L, Vandergheynst P, Clarke S, Maeder P, Meuli R (2003) DTI mapping of human brain connectivity: statistical fibre tracking and virtual dissection. NeuroImage 19:545–554. doi:10.1016/S1053-8119(03)00142-3
Herman SB et al (2012) Overt cleft palate phenotype and TBX1 genotype correlations in Velo-cardio-facial/DiGeorge/22q11.2 deletion syndrome patients. Am J Med Genet Part A 158A:2781–2787. doi:10.1002/ajmg.a.35512
Hubl D, Koenig T, Strik W et al (2004) Pathways that make voices: white matter changes in auditory hallucinations. Arch Gener Psychiatry 61:658–668. doi:10.1001/archpsyc.61.7.658
Jalbrzikowski M, Jonas R, Senturk D, Patel A, Chow C, Green MF, Bearden CE (2013) Structural abnormalities in cortical volume, thickness, and surface area in 22q11.2 microdeletion syndrome: relationship with psychotic symptoms. NeuroImage Clin 3:405–415. doi:10.1016/j.nicl.2013.09.013
Jalbrzikowski M, Villalon-Reina JE, Karlsgodt KH, Senturk D, Chow C, Thompson PM, Bearden CE (2014) Altered white matter microstructure is associated with social cognition and psychotic symptoms in 22q11.2 microdeletion syndrome. Front Behav Neurosci. doi:10.3389/fnbeh.2014.00393
Jansen PW, Duijff SN, Beemer FA, Vorstman JAS, Klaassen PWJ, Morcus MEJ, Heineman-de Boer JA (2007) Behavioral problems in relation to intelligence in children with 22q11.2 deletion syndrome: a matched control study. Am J Med Genet Part A 143A:574–580. doi:10.1002/ajmg.a.31623
Karbasforoushan H, Woodward ND (2012) Resting-State Networks in Schizophrenia. Top Med Chem 12:2404–2414
Kates WR et al (2011) Neuroanatomic predictors to prodromal psychosis in velocardiofacial syndrome (22q11.2 deletion syndrome): a longitudinal study. Biol Psychiatry 69:945–952. doi:10.1016/j.biopsych.2010.10.027
Kates WR et al (2015) White matter microstructural abnormalities of the cingulum bundle in youths with 22q11.2 deletion syndrome: associations with medication, neuropsychological function, and prodromal symptoms of psychosis. Schizophr Res 161:76–84. doi:10.1016/j.schres.2014.07.010
Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276
Keshavan MS, Tandon R, Boutros NN, Nasrallah HA (2008) Schizophrenia, “just the facts”: what we know in 2008: Part 3: Neurobiology. Schizophr Res 106:89–107. doi:10.1016/j.schres.2008.07.020
Kikinis Z et al (2012) Reduced fractional anisotropy and axial diffusivity in white matter in 22q11.2 deletion syndrome: a pilot study. Schizophr Res 141:35–39. doi:10.1016/j.schres.2012.06.032
Kikinis Z et al (2013) Genetic contributions to changes of fiber tracts of ventral visual stream in 22q11.2 deletion syndrome. Brain Imaging Behav 7:316–325. doi:10.1007/s11682-013-9232-5
Kiley-Brabeck K, Sobin C (2006) Social skills and executive function deficits in children with the 22q11 Deletion Syndrome. Appl Neuropsychol 13:258–268. doi:10.1207/s15324826an1304_7
Latora V, Marchiori M (2001) Efficient behavior of small-world networks. Phys Rev Lett 87:198701
Machado AMC, Simon TJ, Nguyen V, McDonald-McGinn DM, Zackai EH, Gee JC (2007) Corpus callosum morphology and ventricular size in chromosome 22q11.2 deletion syndrome. Brain Res 1131:197–210. doi:10.1016/j.brainres.2006.10.082
Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J (1995) A probabilistic atlas of the human brain: theory and rationale for its development: the international consortium for brain mapping (ICBM). NeuroImage 2:89–101. doi:10.1006/nimg.1995.1012
