Abstract
Background
Up to 35% of aneurysmal subarachnoid haemorrhage (aSAH) cases may present as poor grade, defined as World Federation of Neurosurgical Societies (WFNS) grades IV and V. In this study, we evaluate functional outcomes and prognostic factors.
Methods
This prospective study included all patients referred to a national, centralized neurosurgical service with a diagnosis of poor-grade aSAH between 01/01/2016 and 31/12/2019. Multivariable logistic regression models were used to estimate probability of poor functional outcomes, defined as a Glasgow Outcome Scale (GOS) of 1–3 at 3 months.
Results
Two hundred fifty-seven patients were referred, of whom 116/257 (45.1%) underwent treatment of an aneurysm, with 97/116 (84%) treated within 48 h of referral. Median age was 62 years (IQR 51–69) with a female predominance (167/257, 65%). Untreated patients tended to be older; 123/141 (87%) had WFNS V, 60/141 (45%) unreactive pupils and 21/141 (16%) circulatory arrest. Of all referred patients, poor outcome occurred in 169/230 (73.5%). Unreactive pupils or circulatory arrest conferred a universally poor prognosis, with mortality in 55/56 (98%) and 19/19 (100%), respectively. The risk of a poor outcome was 14.1% (95% CI 4.5–23.6) higher in WFNS V compared with WFNS IV. Age was important in patients without circulatory arrest or unreactive pupils, with risk of a poor outcome increasing by 1.8% per year (95% CI 1–2.7). In patients undergoing aneurysm securement, 48/101 (47.5%) had a poor outcome, with age, rebleeding, vasospasm and cerebrospinal fluid (CSF) diversion being important prognosticators. The addition of serum markers did not add significant discrimination beyond the clinical presentation.
Conclusions
The overall outcomes of WFNS IV and V aSAH remain poor, mainly due to the devastating effects of the original haemorrhage. However, in patients selected for aneurysm securement, good outcomes can be achieved in more than half of patients. Age, pre-intervention rebleeding, vasospasm, and CSF diversion are important prognostic factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aneurysmal subarachnoid haemorrhage (aSAH) is a devastating neurological event characterized by the rupture of an intracranial aneurysm with bleeding into the subarachnoid space [58]. SAH accounts for 5–10% of all strokes [37, 58], with an estimated worldwide incidence of 7.9 cases per 100,000 patient-years [15]. Up to 35% of cases present as poor-grade [8, 48, 56], most commonly defined via the World Federation of Neurosurgical Societies (WFNS) grade [2] as grades IV and V, corresponding to a Glasgow Coma Scale of less than 13.
This historically carries a poor prognosis, with poor functional outcomes in a majority of patients and a mortality rate of up to 50% [28, 33, 39, 54, 60]. While early intervention has been well established in good-grade aSAH, patients with poor-grade aSAH have been under-represented in clinical trials [67], and there remains uncertainty as to which patients, if any, will benefit from early treatment. Traditionally, patients with poor-grade presentations are often observed for improvement before definitive aneurysm treatment. However, more recent evidence has demonstrated heterogeneity in clinical presentation and subsequent outcome within this group [50]. Some series have even demonstrated improving outcomes in patients treated aggressively and early [22, 23].
Accurate prognostication of patients with poor grade aSAH would allow appropriate patient selection for early intervention, facilitating treatment of those patients likely to improve with a significant possibility of a good functional outcome. Therefore, in this study, we evaluate prognostic factors associated with longitudinal functional outcome in an unselected cohort of patients with poor-grade SAH presenting to a national, centralized neurosurgical service.
Methods
This prospective, observational study is reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement [63] and set in a centralized neurosurgical service serving a catchment of approximately 3.9 million people. All patients with aSAH within this catchment are referred to the National Neurosurgical Centre, with most transferred for aneurysm intervention [8]. Demographic, clinical and radiological details of all patients referred to the national neurosurgical centre in Ireland between 01/01/2016 and 31/12/2019 were prospectively recorded. This included all patients referred, including those not accepted for treatment. We identified patients who died pre-hospital via the Central Statistics Office (CSO).
