Abstract
Alzheimer’s disease is a major worldwide health problem with no effective therapy. Deep brain stimulation (DBS) has emerged as a useful therapy for certain movement disorders and is increasingly being investigated for treatment of other neural circuit disorders. Here we review the rationale for investigating DBS as a therapy for Alzheimer’s disease. Phase I clinical trials of DBS targeting memory circuits in Alzheimer’s disease patients have shown promising results in clinical assessments of cognitive function, neurophysiological tests of cortical glucose metabolism, and neuroanatomical volumetric measurements showing reduced rates of atrophy. These findings have been supported by animal studies, where electrical stimulation of multiple nodes within the memory circuit have shown neuroplasticity through stimulation-enhanced hippocampal neurogenesis and improved performance in memory tasks. The precise mechanisms by which DBS may enhance memory and cognitive functions in Alzheimer’s disease patients and the degree of its clinical efficacy continue to be examined in ongoing clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction: scope of the problem
Alzheimer’s disease (AD) is a neurodegenerative disorder and the major cause of dementia. There are no effective therapies. It is characterized pathophysiologically by hyperphosphorylated tau protein that generates neurofibrillary tangles, deposition of fibrillar forms of amyloid beta protein, neuronal and synaptic loss, and defects in neurotransmission (Zibly et al. 2014; Watt et al. 2013; Buckner et al. 2005; Querfurth and LaFerla 2010; Palop and Mucke 2010). Pathological studies have revealed that these processes result in atrophy in widespread brain regions, including frontal, temporal, and parietal areas, but with a predilection for neural circuits serving memory, including the circuit of Papez (Sperling et al. 2010). Both morphological and volumetric structural changes, particularly in the entorhinal cortex and hippocampus, have been shown by neuroimaging to predate AD symptoms and track with disease severity (Petersen and Jack 2009). Furthermore, these structural alterations are coupled with functional disturbances, as seen with regional reduction in glucose metabolism in the temporal lobe and posterior cingulum on positron emission tomography (PET) and single photon emission computed tomography in patients early in the disease (Smith et al. 1992; Minoshima et al. 1997; Reiman et al. 1996).
Clinically, these neural substrate alterations produce a progressive disturbance in cognitive functions, particularly those involving memory. There are three categories in AD diagnosis—possible, probable, and pathophysiologically proven AD (McKhann et al. 2011)—with the core clinical criteria of probable AD as follows: (1) declining function in various cognitive domains, such as memory, reasoning, judgement, executive function, not attributable to delirium or other major psychiatric disorder; (2) gradual onset; (3) cognitive worsening over time; (4) initial and most prominent cognitive deficits are amnestic (most common) or non-amnestic (language, visuospatial or executive dysfunction), and (5) no concomitant disease with substantial effect on cognition. Possible AD applies to cases with an atypical course or mixed etiology (McKhann et al. 2011). Pathophysiologically proved AD is restricted to cases that satisfy criteria for probable AD and have characteristic pathologic findings of widespread neocortical neuritic plaques and neurofibrillary tangles (McKhann et al. 2011). Alternatively, possible and probable AD may be supplemented with the descriptor “with evidence of AD pathophysiologic process.” This refers to two categories of AD biomarkers: (1) amyloid beta protein deposition, marked by low cerebrospinal fluid Ab42 and positive PET amyloid imaging, and (2) downstream neuronal degeneration or injury, marked by elevated cerebrospinal fluid tau, decreased fluorodeoxyglucose (FDG) uptake in temporal–parietal cortex on PET, and disproportionate atrophy on MRI in medial, basal and lateral temporal lobe and medial parietal cortex (McKhann et al. 2011; Blennow 2004).
Despite this progress in understanding its pathogenesis and diagnosis, however, there are no current therapies to prevent, arrest, or cure AD (Ihl et al. 2015). Management options are limited to medications to potentiate cholinergic pathways (acetyl-cholinesterase inhibitors: donepezil, galantamine, rivastigmine) and modulate glutamatergic pathways (N-methyl d-aspartate receptor blockers: memantine) (Qaseem et al. 2008). At the same time, the number of people affected by AD continues to grow, from 26.6 million in 2006 to 36 million in 2014, with a projection to quadruple in number by 2050 (Brookmeyer et al. 2007). Novel therapeutic strategies are therefore urgently needed for patients with AD (Qaseem et al. 2008; Ihl et al. 2015).
Electrical neural stimulation for Alzheimer’s disease
Alzheimer’s disease, in addition to being a neurodegenerative disorder, can be considered a neural circuit disorder since it affects several integrated pathways linking specific cortical and subcortical sites, especially those serving aspects of memory and cognition. Consequently, there has been growing interest in modulating the activity of these dysfunctional circuits in order to maximize their function (Laxton et al. 2010, 2014; Laxton and Lozano 2013; Sharma et al. 2015).