Menon V (2011) Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci 15:483–506. doi:10.1016/j.tics.2011.08.003
Menon V, Uddin LQ (2010) Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214:655–667. doi:10.1007/s00429-010-0262-0
Meskaldji DE et al (2011) Adaptive strategy for the statistical analysis of connectomes. PLoS One 6:e23009. doi:10.1371/journal.pone.0023009
Mukherjee P, McKinstry RC (2006) Diffusion tensor imaging and tractography of human brain development. Neuroimag Clin N Am 16:19–43. doi:10.1016/j.nic.2005.11.004
Murphy K, Jones L, Owen M (1999) High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry 56:940–945
Murray RJ, Schaer M, Debbané M (2012) Degrees of separation: a quantitative neuroimaging meta-analysis investigating self-specificity and shared neural activation between self- and other-reflection. Neurosci Biobehav Rev 36:1043–1059. doi:10.1016/j.neubiorev.2011.12.013
Nekovarova T, Fajnerova I, Horacek J, Spaniel F (2014) Bridging disparate symptoms of schizophrenia: a triple network dysfunction theory Front. Behav Neurosci 8:171. doi:10.3389/fnbeh.2014.00171
Niklasson L, Gillberg C (2010) The neuropsychology of 22q11 deletion syndrome. A neuropsychiatric study of 100 individuals. Res Dev Disabil 31:185–194. doi:10.1016/j.ridd.2009.09.001
Oskarsdottir S (2004) Incidence and prevalence of the 22q11 deletion syndrome: a population-based study in Western Sweden. Arch Dis Childhood 89:148–151. doi:10.1136/adc.2003.026880
Oskarsdottir S, Persson C, Eriksson BO, Fasth A (2005) Presenting phenotype in 100 children with the 22q11 deletion syndrome. Eur J Pediatr 164:146–153. doi:10.1007/s00431-004-1577-8
Ottet M-C (2013) Analyzing quantitatively and topologically the white matter organization in 22q11.2DS. Doctoral thesis University of Geneva, Geneva, Switzerland
Ottet MC, Schaer M, Cammoun L, Schneider M, Debbane M, Thiran JP, Eliez S (2013a) Reduced fronto-temporal and limbic connectivity in the 22q11.2 deletion syndrome: vulnerability markers for developing schizophrenia? PLoS One 8:e58429. doi:10.1371/journal.pone.0058429
Ottet MC, Schaer M, Debbane M, Cammoun L, Thiran JP, Eliez S (2013b) Graph theory reveals dysconnected hubs in 22q11DS and altered nodal efficiency in patients with hallucinations. Front Hum Neurosci 7:402. doi:10.3389/fnhum.2013.00402
Padula MC, Schaer M, Scariati E, Schneider M, Van De Ville D, Debbané M, Eliez S (2015) Structural and functional connectivity in the default mode network in 22q11.2 deletion syndrome. J Neurodev Dis. doi:10.1186/s11689-015-9120-y
Perlstein MD et al (2014) White matter abnormalities in 22q11.2 deletion syndrome: preliminary associations with the Nogo-66 receptor gene and symptoms of psychosis. Schizophr Res 152:117–123. doi:10.1016/j.schres.2013.11.015
Raichle ME (2001) Cognitive neuroscience: bold insights. Nature 412:128–130
Raichle ME (2009) A paradigm shift in functional brain imaging. J Neurosci 29:12729–12734. doi:10.1523/JNEUROSCI.4366-09.2009
Rapoport JL, Addington AM, Frangou S (2005) Psych MRC (2005) The neurodevelopmental model of schizophrenia: update. Mol Psychiatry 10:434–449
Rosazza C, Minati L (2011) Resting-state brain networks: literature review and clinical applications. Neurol Sci 32:773–785. doi:10.1007/s10072-011-0636-y