Participants
Patients were diagnosed with aSAH via the presence of subarachnoid blood on computed tomography (CT) and/or xanthochromic cerebrospinal fluid (CSF) on lumbar puncture. The presence of cerebral aneurysms was diagnosed with CT angiography (CTA) and/or digital subtraction angiography (DSA). Patients aged ≥ 18 years with SAH and a poor-grade presentation defined by WFNS grade IV or V at the time of referral were included.
Variables
We prospectively recorded demographic, clinical, radiological and treatment details including age, sex, WFNS grade [2], pupillary reactivity, cardiorespiratory arrest, pre-intervention aneurysm rebleeding, Fisher grade [16], aneurysm location, method of aneurysm repair, need for cerebrospinal fluid (CSF) diversion and symptomatic vasospasm. We also recorded several serum markers, including C-reactive protein (CRP), total protein and white cell count (WCC), measured on admission. Outcomes were assessed using the Glasgow Outcome Score (GOS) [30] at discharge and at 3 months by nurse specialists. The primary outcome was the GOS at 3 months. We considered a GOS of 1–3 to be a poor outcome.
Statistical analysis
All analysis was performed in R v4.2.2 [51] (The R Foundation, Vienna, Austria). First, we fit a multivariable logistic regression model for a poor 3-month outcome in the entire cohort of patients with poor-grade ASAH. Variables included were those a priori assumed to moderate prognosis [24], including age, WFNS grade, Fisher grade, pupil reactivity, circulatory arrest and pre-intervention rebleeding. We used predictions to estimate the risk of a poor outcome under various combinations of risk factors and generated UpSet [6] plots. In these models, missing data was minimal and we considered that data may be missing not-at-random (MNAR) [46]. Therefore, this analysis used a listwise deletion approach.
We then fit a second multivariable logistic regression model in patients accepted for transfer. We included factors a priori expected to influence prognosis following admission including need for CSF diversion, symptomatic vasospasm and further rebleeding, adjusted for age, WFNS and Fisher grades. We additionally fit proportional odds models, in which the variable in question was additionally adjusted for age, WFNS grade and Fisher grade. From this, we report the common odds ratio (OR), which represents the odds ratio associated with a one-point upward shift in the GOS, and thus odds ratios > 1 indicate better outcomes [53].
We assessed whether serum markers may be associated with poorer outcomes by adding them to our prognostic model and calculating their marginal effect [47], which reflects the percentage increase in absolute risk of a poor outcome averaged across all values of the biomarker. We additionally calculated the incremental change in the area under the receiver operating curve (iAUC), which reflects the change in AUC for a model including important clinical factors, with versus without the serum marker. We calculated the 95% CI around the iAUC using bootstraps. We took this approach to establish whether the serum marker adds incremental value to the clinical presentation. In these analyses, we assumed data were missing-at-random (MAR) and therefore performed all analyses on five multiply-imputed datasets, pooling results using Rubin’s rules [40, 46]. Anonymised datasets and statistical code are available upon reasonable request to the corresponding authors.
Results
Two hundred fifty-seven patients with poor grade aSAH were referred during the 4-year study period, of whom 154/257 (60%) were accepted for transfer and 116/257 (45.1%) underwent treatment of an aneurysm (Fig. 1). Median age was 62 years (IQR 51 to 69), with an expected female predominance (167/257, 65%). When assessing the entire cohort, poor outcomes (GOS 1–3) were observed in 169/230 (73.5%), with mortality in 139/230 (60%) (Table 1). All included patients had imaging evidence of aSAH on a CT scan. In those patients who underwent aneurysm securement, poor 3-month outcomes were observed in 48/101 (48%). The characteristics of patients undergoing aneurysm intervention are shown in Table 1.