Among the earliest targets for electrical stimulation in AD was the nucleus basalis of Meynert (NBM). AD results in severe atrophy of the basal forebrain cholinergic system, particularly within the NBM, leading to a reduction of cholinergic innervation of the neocortex and hippocampus (Davies and Maloney 1976; Grothe et al. 2012; Pearson et al. 1983; Whitehouse et al. 1982). This cholinergic denervation is considered integral to the pathophysiological cascade of cognitive decline in AD (Schliebs and Arendt 2011). In 1984, attempting to augment the remaining cholinergic tone to the cortex and increase associated cortical metabolic activity, Turnbull et al. (1985) implanted DBS into the left NBM of a 74-year-old man with probable AD. Targeting was planned according to a cadaveric study prior to the procedure with final coordinates being 8 mm posterior to the anterior commissure, 11 mm lateral to midline, and 5 mm below the intercommissural line. At the procedure, the authors confirmed the diagnosis by biopsy, which revealed senile plaques, neurofibrillary tangles, and amyloid angiopathy. Postoperative CT demonstrated the lead at the target and stimulation parameters were 3 V, 50 Hz, 210 µs, cycling between 15 s on and 12 min off. After 8 months of stimulation, the authors concluded no clinical response, though they did not use detailed neuropsychological assessments with standardized measures. Perhaps more importantly, however, the authors did show an effect on cortical glucose metabolism. Comparing FDG-PET studies 4 months prior to the operation vs. 2 months after stimulation (6 months interval), the unstimulated right hemisphere showed reduced glucose use of 21, 24, 10, and 7.5 % in the frontal, temporal, parietal, and occipital lobes, respectively, while the stimulated left hemisphere showed reduction of only 12 % in frontal and 4 % in the occipital lobes, no change in the parietal lobe, and an increase of 1.5 % in the temporal lobe. In other words, despite concluding no clinical response, they demonstrated a possibly pathophysiologically relevant biological effect.
Subsequently, the next case study of NBM DBS for AD was not until 2009, when Freund et al. (2009) simultaneously implanted bilateral NBM and STN electrodes for a patient with Parkinson’s disease dementia, with the goal of addressing both the motor and cognitive aspects of his condition. Target coordinates for NBM were slightly different: 12.5 mm lateral to the third ventricle wall, 4 mm posterior to the anterior commissure, and 5 mm below the intercommissural line (Fig. 1a). This placed the ventral two electrode contacts within NBM: stimulation configuration was set with both of these contacts as cathodes and the case as anode, with parameters of 1 V, 20 Hz, and 120 µs. STN DBS was initiated first and resulted in improved motor function, but cognitive impairment was unchanged. After NBM stimulation was initiated, the patient experienced almost immediate cognitive improvement as demonstrated in multiple tasks, including the Rey Auditory Verbal Learning test, the clock drawing task and the trail making task-part A. This was maintained in testing sessions over the next 2 months while stimulation was on. The necessity of NBM stimulation for this improvement was demonstrated when the authors subsequently turned off the stimulator for 1 week and then turned it back on without informing the patient. With NBM DBS off, the patient returned to his baseline poor cognitive state; with the NBM DBS on, the patient regained his cognitive improvement as before. These promising results led these authors to initiate a phase I trial of NBM DBS in six patients with mild to moderate AD, which has recently been completed and published (Kuhn et al. 2015).
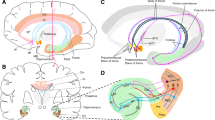
Nucleus basalis of Meynert DBS for Alzheimer’s disease. a Axial and coronal magnetic resonance images show DBS lead position in the lateral and posterior portion of NBM in patients four, five, and six from the phase I study of NBM DBS for AD (Kuhn et al. 2015). Yellow-optic tract; peach-anterior commissure; other colors-subdivisions of NBM. b Cerebral glucose utilization (normalized to cerebellum) as measured by FDG-PET in patients four, five, and six relative to an age-matched control collective (Higgins and Mufson 1989). Note the parieto-temporal hypometabolism on pre-operative scans, typical for AD. Glucose consumption improved for these patients after 1 year of DBS. Heat gradient indicates glucose utilization as a z value relative to control. Figure adapted with permission from Kuhn et al. (2015)
Briefly, this was a phase I study of bilateral low-frequency DBS of the NBM, with a 4-week double-blind sham-controlled phase (three patients with 2 weeks ON followed by 2 weeks OFF, three patients with 2 weeks OFF followed by 2 weeks ON), then an 11 months follow-up open label period (Kuhn et al. 2015). The primary outcome measure was change in Alzheimer’s disease assessment scale–cognitive subscale (ADAS-Cog) (Rosen et al. 1984), which worsened by an average of three points over 1 year, representing a non-significant change. Four of six patients were considered responders based on a stable or improved ADAS-Cog score at 1 year. Mini-mental status examination (MMSE) was a secondary outcome measure and this also showed no change on average over 1 year. These results point to a rather slow disease progression during the year of stimulation. Additionally, FDG-PET studies showed a global increase of 2–5 % in cortical glucose metabolism, most pronounced in the amygdalo-hippocampal and temporal regions, in three of four patients examined by PET under DBS (Fig. 1b). This is in contrast to the untreated course of AD, characterized by an average decrease of FDG uptake of 5.2 % per year (Lo et al. 2011).