Samartzis L, Dima D, Fusar-Poli P, Kyriakopoulos M (2014) White matter alterations in early stages of schizophrenia: a systematic review of diffusion tensor imaging studies. J Neuroimaging 24:101–110. doi:10.1111/j.1552-6569.2012.00779.x
Scariati E, Schaer M, Richiardi J, Schneider M, Debbane M, Van De Ville D, Eliez S (2014) Identifying 22q11.2 deletion syndrome and psychosis using resting-state connectivity patterns. Brain Topogr 27:808–821. doi:10.1007/s10548-014-0356-8
Schaer M, Debbane M, Bach Cuadra M, Ottet MC, Glaser B, Thiran JP, Eliez S (2009) Deviant trajectories of cortical maturation in 22q11.2 deletion syndrome (22q11DS): a cross-sectional and longitudinal study. Schizophr Res 115:182–190. doi:10.1016/j.schres.2009.09.016
Schneider M, Debbane M, Lagioia A, Salomon R, d’Argembeau A, Eliez S (2012) Comparing the neural bases of self-referential processing in typically developing and 22q11.2 adolescents. Dev Cogn Neurosci 2:277–289. doi:10.1016/j.dcn.2011.12.004
Schneider M et al (2014a) Psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome: results from the international consortium on brain and behavior in 22q11.2 deletion syndrome. Am J Psychiatry 171:627–639. doi:10.1176/appi.ajp.2013.13070864
Schneider M, Schaer M, Mutlu AK, Menghetti S, Glaser B, Debbane M, Eliez S (2014b) Clinical and cognitive risk factors for psychotic symptoms in 22q11.2 deletion syndrome: a transversal and longitudinal approach. Eur Child Adolesc Psychiatry 23:425–436. doi:10.1007/s00787-013-0469-8
Schneider M, Van der Linden M, Menghetti S, Glaser B, Debbane M, Eliez S (2014c) Predominant negative symptoms in 22q11.2 deletion syndrome and their associations with cognitive functioning and functional outcome. J Psychiatr Res 48:86–93. doi:10.1016/j.jpsychires.2013.10.010
Schreiner MJ, Karlsgodt KH, Uddin LQ, Chow C, Congdon E, Jalbrzikowski M, Bearden CE (2014) Default mode network connectivity and reciprocal social behavior in 22q11.2 deletion syndrome. Soc Cogn Affect Neurosci 9:1261–1267. doi:10.1093/scan/nst114
Shashi V, Muddasani S, Santos CC, Berry MN, Kwapil TR, Lewandowski E, Keshavan MS (2004) Abnormalities of the corpus callosum in nonpsychotic children with chromosome 22q11 deletion syndrome. Neuroimage 21:1399–1406. doi:10.1016/j.neuroimage.2003.12.004
Shashi V et al (2012) Increased corpus callosum volume in children with chromosome 22q11.2 deletion syndrome is associated with neurocognitive deficits and genetic polymorphisms. Eur J Hum Genet 20:1051–1057. doi:10.1038/ejhg.2012.138
Simon TJ, Ding L, Bish JP, McDonald-McGinn DM, Zackai EH, Gee J (2005) Volumetric, connective, and morphologic changes in the brains of children with chromosome 22q11.2 deletion syndrome: an integrative study. Neuroimage 25:169–180. doi:10.1016/j.neuroimage.2004.11.018
Simon TJ, Wu Z, Avants B, Zhang H, Gee JC, Stebbins GT (2008) Atypical cortical connectivity and visuospatial cognitive impairments are related in children with chromosome 22q11.2 deletion syndrome. Behav Brain Funct 4:25. doi:10.1186/1744-9081-4-25
Song S-K, Sun S-W, Ju W-K, Lin S-J, Cross AH, Neufeld AH (2003) Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. NeuroImage 20:1714–1722. doi:10.1016/S1053-8119(03)00440-3
Song S-K, Yoshino J, Le TQ, Lin S-J, Sun S-W, Cross AH, Armstrong RC (2005) Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage 26:132–140. doi:10.1016/j.neuroimage.2005.01.028