Prognostic factors for all patients at initial presentation
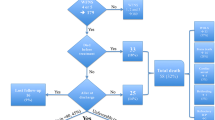
The presence of unreactive pupils or circulatory arrest conferred a uniformly poor prognosis (Fig. 2). Patients presenting with unreactive pupils had an in-hospital mortality of 55/56 (98%), while patients with circulatory arrest had a mortality of 19/19 (100%). Age was not an important factor in this group (Fig. 3). While pre-intervention rebleeding also increased risk of a poor outcome (Table 2), age remained an important prognosticator (Fig. 2).
Line plots showing the risk of a poor outcome at three months in relationship to age, in patients with WFNS grade V and Fisher grade IV ASAH. A Patients with reactive pupils, no arrest and no rebleeding, B patients with unreactive pupils, C patients with an arrest and D patients with a rebleed, but reactive pupils and no arrest
In patients without unreactive pupils, circulatory arrest or pre-intervention rebleeding, we found that age, WFNS grade and Fisher grade were important prognosticators. On average, the absolute risk of a poor outcome at 3 months was increased by 14.1% (95% CI 4.5 to 23.6) in WFNS V SAH compared with WFNS IV (Table 3). In these patients, we also found that, on average, the risk of a poor outcome increased by an average of 1.4% (95% CI 0.7 to 2) per year older in patients with WFNS V SAH (Table 2).
Prognostic factors in patients who underwent aneurysm securement
In patients undergoing aneurysm treatment, patients with WFNS V (vs. WFNS IV) aSAH had a lower GOS at 3 months (age-adjusted OR 0.51, 95% CI 0.33 to 0.98). In our cohort, Fisher IV aSAH was not strongly associated with poorer outcome (age-adjusted OR 0.63, 95% CI 0.2 to 2), though 241/257 (94%) of patients had Fisher IV aSAH. We observed a similar trend in patients who had an aneurysm secured (Fig. 4), with risk of a poor outcome increasing with age (Table 4). We found that need for CSF diversion (adjusted OR 0.49, 95% CI 0.25 to 0.99) was associated with a lower GOS at 3 months. We did not find evidence that radiological vasospasm (adjusted OR 0.65, 95% CI 0.29 to 1.45), symptomatic vasospasm (adjusted OR 0.88, 95% CI 0.38 to 2.04) or rebleeding (adjusted OR 0.38, 95% CI 0.12 to 1.21) were strongly associated with outcome (Table 5). However, there was a cumulative increased risk in patients with multiple prognostic factors (Fig. 4). For example, in a hypothetical patient aged 60 years, risk of a poor outcome increases from 43% (95% CI 25 to 62) without need for CSF diversion, rebleeding or symptomatic vasospasm to 57% (95% CI 38 to 75) with CSF diversion alone and 78% (95% CI 49 to 100) with symptomatic vasospasm, rebleeding and need for CSF diversion.
Timing of aneurysm securement
In our study, 97/116 (84%) of treated patients were treated within 48 h of ictus. Overall, we did not observe an important difference in outcomes or rebleeding in patients treated earlier (Table 6). Poor outcomes occurred in 39/84 (46%) of patients treated within 48 h compared with 9/17 (53%) of patients treated later. Adjustment for WFNS grade did not influence the outcome within 48 (OR 0.75, 95% CI 0.37 to 1.56) or 24 h (OR 0.38, 95% CI 0.14 to 1.05). Pre-intervention rebleeding occurred in 5/97 (5.2%) of patients treated within 48 h compared with 2/19 (10.5%) of patients treated later.
Utility of serum markers
We did not find evidence that addition of serum markers including CRP, WCC and total protein improved discrimination of outcomes when added to a model adjusting for clinical prognosticators. We found only marginal increases in the AUC for CRP (+ 1%, 95% CI − 1.1 to 3.2), WCC (+ 0.1%, 95% CI − 1 to 1.2) and total protein (+ 1.8%, 95% CI − 1.2 to 4.9), with only adjusted increases in risk with increases in serum markers (Table 6). Any degree of discrimination observed appeared to be most pronounced in patients without clinical prognosticators (Fig. 5). However, in a subgroup of patients who had an aneurysm secured, the addition of CRP to a model adjusted for clinical factors resulted in a small increase in AUC of 8.7% (95% CI 0.8 to 17) (Table 7).