NBM DBS for AD is also supported by mechanistic studies in animals. In awake behaving rats, the nucleus basalis system has been shown to play a key role in neocortical arousal by both directly activating the neocortex and suppressing rhythm generation in the reticular nucleus–thalamocortical circuit; deprivation of cholinergic input in these rats compromised information transmission to the cortex (Buzsaki et al. 1988). Furthermore, stimulation of the nucleus basalis in rats has been shown to drive massive reorganization of auditory receptive fields when paired with auditory stimuli, demonstrating the importance of the cholinergic forebrain in cortical learning and plasticity (Kilgard and Merzenich 1998).
A serendipitous finding and new DBS target for AD
In 2002, as part of a trial assessing the hypothalamus as a DBS target for obesity, a 50-year-old man with life-long, treatment-resistant obesity and normal cognitive function underwent bilateral hypothalamic DBS (Hamani et al. 2008). During the procedure, stimulation of electrode contacts on either side (3–5 V, 130 Hz, 60 µs) evoked vivid deja-vu experiences that the patient relayed as detailed autobiographical memories. He reported the sudden perception of being in a park with friends: he was around 20 years old and able to recognize his epoch-appropriate girlfriend. As stimulation intensity was increased from 3 to 5 V, the patient reported that the details in the scene became more vivid. These same perceptions were obtained during multiple sequential on–off trials conducted in a blinded manner, with all perceptions time-locked with stimulation and specific to the electrode contact and threshold stimulation parameters. A neuropsychological assessment battery conducted pre- and post-operatively as part of the trial further validated the memory-enhancing effects of stimulation. After 3 weeks of continuous hypothalamic DBS, there were significant improvements on the California Verbal Learning Test (above the 95 % confidence interval for reliable change), which tests word list memory, and the Spatial Associative Learning test (postoperative score improved >1.5 standard deviations), another test of declarative memory. To localize the stimulation site responsible for these changes, the Medtronic FrameLink system (Medtronic, Minneapolis, MN, USA) was loaded with postoperative images to calculate the stereotactic coordinates of the active DBS contacts. These coordinates were then plotted on the Schaltenbrand–Wahren brain atlas (Schaltenbrand and Wahren 1977) and estimated to be adjacent to the columns of the fornix (Fig. 2a). The fornix is a major fiber bundle within the memory circuit of Papez that interconnects the subiculum and hippocampus to the mammillary nuclei and septal area, and is thereby a fundamental structure in the neuroanatomy of declarative memory (Tsivilis et al. 2008). Fornix lesions produce severe memory impairments (Browning et al. 2010; Wilson et al. 2008). Notably, the progression to AD has been tightly associated with axonal degeneration and dysfunction in the fornix (Mielke et al. 2012). Based on these observations, a hypothesis was put forth: fornix DBS could be applied in patients with dementia in order to augment activity within the circuit of Papez and restore memory and cognitive function. This hypothesis was tested in a phase I clinical trial of fornix DBS in patients with mild AD (Laxton et al. 2010).
Fornix DBS for Alzheimer’s disease. a Sagittal magnetic resonance image shows DBS lead positioned anterior and parallel to the columns of the fornix within the hypothalamus. The proximity to the fornix can be estimated with the lead projected onto a stereotactic atlas 3.5 mm from the midline. b An averaged standardized low-resolution electromagnetic tomography (sLORETA) three-dimensional reconstruction during fornix/hypothalamic stimulation shows activation of ipsilateral hippocampal structures with latency of ~50 ms after stimulation in six patients. c Positron emission tomography scans of glucose metabolism with voxel-wise (SPM5) results displayed on a three-dimensional magnetic resonance rendering of a representative subject showing comparison of AD patient vs. control (top), 1 month of DBS vs. baseline (middle), and 1 year of DBS vs. baseline. Blue represents areas of decreased and red areas of increased glucose metabolism, scaled to mg/100 g tissue/min. Figure adapted with permission from Laxton et al. (2010)
The phase I trial of fornix DBS enrolled six patients with mild to moderate AD. These were considered the most suitable candidates for DBS due to relative preservation of structural integrity within this memory circuit early in the disease. Leads were placed 2 mm anterior to the fornix with a trajectory parallel to the columns of the fornix, with the most ventral contacts 2 mm above the dorsal surface of the optic tract, ~5 mm lateral to the midline (Fig. 2a) (Laxton et al. 2010). Based on the results from the original case study in a obese patient, ventral contacts were stimulated in monopolar configuration at 3–5 V, 130 Hz, and 90 µs. Functional imaging, including pre- and postoperative FDG-PET studies and postoperative standardized low-resolution electromagnetic tomography (sLORETA), was performed (Fig. 2b, c) (discussed in detail below). Extensive neuropsychological and clinical assessments were conducted before and at 1, 6, and 12 months after surgery.