Sporns O (2006) Small-world connectivity, motif composition, and complexity of fractal neuronal connections. Biosystems 85:55–64. doi:10.1016/j.biosystems.2006.02.008
Sporns O, Tononi G, Kötter R (2005) The human connectome: a structural description of the human brain. PLoS Comput Biol 1:e42. doi:10.1371/journal.pcbi.0010042
Sporns O, Honey CJ, Kötter R (2007) Identification and classification of hubs in brain networks. PLoS One 2:e1049. doi:10.1371/journal.pone.0001049
Sundram F et al (2010) White matter microstructure in 22q11 deletion syndrome: a pilot diffusion tensor imaging and voxel-based morphometry study of children and adolescents. J Neurodev Disord 2:77–92. doi:10.1007/s11689-010-9043-6
Swillen A, Devriendt K, Legius E, Eyskens B, Dumoulin M, Gewillig M, Fryns J (1997) Intelligence and psychosocial adjustment in velocardiofacial syndrome: a study of children and adolescents with VCFS. J Med Genet 34:453–458
Takao H, Hayashi N, Kabasawa H, Ohtomo K (2012) Effect of scanner in longitudinal diffusion tensor imaging studies. Hum Brain Mapp 33:466–477. doi:10.1002/hbm.21225
Tan GM, Arnone D, McIntosh AM, Ebmeier KP (2009) Meta-analysis of magnetic resonance imaging studies in chromosome 22q11.2 deletion syndrome (velocardiofacial syndrome). Schizophr Res 115:173–181. doi:10.1016/j.schres.2009.09.010
Tomescu MI et al (2014) Deviant dynamics of EEG resting state pattern in 22q11.2 deletion syndrome adolescents: a vulnerability marker of schizophrenia? Schizophr Res 157:175–181. doi:10.1016/j.schres.2014.05.036
Tomescu MI et al (2015) Schizophrenia patients and 22q11.2 deletion syndrome adolescents at risk express the same deviant patterns of resting state EEG microstates: a candidate endophenotype of schizophrenia. Schizophrenia Res. doi:10.1016/j.scog.2015.04.005
van den Heuvel MP, Sporns O (2013) Network hubs in the human brain. Trends Cogn Sci 17:683–696. doi:10.1016/j.tics.2013.09.012
Villalon-Reina J, Jahanshad N, Beaton E, Toga AW, Thompson PM, Simon TJ (2013) White matter microstructural abnormalities in girls with chromosome 22q11.2 deletion syndrome, Fragile X or Turner syndrome as evidenced by diffusion tensor imaging. Neuroimage 81:441–454. doi:10.1016/j.neuroimage.2013.04.028
Vorstman JA et al (2015) Cognitive decline preceding the onset of psychosis in patients with 22q11.2 deletion syndrome. JAMA Psychiatry 72:377–385. doi:10.1001/jamapsychiatry.2014.2671
Watts DJ, Strogatz SH (1998) Collective dynamics of/`small-world/’ networks. Nature 393:440–442
Wernicke C (1906) Grundrisse der Psychiatrie Leipzig. Thieme, Germany
Woodin M, Wang P, Aleman D, McDonald-McGinn D, Zackai E, Moss E (2001) Neuropsychological profile of children and adolescents with the 22q11.2 microdeletion. Genet Med 3:34–39
Acknowledgements
This work is supported by the National Center of Competence in Research (NCCR) Synapsy-The Synaptic Bases of Mental Diseases (SNF) and by grants of the Swiss National Foundation to S. Eliez (324730_121996 and 324730_144260). Individual fellowships from the Swiss National Foundation of Science support Marie Schaer (#145760) and Elisa Scariati (#145250). We would like to thank Angeline Mihailov for the manuscript proof reading.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflict of interest to report.