Association of serum markers with absolute risk of a poor outcome, stratified by clinical risk factors, derived from logistic regression models adjusted for age, WFNS grade, Fisher grade and the presence of rebleeding, unreactive pupils or circulatory arrest. Shaded areas represent the 95% confidence interval of the estimate. A–C The entire cohort of patients. D–F Patients undergoing treatment of an aneurysm only
Pre-hospital mortality
In addition to the patients included this study, data suggested an additional 184 patients with pre-hospital mortality due to spontaneous SAH during the study period. If we assume that the cause of SAH in all these patients was a ruptured aneurysm and include them in the present study, poor outcomes would have been observed in 353/414 (85.3%) and mortality in 323/414 (78%).
Discussion
Multiple recent publications have suggested that good outcomes may be achievable in patients with poor grade aSAH, attributing this improvement to endovascular techniques and/or earlier aneurysm treatment [1, 5, 7, 11, 12, 25, 31, 36, 39, 59, 65, 66]. Some studies have suggested that good outcomes may be achieved in 30–40% of patients [1, 26, 31, 65, 66] and mortality in less than 20% [31]. Our data suggests that, unfortunately, the outcomes of poor-grade aSAH remain poor, with mortality observed in 60% of patients and poor outcome (GOS 1–3) in 73.5% at 3 months. Our findings are consistent with other published data in unselected patients, showing similar risks of mortality and poor outcomes [28, 33, 39, 54]. This suggests that better outcomes in more recent publications may be at least partly attributable to selection bias, with exclusion of patients with factors such as unreactive pupils or circulatory arrest [17, 29, 49, 64]. Another important factor is the exclusion of untreated patients, who make up a large proportion of patients in unselected cohorts and have a universally poor outcome [20, 27].
In our study, unreactive pupils were associated with mortality in 98% and poor functional outcome in 100%, along with 100% mortality in patients with circulatory arrest. This is broadly consistent with previous publications [13, 35, 44, 52], and some authors have suggested a modification of the WFNS grading scale to account for the absence of brainstem reflexes [50]. Occasional good outcomes in patients with unreactive pupils have been reported [26], and therefore, some may argue that the poor outcomes in such patients may be because they were not accepted for active management and therefore the result of a self-fulfilling prophecy. As we did not treat these patients actively, we cannot comment on what the outcome might have been if they had been accepted for neurocritical care and neurosurgical management. However, in one study examining early and aggressive intervention in patients thought to have a poor prognosis, even some patients with bilaterally unreactive pupils, outcomes remained poor with a 1-year mortality of 65.8% [38].
We found that in patients selected for aneurysm intervention, good outcomes (GOS 4–5) were achieved with 53% of cases being independent at 3 months. This further improved in younger patients aged less than 65 years old to 63%, which highlights that good outcomes are achievable in carefully selected patients. In addition, patients with poor grade aSAH, particularly younger patients, may continue to improve functionally beyond our 3-month timeframe [4], and thus, the proportion achieving good longer term outcomes may be higher than our study suggests. It is also worth noting that a very small proportion of patients (2% in the treated group and 2.3% in the untreated group) survived in a vegetative state (GOS2). In patients who did not have unreactive pupils, circulatory arrest or pre-intervention rebleeding, we found that WFNS grade, Fisher grade and age were important prognostic factors. In those who underwent aneurysm securement, the need for CSF diversion was also an important factor. However, it is important to note that good outcomes were observed in 8/141 (6.3%) of untreated patients, which highlights the need for refinement of the current patient selection criteria.
Our finding that increasing age is associated with an escalating risk of a poor outcome is consistent with previous studies, which have demonstrated poor outcomes in the elderly [13, 19], even in good-grade SAH [62]. However, an important caveat is that age may be acting as a surrogate for general comorbidity burden. The applicability of this finding to otherwise healthy older adults with a good functional baseline is unclear.