The study tracked changes in ADAS-Cog and MMSE scores as primary outcome measures for the effects of fornix DBS on AD disease severity (Laxton et al. 2010). At 1 month after surgery, three patients showed slight worsening (increased ADAS-Cog score) and three patients showed mild improvement compared to 1 month prior to surgery, demonstrating that surgery was well tolerated. After 6 months of stimulation, four of six patients showed improvement in ADAS-Cog scores ranging from 1.3 to 4 point reductions. By 12 months, one patient continued to show improved ADAS-Cog score (4.4 point reduction) and the other five had slightly worsened scores (increase of 2 points in two patients, 5 points in one patient, and >5 points in two patients). Overall, this represented a mean increase across the six patients of 4.2 points in the ADAS-Cog over 12 months, which compares favorably with historical figures in AD patients suggesting a mean increase of 6–7 points per year, with a range of 3–10 points per year (Mayeux and Sano 1999; Ito et al. 2010). Changes in MMSE scores (Folstein et al. 1975) were also tracked from baseline to 12 months. Comparing the rate of decline in the 11 months preceding surgery to the 11 months after surgery, there was a decrease in the rate of decline from a mean of 2.8–0.8 points per year across the six patients. This favors comparably to the average expected rate of decline of three points per year in the AD population (Mayeux and Sano 1999; Ito et al. 2010). Still, although these results appear promising, they should be treated cautiously: the rate of change in both ADAS-Cog and MMSE scores in AD is variable and nonlinear, which together with the small number of patients in this study, precludes conclusions about efficacy. This study did, however, show that fornix DBS was well tolerated and safe in the AD population: no patient developed seizures, sleep disturbances, weight changes, hypothalamic dysfunction, or metabolic or endocrine alterations during 1 year of DBS. The results of the phase I trial prompted a double-blind, randomized controlled phase II trial of fornix DBS for AD, in which patients are randomly assigned to early vs. 6-month postoperative initiation of stimulation, to better address efficacy. This study (NCT01608061) is nearing completion.
Two other studies of fornix stimulation, conducted by independent groups, corroborate these findings. Fontaine et al. (2013) performed chronic bilateral fornix DBS in a single patient with early AD and demonstrated stabilization of several clinical measures of memory and cognition (ADAS-Cog, MMSE, Free and Cued Selective Reminding test) for up to 1 year. Koubeissi et al. (2013) performed low-frequency stimulation of the fornix using depth electrodes implanted in 11 patients with intractable epilepsy and found that MMSE scores improved over a 4-h period. However, neither study was controlled so the results should be interpreted cautiously.
DBS for AD proof of concept: neurophysiological changes
The significance of these early trials of DBS for AD, beyond promising clinical results, was the demonstration of long-lasting neurophysiological changes in pathologically relevant neural circuits. Standardized low-resolution electromagnetic tomography (sLORETA) is a functional imaging modality based on electroencephalographic source localization. It presents blurred images of statistically standardized current density distributions on a cortical grid of >6200 voxels with accurate localization (Pascual-Marqui 2002). This can be used to map brain areas affected by deep brain stimulation. In the original case study of DBS in the obese patient, sLORETA revealed that fornix stimulation resulted in localized changes in the activity of ipsilateral mesial temporal lobe structures, mainly the hippocampal formation and the parahippocampal gyrus (Hamani et al. 2008). These findings were corroborated and further dissected in the phase I study (Laxton et al. 2010). sLORETA showed temporally specific activation of downstream projection structures with fornix stimulation. The peak of the first significant evoked response after stimulation was localized to the ipsilateral hippocampus and parahippocampal gyrus, with a latency of 38–52 ms, followed by significant activation of the ipsilateral cingulum and precuneus area of the parietal lobe, at latencies of 102–256 ms (Fig. 2b). These results demonstrate that fornix stimulation produces direct and trans-synaptic sequential activation of downstream targets within the memory circuits affected in AD.
In addition, FDG-PET was used in the phase I fornix DBS study to measure quantitative and topographic changes in cerebral glucose metabolism, allowing visualization of activity changes in brain networks, pre- vs. postoperatively (Fig. 2c). Preoperative scans of the participants showed expected decreases in temporal–parietal glucose metabolism compared with healthy controls, consistent with characteristic findings in AD. However, at 1 month postoperatively (2 weeks after stimulation initiated), glucose metabolism in temporal–parietal regions was increased in comparison to preoperative scans. Increased metabolism was also seen in primary sensory and motor areas and the cerebellum, while decreased metabolism was seen in some frontal cortical areas, the left caudate, and the medial dorsal nuclei of the thalamus. Importantly, the increased metabolism in the temporal–parietal areas was maintained with chronic fornix DBS at 1 year postoperatively. Fornix DBS was therefore able to induce large and sustained neurophysiological changes in cognitive and limbic brain regions adversely affected in AD. These findings help to reshape our traditional concept of the DBS target—from a single anatomical structure to an access point to an integrated neuronal circuit.