Additional information
E. Scariati and M. C. Padula contributed equally to this work.
Appendices
Appendix
BOX 1: Summary of methods used to measure connectivity in 22q11DS:
Methods for structural connectivity analysis using diffusion tensor imaging (DTI):
-
Voxel-based measures Measures that describe the properties of water diffusion. In white matter, water molecules are constrained by the cell’s structures to diffuse preferentially along the axon axis. Such an environment is called anisotropic, as the probability of water diffusion is not equal in all directions (Basser et al. 1994).
-
Fractional anisotropy (FA) FA is a scalar value between 0 and 1 and reflects the degree of anisotropy in an environment (Armitage and Bastin 2000). It gives information about the microarchitecture of the white matter (Mukherjee and McKinstry 2006).
-
Axial diffusivity (AD) AD measures the amplitude of water diffusion along the main diffusion direction. Animal studies suggest that AD is a measure of axonal integrity (Budde et al. 2009; Song et al. 2003; Song et al. 2005).
-
Radial diffusivity (RD) RD measures the amplitude of water diffusion perpendicular to the main diffusion direction. Studies in mouse models suggest that increased RD reflects reduced myelination (Song et al. 2003; Song et al. 2005).
-
-
Tractography Tractography algorithms are used to reconstruct the anatomy of the white matter bundles. With this method, virtual fibres (also called streamlines) are initiated in each point of the image and are grown in both directions, point by point, following the main diffusion direction. Thus, the pathway of the major white matter tracts can be reconstructed and the presence of a white matter bundle between two brain regions can be measured by the number of streamlines that connect them (Bammer et al. 2003; Hagmann et al. 2003).
Methods for functional connectivity analysis using resting-state fMRI:
Resting-state fMRI records spontaneous brain activity that takes place during rest, providing information about the intrinsic structure of the functional brain network through the measure of synchrony among brain regions (Biswal et al. 1995; Greicius 2008). Two methods have principally been used in studies about 22q11DS:
-
Independent Component Analysis (ICA) ICA is a data-driven approach retrieving a set of networks (components) that include regions with a coherent temporal activity (Calhoun et al. 2009; Raichle 2009). These networks are highly consistent across populations and sustain different brain functions, example of such resting state networks include the saliency, auditory, central executive or default mode networks (Rosazza and Minati 2011).
-
Correlation analysis Correlation analyses are based on the computation of the Pearson’s correlation coefficient between time series of predefined regions of interest (ROIs). It can be computed from one ROI to all the points on the brain surface resulting in a complete brain map representing the connectivity of a particular region. Alternatively, the correlation can be computed for a set of ROIs that can eventually cover the whole brain.
Glossary
- Connectome :
-
Complete map of brain connections where the brain network is represented as a set of nodes that correspond to brain regions (Hagmann 2005; Sporns et al. 2005). These nodes are connected by edges that can be either structural connectivity (for example tractography measures between a pair of nodes) or functional connectivity measurements (for example correlation analysis between the nodes’ time series)
- Degree :
-
of a node is the number of edges connecting that node
- Hubs :
-
Highly connected nodes providing necessary shortcuts between distant network nodes (Sporns et al. 2007)
- Local clustering coefficient :
-
Number of connections between the neighbours of a node (Watts and Strogatz 1998)
- Path length :
-
Shortest path connecting pair of regions in the network (Sporns 2006)
- Global efficiency :
-
Inverse of the path length (Latora and Marchiori 2001)
Rights and permissions
About this article
Cite this article
Scariati, E., Padula, M.C., Schaer, M. et al. Long-range dysconnectivity in frontal and midline structures is associated to psychosis in 22q11.2 deletion syndrome. J Neural Transm 123, 823–839 (2016). https://doi.org/10.1007/s00702-016-1548-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-016-1548-z