Need for CSF diversion was the only factor that appeared to be an important prognosticator in our cohort, which likely reflects the presence of hydrocephalus [69]. While symptomatic vasospasm or rebleeding did not influence risk of poor outcomes in isolation, their effects appeared to be cumulative, especially in older patients (Fig. 4). Pre-intervention rebleeding occurred in 26/257 (10%) of our patients with poor grade aSAH. In the treated group, data was captured on a daily basis prospectively, and therefore, it is unlikely for rebleeds to have been missed in this group. Rebleeding may have been missed from unsecured aneurysms in the untreated group of patients as these patients were not transferred to the neurosurgical centre. The prognostic importance of rebleeding in the overall patient cohort with poor-grade aSAH therefore remains unclear in our study.
The timing of aneurysm treatment in poor-grade aSAH has been the subject of much debate with some advocating early treatment to minimize risk of rebleeding [10], delaying treatment until after the vasospasm period and selecting patients who recover well [32]. Traditionally, delayed aneurysm treatment was performed at our centre, but there has been a shift towards early treatment [8]. During the study period, our centre’s approach has been to secure the aneurysm early (within 48 h of ictus) in poor-grade aSAH patients. There were some variations in practice between different neurosurgeons. For example, some neurosurgeons would lighten sedation and assess the patient’s neurological function prior to a decision regarding intervention, whereas others would proceed with aneurysm securement without this neurological assessment. However, despite these variations, 84% of our patients underwent treatment within 48 h. Patients with poor-grade SAH are thought to be at particularly high risk of early rebleeding [14], which makes early treatment appealing in this group [68]. Rebleeding was uncommon in our cohort, which may be partially attributable to the aforementioned shift towards earlier treatment, although underreporting in patients not accepted for treatment may also be a factor.
In our cohort, treatment within 48 h was not associated with outcome. This is likely a consequence of few undergoing late treatment after 48 h (19/116, 16%) and the selected nature of this group. Patients treated late are likely those who improved after referral, with late intervention [32, 67]. Improvement prior to treatment is associated with an improved prognosis [13], and thus, this cohort may provide a false inverse association between prolonged time to treatment and improved outcome. As a result, any benefit of early treatment may not be reflected in our study in this cohort of patients. It is important to additionally note, however, that our findings do not preclude a benefit of much earlier (for example within 6 h) treatment.
An array of serum biomarkers have been proposed as prognostic in SAH including CRP [3, 18, 61], WCC [21, 42, 45] and haemoglobin [41, 55]. Studies to date have primarily assessed these markers in univariable models. In our study, there was no incremental benefit of serum markers over clinical presentation alone. Our results suggest that any benefit is likely too small to be clinically useful. However, our study cannot comment on their utility in the wider population with SAH. We observed marginally increasing benefit in patients with better clinical presentation. In particular, the addition of CRP appeared to slightly improve prognostication in patients undergoing aneurysm securement.
An important issue when considering outcomes of poor-grade SAH is in relation to pre-hospital mortality. These patients are typically not counted in studies examining poor grade SAH but account for 15–25% of all SAH cases [9, 34, 43, 57]. In one large study examining over 79 million person-years of follow-up, sudden deaths accounted for 26% of all SAH cases [34]. These cases are liable to be missed in studies examining aSAH referred to neurosurgical centres, and thus, the true incidence and mortality attributable to poor-grade aSAH is likely underestimated. In our study, inclusion of patients who died pre-hospital would have increased the risk of poor outcomes and mortality to 85.3% and 78%, respectively.
Limitations
This is observational and is therefore subject to confounding despite our efforts to include as unselected sample as possible. One source of bias in our study is that patients with factors associated with a very poor prognosis such as unreactive pupils or circulatory arrest were typically not treated.
Generalizability
This study is an unselected cohort and thus may be expected to generalize reasonably to areas with similar population structure. The neurosurgical service in Ireland is highly centralized with only 2 neurosurgical centres for the entire population of 5 million people. Our centre provides neurosurgery to three-quarters of Ireland and receives all neurosurgical referrals from the hospitals within this catchment area (with a population of 3.8 million people). We additionally accounted for pre-hospital mortalities. Therefore, we are confident that very few, if any, cases of aSAH would have been missed.