DBS for AD proof of concept: neuroanatomical changes
Neurogenesis persists in the adult hippocampal dentate gyrus in both rodents (Aimone et al. 2014; Jessberger and Gage 2014) and humans (Eriksson et al. 1998). In rodent studies, DBS of several nodes of the memory circuit, including the anterior nucleus of the thalamus (Hamani et al. 2011; Encinas et al. 2011; Toda et al. 2008), the hippocampus (Bruel-Jungerman et al. 2006; Derrick et al. 2000), and the entorhinal cortex/performant path (Stone et al. 2011; Chun et al. 2006; Kitamura et al. 2010), stimulates increased neurogenesis in the dentate gyrus. Intriguingly, evidence suggests that stimulation-induced neurogenesis following DBS of either the entorhinal cortex (Stone et al. 2011) or anterior nucleus of the thalamus (Hamani et al. 2011) may have memory enhancing effects.
Based on these animal studies and the sustained increase in cortical glucose metabolism observed in AD patients with fornix DBS (Laxton et al. 2010), Sankar et al. (2015) hypothesized that fornix DBS might induce neuroanatomical changes within the memory circuits. The study, a follow-up analysis of the phase I fornix DBS trial, quantitatively analyzed serial structural MRI scans obtained 1 day and 1 year after fornix DBS in the six study patients. The volumes of the hippocampus, fornix and mammillary bodies were measured at two time points by manual segmentation. In addition, using deformation-based morphometry, the study looked in a hypothesis-free manner for brain-wide structural changes. Remarkably, in contrast to expected progressive atrophy of the hippocampus which characterizes AD, two of six patients showed increases in bilateral hippocampal volume after 1 year of fornix DBS (Sankar et al. 2015). Furthermore, these two patients also demonstrated the least atrophy in the fornix and mammillary bodies. Volume changes in fornix and mammillary bodies were highly correlated with hippocampal volume change across all study patients, suggesting a circuit-wide structural effect. The two patients with hippocampal enlargement also had the least deterioration in ADAS-Cog score, with a trend towards a significant correlation between percentage change in hippocampal volume and percentage change in ADAS-cog score across the study population. There was a significant correlation between percentage change in hippocampal volume and percentage change in hippocampal glucose metabolism, suggestive of a trophic mechanism for hippocampal enlargement. Additionally, deformation-based morphometry revealed several clusters of volume expansion throughout the brain at the group level with fornix DBS, including several areas known to atrophy in AD, such as the bilateral parahippocampal gyri, right superior temporal gyrus, left inferior parietal lobule, and bilateral precuneus (Sankar et al. 2015). Finally, the study showed that hippocampal volume loss over 1 year was significantly higher in a well-matched cohort of AD patients not receiving fornix DBS compared to the six study patients (−9.5 ± 1.2 vs. −2.6 ± 3.3 %; p = 0.027). These findings represent the first evidence of structural plasticity in the human brain with DBS and are further supported by the aforementioned animal studies. Moving forward, these results should be validated by independent groups and in larger cohorts of patients (e.g. patients from the phase II fornix DBS for AD trial now nearing completion).
Conclusions
Alzheimer’s disease is a major worldwide health problem with no effective therapy. It is associated with significant costs to the individual and society. A recent cost-effectiveness study found that the clinical and economic thresholds required for DBS to be considered cost-effective for AD are relatively low (Mirsaeedi-Farahani et al. 2015). Compared to standard treatment, DBS needs only a success rate of 3 % to overcome effects of potential surgical complications on quality of life. At a success rate of 20 %, DBS can be considered cost-effective for mild AD at the price of $200K per quality-adjusted life year (QALY), while at success rates of 74 %, that price drops to $50K/QALY. Above 80 % success rate, DBS is both clinically more effective and more cost-effective than standard treatment (Mirsaeedi-Farahani et al. 2015). Indeed, phase I trials of DBS for AD, targeting either the fornix (Laxton et al. 2010) or NBM (Kuhn et al. 2015), have shown that DBS is safe and well tolerated in AD patients, with promising early data for cognitive improvement. Functional imaging revealed that DBS can modulate neuronal activity within memory circuits and alter pathological cortical physiology (Figs. 1b, 2c). Remarkably, new evidence suggests the potential for long-term structural plasticity, including hippocampal enlargement, invoked by fornix DBS (Sankar et al. 2015). Consistent with these promising early trials, the clinicaltrials.gov registry shows five ongoing clinical trials of DBS for AD, including a phase II trial of fornix DBS nearing completion (NCT01608061). Further investigation of DBS for AD is clearly warranted and underway.
Abbreviations
- AD:
-
Alzheimer’s disease
- ADAS-Cog:
-
Alzheimer’s disease assessment scale-cognitive subscale
- DBS:
-
Deep brain stimulation
- FDG:
-
Fluorodeoxyglucose
- MMSE:
-
Mini-mental state examination
- NBM:
-
Nucleus basalis of Meynert
- PET:
-
Positron emission tomography
- QALY:
-
Quality-adjusted life year
- sLORETA:
-
Standardized low-resolution electromagnetic tomography
- STN:
-
Subthalamic nucleus
References
Aimone JB, Li Y, Lee SW, Clemenson GD, Deng W, Gage FH (2014) Regulation and function of adult neurogenesis: from genes to cognition. Physiol Rev 94(4):991–1026. doi:10.1152/physrev.00004.2014
Blennow K (2004) Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. NeuroRx J Am Soc Exp NeuroTher 1(2):213–225. doi:10.1602/neurorx.1.2.213
Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM (2007) Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc 3(3):186–191. doi:10.1016/j.jalz.2007.04.381
Browning PG, Gaffan D, Croxson PL, Baxter MG (2010) Severe scene learning impairment, but intact recognition memory, after cholinergic depletion of inferotemporal cortex followed by fornix transection. Cereb Cortex 20(2):282–293. doi:10.1093/cercor/bhp097
Bruel-Jungerman E, Davis S, Rampon C, Laroche S (2006) Long-term potentiation enhances neurogenesis in the adult dentate gyrus. J Neurosci 26(22):5888–5893. doi:10.1523/JNEUROSCI.0782-06.2006
Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA (2005) Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci 25(34):7709–7717. doi:10.1523/JNEUROSCI.2177-05.2005
Buzsaki G, Bickford RG, Ponomareff G, Thal LJ, Mandel R, Gage FH (1988) Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J Neurosci 8(11):4007–4026
Chun SK, Sun W, Park JJ, Jung MW (2006) Enhanced proliferation of progenitor cells following long-term potentiation induction in the rat dentate gyrus. Neurobiol Learn Mem 86(3):322–329. doi:10.1016/j.nlm.2006.05.005
Davies P, Maloney AJ (1976) Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet 2(8000):1403
Derrick BE, York AD, Martinez JL Jr (2000) Increased granule cell neurogenesis in the adult dentate gyrus following mossy fiber stimulation sufficient to induce long-term potentiation. Brain Res 857(1–2):300–307
Encinas JM, Hamani C, Lozano AM, Enikolopov G (2011) Neurogenic hippocampal targets of deep brain stimulation. J Comp Neurol 519(1):6–20. doi:10.1002/cne.22503
Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH (1998) Neurogenesis in the adult human hippocampus. Nat Med 4(11):1313–1317. doi:10.1038/3305
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12(3):189–198
Fontaine D, Deudon A, Lemaire JJ, Razzouk M, Viau P, Darcourt J, Robert P (2013) Symptomatic treatment of memory decline in Alzheimer’s disease by deep brain stimulation: a feasibility study. J Alzheimers Dis JAD 34(1):315–323. doi:10.3233/JAD-121579
Freund HJ, Kuhn J, Lenartz D, Mai JK, Schnell T, Klosterkoetter J, Sturm V (2009) Cognitive functions in a patient with Parkinson-dementia syndrome undergoing deep brain stimulation. Arch Neurol 66(6):781–785. doi:10.1001/archneurol.2009.102
Grothe M, Heinsen H, Teipel SJ (2012) Atrophy of the cholinergic Basal forebrain over the adult age range and in early stages of Alzheimer’s disease. Biol Psychiatry 71(9):805–813. doi:10.1016/j.biopsych.2011.06.019
Hamani C, McAndrews MP, Cohn M, Oh M, Zumsteg D, Shapiro CM, Wennberg RA, Lozano AM (2008) Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Ann Neurol 63(1):119–123. doi:10.1002/ana.21295
Hamani C, Stone SS, Garten A, Lozano AM, Winocur G (2011) Memory rescue and enhanced neurogenesis following electrical stimulation of the anterior thalamus in rats treated with corticosterone. Exp Neurol 232(1):100–104. doi:10.1016/j.expneurol.2011.08.023
Higgins GA, Mufson EJ (1989) NGF receptor gene expression is decreased in the nucleus basalis in Alzheimer’s disease. Exp Neurol 106(3):222–236
Ihl R, Bunevicius R, Frolich L, Winblad B, Schneider LS, Dubois B, Burns A, Thibaut F, Kasper S, Moller HJ, WFSBP Task Force on Mental Disorders in Primary Care, WFSBP Task Force on Dementia (2015) World Federation of Societies of Biological Psychiatry guidelines for the pharmacological treatment of dementias in primary care. Int J Psychiatry Clin Pract 19(1):2–7. doi:10.3109/13651501.2014.961931