Conclusion
Unfortunately, despite the many advances in aSAH treatment, the overall outcomes of poor-grade SAH remain poor due to the high mortality and irreversible brain injury at the time of initial haemorrhage. However, in selected patients who undergo aneurysm securement, good outcomes (GOS 4–5 at 3 months) can be achieved in 53% of patients. Increasing age, WFNS grade V, rebleeding, vasospasm and need for CSF diversion were risk factors for poor outcome. In patients with poor-grade SAH, the use of CRP, total protein and WCC adds only minimal predictive discrimination beyond of the clinical presentation.
References
(1988) Report of World Federation of Neurological Surgeons Committee on a universal subarachnoid hemorrhage grading scale. J Neurosurg 68(6):985–986
Alessandro O, Rene W, Stefan W, Miodrag F, Martin S, Oliver B, Urs P (2022) C-reactive protein elevation predicts in-hospital deterioration after aneurysmal subarachnoid hemorrhage: a retrospective observational study. Acta Neurochir (Wien) 164(7):1805–1814
Al-Khindi T, Macdonald RL, Schweizer TA (2010) Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke 41(8):e519-536
Al-Mufti F, Mayer SA, Kaur G et al (2021) Neurocritical care management of poor-grade subarachnoid hemorrhage: unjustified nihilism to reasonable optimism. Neuroradiol J 34(6):542–551
Ballarini NM, Chiu Y-D, König F, Posch M, Jaki T (2020) A critical review of graphics for subgroup analyses in clinical trials. Pharm Stat 19(5):541–560
Chen J, Zhu J, He J, Wang Y, Chen L, Zhang C, Zhou J, Yang L (2016) Ultra-early microsurgical treatment within 24 h of SAH improves prognosis of poor-grade aneurysm combined with intracerebral hematoma. Oncol Lett 11(5):3173–3178
Choudhry A, Murray D, Corr P et al (2022) Timing of treatment of aneurysmal subarachnoid haemorrhage: are the goals set in international guidelines achievable? Ir J Med Sci 191(1):401–406
Chu KH, Mahmoud I, Hou X-Y, Winter CD, Jeffree RL, Brown NJ, Brown AF (2018) Incidence and outcome of subarachnoid haemorrhage in the general and emergency department populations in Queensland from 2010 to 2014. Emerg Med Australas 30(4):503–510
Connolly ES, Rabinstein AA, Carhuapoma JR et al (2012) Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke 43(6):1711–1737
Das KK, Singh S, Sharma P, Mehrotra A, Bhaisora K, Sardhara J, Srivastava AK, Jaiswal AK, Behari S, Kumar R (2017) Results of proactive surgical clipping in poor-grade aneurysmal subarachnoid hemorrhage: pattern of recovery and predictors of outcome. World Neurosurg 102:561–570
de Oliveira Manoel AL, Mansur A, Silva GS et al (2016) Functional outcome after poor-grade subarachnoid hemorrhage: a single-center study and systematic literature review. Neurocrit Care 25(3):338–350
de Winkel J, Cras TY, Dammers R, van Doormaal P-J, van der Jagt M, Dippel DWJ, Lingsma HF, Roozenbeek B (2022) Early predictors of functional outcome in poor-grade aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. BMC Neurol 22(1):239
Doherty RJ, Henry J, Brennan D, Javadpour M (2022) Predictive factors for pre-intervention rebleeding in aneurysmal subarachnoid haemorrhage: a systematic review and meta-analysis. Neurosurg Rev 46(1):24
Etminan N, Chang H-S, Hackenberg K, de Rooij NK, Vergouwen MDI, Rinkel GJE, Algra A (2019) Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: a systematic review and meta-analysis. JAMA Neurol 76(5):588–597