Ito K, Ahadieh S, Corrigan B, French J, Fullerton T, Tensfeldt T, Alzheimer’s Disease Working Group (2010) Disease progression meta-analysis model in Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc 6(1):39–53. doi:10.1016/j.jalz.2009.05.665
Jessberger S, Gage FH (2014) Adult neurogenesis: bridging the gap between mice and humans. Trends Cell Biol 24(10):558–563. doi:10.1016/j.tcb.2014.07.003
Kilgard MP, Merzenich MM (1998) Cortical map reorganization enabled by nucleus basalis activity. Science 279(5357):1714–1718
Kitamura T, Saitoh Y, Murayama A, Sugiyama H, Inokuchi K (2010) LTP induction within a narrow critical period of immature stages enhances the survival of newly generated neurons in the adult rat dentate gyrus. Mol Brain 3:13. doi:10.1186/1756-6606-3-13
Koubeissi MZ, Kahriman E, Syed TU, Miller J, Durand DM (2013) Low-frequency electrical stimulation of a fiber tract in temporal lobe epilepsy. Ann Neurol 74(2):223–231. doi:10.1002/ana.23915
Kuhn J, Hardenacke K, Lenartz D, Gruendler T, Ullsperger M, Bartsch C, Mai JK, Zilles K, Bauer A, Matusch A, Schulz RJ, Noreik M, Buhrle CP, Maintz D, Woopen C, Haussermann P, Hellmich M, Klosterkotter J, Wiltfang J, Maarouf M, Freund HJ, Sturm V (2015) Deep brain stimulation of the nucleus basalis of Meynert in Alzheimer’s dementia. Mol Psychiatry 20(3):353–360. doi:10.1038/mp.2014.32
Laxton AW, Lozano AM (2013) Deep brain stimulation for the treatment of Alzheimer disease and dementias. World Neurosurg 80(3–4):S28.(e21–28). doi:10.1016/j.wneu.2012.06.028
Laxton AW, Tang-Wai DF, McAndrews MP, Zumsteg D, Wennberg R, Keren R, Wherrett J, Naglie G, Hamani C, Smith GS, Lozano AM (2010) A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease. Ann Neurol 68(4):521–534. doi:10.1002/ana.22089
Laxton AW, Stone S, Lozano AM (2014) The neurosurgical treatment of Alzheimer’s disease: a review. Stereotact Funct Neurosurg 92(5):269–281. doi:10.1159/000364914
Lo RY, Hubbard AE, Shaw LM, Trojanowski JQ, Petersen RC, Aisen PS, Weiner MW, Jagust WJ, Alzheimer’s Disease Neuroimaging I (2011) Longitudinal change of biomarkers in cognitive decline. Arch Neurol 68(10):1257–1266. doi:10.1001/archneurol.2011.123
Mayeux R, Sano M (1999) Treatment of Alzheimer’s disease. N Engl J Med 341(22):1670–1679. doi:10.1056/NEJM199911253412207
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc 7(3):263–269. doi:10.1016/j.jalz.2011.03.005
Mielke MM, Okonkwo OC, Oishi K, Mori S, Tighe S, Miller MI, Ceritoglu C, Brown T, Albert M, Lyketsos CG (2012) Fornix integrity and hippocampal volume predict memory decline and progression to Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc 8(2):105–113. doi:10.1016/j.jalz.2011.05.2416
Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE (1997) Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol 42(1):85–94. doi:10.1002/ana.410420114
Mirsaeedi-Farahani K, Halpern CH, Baltuch GH, Wolk DA, Stein SC (2015) Deep brain stimulation for Alzheimer disease: a decision and cost-effectiveness analysis. J Neurol 262(5):1191–1197. doi:10.1007/s00415-015-7688-5
Palop JJ, Mucke L (2010) Synaptic depression and aberrant excitatory network activity in Alzheimer’s disease: two faces of the same coin? NeuroMol Med 12(1):48–55. doi:10.1007/s12017-009-8097-7
Pascual-Marqui RD (2002) Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find Exp Clin Pharmacol 24 (Suppl D):5–12
Pearson RC, Sofroniew MV, Cuello AC, Powell TP, Eckenstein F, Esiri MM, Wilcock GK (1983) Persistence of cholinergic neurons in the basal nucleus in a brain with senile dementia of the Alzheimer’s type demonstrated by immunohistochemical staining for choline acetyltransferase. Brain Res 289(1–2):375–379
Petersen RC, Jack CR Jr (2009) Imaging and biomarkers in early Alzheimer’s disease and mild cognitive impairment. Clin Pharmacol Ther 86(4):438–441. doi:10.1038/clpt.2009.166
Qaseem A, Snow V, Cross JT Jr, Forciea MA, Hopkins R Jr, Shekelle P, Adelman A, Mehr D, Schellhase K, Campos-Outcalt D, Santaguida P, Owens DK, American College of Physicians/American Academy of Family Physicians Panel on D (2008) Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 148(5):370–378