Fisher CM, Kistler JP, Davis JM (1980) Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery 6(1):1–9
Fukuda H, Hayashi K, Moriya T, Nakashita S, Lo BWY, Yamagata S (2015) Intrasylvian hematoma caused by ruptured middle cerebral artery aneurysms predicts recovery from poor-grade subarachnoid hemorrhage. J Neurosurg 123(3):686–692
Gaastra B, Barron P, Newitt L, Chhugani S, Turner C, Kirkpatrick P, MacArthur B, Galea I, Bulters D (2021) CRP (C-reactive protein) in outcome prediction after subarachnoid hemorrhage and the role of machine learning. Stroke 52(10):3276–3285
Goldberg J, Schoeni D, Mordasini P, Z’Graggen W, Gralla J, Raabe A, Beck J, Fung C (2018) Survival and outcome after poor-grade aneurysmal subarachnoid hemorrhage in elderly patients. Stroke 49(12):2883–2889
Goldberg J, Z’Graggen WJ, Hlavica M et al (2023) Quality of life after poor-grade aneurysmal subarachnoid hemorrhage. Neurosurgery. https://doi.org/10.1227/neu.0000000000002332
Gusdon AM, Savarraj JPJ, Shihabeddin E, Paz A, Assing A, Ko S-B, McCullough LD, Choi HA (2021) Time course of peripheral leukocytosis and clinical outcomes after aneurysmal subarachnoid hemorrhage. Front Neurol 12:694996
Han Y, Ye F, Long X, Li A, Xu H, Zou L, Yang Y, You C (2018) Ultra-early treatment for poor-grade aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. World Neurosurg 115:e160–e171
Hanalioglu S, Sahin B, Sayyahmelli S, Ozaydin B, Erginoglu U, Aycan A, Baskaya MK (2022) The role of microsurgery for poor-grade aneurysmal subarachnoid hemorrhages in the endovascular era. Acta Neurochir (Wien) 164(3):781–793
Heinze G, Wallisch C, Dunkler D (2018) Variable selection - A review and recommendations for the practicing statistician. Biom J 60(3):431–449
Hoogmoed J, van den Berg R, Coert BA, Rinkel GJE, Vandertop WP, Verbaan D (2017) A strategy to expeditious invasive treatment improves clinical outcome in comatose patients with aneurysmal subarachnoid haemorrhage. Eur J Neurol 24(1):82–89
Hoogmoed J, Coert BA, van den Berg R, Roos YBWEM, Horn J, Vandertop WP, Verbaan D (2018) Early treatment decisions in poor-grade patients with subarachnoid hemorrhage. World Neurosurg 119:e568–e573
Hoogmoed J, de Oliveira Manoel AL, Coert BA, Marotta TR, Macdonald RL, Vandertop WP, Verbaan D, Germans MR (2019) Why do patients with poor-grade subarachnoid hemorrhage die? World Neurosurg 131:e508–e513
Hutchinson PJ, Power DM, Tripathi P, Kirkpatrick PJ (2000) Outcome from poor grade aneurysmal subarachnoid haemorrhage–which poor grade subarachnoid haemorrhage patients benefit from aneurysm clipping? Br J Neurosurg 14(2):105–109
Inamasu J, Nakae S, Ohmi T et al (2016) The outcomes of early aneurysm repair in world federation of neurosurgical societies grade V subarachnoid haemorrhage patients with emphasis on those presenting with a glasgow coma scale score of 3. J Clin Neurosci 33:142–147
Jennett B, Bond M (1975) Assessment of outcome after severe brain damage. Lancet 1(7905):480–484
Kaneko J, Tagami T, Unemoto K et al (2019) Functional outcome following ultra-early treatment for ruptured aneurysms in patients with poor-grade subarachnoid hemorrhage. J Nippon Med Sch 86(2):81–90
Kassell NF, Drake CG (1982) Timing of aneurysm surgery. Neurosurgery 10(4):514–519
Kassell NF, Torner JC, Haley EC, Jane JA, Adams HP, Kongable GL (1990) The international cooperative study on the timing of aneurysm surgery. Part 1: overall management results. J Neurosurg 73(1):18–36