Querfurth HW, LaFerla FM (2010) Alzheimer’s disease. N Engl J Med 362(4):329–344. doi:10.1056/NEJMra0909142
Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D (1996) Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med 334(12):752–758. doi:10.1056/NEJM199603213341202
Rosen WG, Mohs RC, Davis KL (1984) A new rating scale for Alzheimer’s disease. Am J Psychiatry 141(11):1356–1364
Sankar T, Chakravarty MM, Bescos A, Lara M, Obuchi T, Laxton AW, McAndrews MP, Tang-Wai DF, Workman CI, Smith GS, Lozano AM (2015) Deep brain stimulation influences brain structure in Alzheimer’s disease. Brain Stimul 8(3):645–654. doi:10.1016/j.brs.2014.11.020
Schaltenbrand G, Wahren W (1977) Atlas for stereotaxy of the human brain. Georg Thieme Verlag, Stuttgart
Schliebs R, Arendt T (2011) The cholinergic system in aging and neuronal degeneration. Behav Brain Res 221(2):555–563. doi:10.1016/j.bbr.2010.11.058
Sharma M, Deogaonkar M, Rezai A (2015) Assessment of potential targets for deep brain stimulation in patients with Alzheimer’s disease. J Clin Med Res 7(7):501–505. doi:10.14740/jocmr2127w
Smith GS, de Leon MJ, George AE, Kluger A, Volkow ND, McRae T, Golomb J, Ferris SH, Reisberg B, Ciaravino J et al (1992) Topography of cross-sectional and longitudinal glucose metabolic deficits in Alzheimer’s disease. Pathophysiologic implications. Arch Neurol 49(11):1142–1150
Sperling RA, Dickerson BC, Pihlajamaki M, Vannini P, LaViolette PS, Vitolo OV, Hedden T, Becker JA, Rentz DM, Selkoe DJ, Johnson KA (2010) Functional alterations in memory networks in early Alzheimer’s disease. NeuroMol Med 12(1):27–43. doi:10.1007/s12017-009-8109-7
Stone SS, Teixeira CM, Devito LM, Zaslavsky K, Josselyn SA, Lozano AM, Frankland PW (2011) Stimulation of entorhinal cortex promotes adult neurogenesis and facilitates spatial memory. J Neurosci 31(38):13469–13484. doi:10.1523/JNEUROSCI.3100-11.2011
Toda H, Hamani C, Fawcett AP, Hutchison WD, Lozano AM (2008) The regulation of adult rodent hippocampal neurogenesis by deep brain stimulation. J Neurosurg 108(1):132–138. doi:10.3171/JNS/2008/108/01/0132
Tsivilis D, Vann SD, Denby C, Roberts N, Mayes AR, Montaldi D, Aggleton JP (2008) A disproportionate role for the fornix and mammillary bodies in recall versus recognition memory. Nat Neurosci 11(7):834–842. doi:10.1038/nn.2149
Turnbull IM, McGeer PL, Beattie L, Calne D, Pate B (1985) Stimulation of the basal nucleus of Meynert in senile dementia of Alzheimer’s type. A preliminary report. Appl Neurophysiol 48(1–6):216–221
Watt AD, Villemagne VL, Barnham KJ (2013) Metals, membranes, and amyloid-beta oligomers: key pieces in the Alzheimer’s disease puzzle? J Alzheimers Dis JAD 33(Suppl 1):S283–S293. doi:10.3233/JAD-2012-129017
Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR (1982) Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science 215(4537):1237–1239
Wilson CR, Baxter MG, Easton A, Gaffan D (2008) Addition of fornix transection to frontal-temporal disconnection increases the impairment in object-in-place memory in macaque monkeys. Eur J Neurosci 27(7):1814–1822. doi:10.1111/j.1460-9568.2008.06140.x
Zibly Z, Shaw A, Harnof S, Sharma M, Graves C, Deogaonkar M, Rezai A (2014) Modulation of mind: therapeutic neuromodulation for cognitive disability. J Clin Neurosci 21(9):1473–1477. doi:10.1016/j.jocn.2013.11.040
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
AML is a consultant to Medtronic, St. Jude, and Boston Scientific. AML serves on the scientific advisory board of Ceregene, Codman, Neurophage, Aleva and Alcyone Life Sciences. AML is cofounder of Functional Neuromodulation Inc. and hold intellectual property in the field of Deep Brain Stimulation. All other authors declare no relevant conflicts.
Rights and permissions
About this article
Cite this article
Mirzadeh, Z., Bari, A. & Lozano, A.M. The rationale for deep brain stimulation in Alzheimer’s disease. J Neural Transm 123, 775–783 (2016). https://doi.org/10.1007/s00702-015-1462-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-015-1462-9