Korja M, Lehto H, Juvela S, Kaprio J (2016) Incidence of subarachnoid hemorrhage is decreasing together with decreasing smoking rates. Neurology 87(11):1118–1123
Kramer AH, Couillard PL, Kromm JA, Ruddell S, Demers-Marcil S, Mitha AP, Sutherland GR, Wong JH (2021) Findings predictive of poor outcome in grade 5 subarachnoid hemorrhage: a cohort study. Can J Neurol Sci 48(6):807–816
Kranthi S, Sahu BP, Aniruddh P (2016) Factors affecting outcome in poor grade subarachnoid haemorrhage: an institutional study. Asian J Neurosurg 11(4):365–371
Krishnamurthi RV, Barker-Collo S, Parag V, Parmar P, Witt E, Jones A, Mahon S, Anderson CS, Barber PA, Feigin VL (2018) Stroke incidence by major pathological type and ischemic subtypes in the Auckland regional community stroke studies: changes between 2002 and 2011. Stroke 49(1):3–10
Lashkarivand A, Sorteberg W, Rosseland LA, Sorteberg A (2020) Survival and outcome in patients with aneurysmal subarachnoid hemorrhage in Glasgow coma score 3–5. Acta Neurochir (Wien) 162(3):533–544
Le Roux PD, Elliott JP, Newell DW, Grady MS, Winn HR (1996) Predicting outcome in poor-grade patients with subarachnoid hemorrhage: a retrospective review of 159 aggressively managed cases. J Neurosurg 85(1):39–49
Li P, Stuart EA, Allison DB (2015) Multiple imputation: a flexible tool for handling missing data. JAMA 314(18):1966–1967
Li R, Lin F, Chen Y et al (2022) Elevated blood hemoglobin on admission as an independent predictor of unfavorable outcomes in patients with aneurysmal subarachnoid hemorrhage. Neurosurg Rev 45(4):2689–2699
Li R, Zhao Y, Chen X, Hao Q (2022) Predictive values of white blood cell count in peripheral blood at admission on in-hospital complications and 90-day outcomes of patients with aneurysmal subarachnoid hemorrhage: insights from the LongTEAM Registry. J Inflamm Res 15:6481–6494
Lindbohm JV, Kaprio J, Jousilahti P, Salomaa V, Korja M (2017) Risk factors of sudden death from subarachnoid hemorrhage. Stroke 48(9):2399–2404
Mack WJ, Hickman ZL, Ducruet AF et al (2008) Pupillary reactivity upon hospital admission predicts long-term outcome in poor grade aneurysmal subarachnoid hemorrhage patients. Neurocrit Care 8(3):374–379
Mahta A, Azher AI, Moody S et al (2021) Association of Early white blood cell trend with outcomes in aneurysmal subarachnoid hemorrhage. World Neurosurg 151:e803–e809
Newgard CD, Lewis RJ (2015) Missing data: how to best account for what is not known. JAMA 314(9):940–941
Norton EC, Dowd BE, Maciejewski ML (2019) Marginal effects-quantifying the effect of changes in risk factors in logistic regression models. JAMA 321(13):1304–1305
Post R, Germans MR, Tjerkstra MA et al (2021) Ultra-early tranexamic acid after subarachnoid haemorrhage (ULTRA): a randomised controlled trial. Lancet 397(10269):112–118
Raabe A, Beck J, Goldberg J et al (2022) Herniation World Federation of Neurosurgical Societies scale improves prediction of outcome in patients with poor-grade aneurysmal subarachnoid hemorrhage. Stroke 53(7):2346–2351
R Core Team (2022) R: a language and environment for statistical computing
Ridwan S, Kristof R (2019) Cardiac arrest in patients with poor-grade aneurysmal subarachnoid hemorrhage: a single-center experience. J Neurol Surg A Cent Eur Neurosurg 80(6):409–412
Roozenbeek B, Lingsma HF, Perel P, Edwards P, Roberts I, Murray GD, Maas AI, Steyerberg EW, IMPACT (International Mission on Prognosis and Clinical Trial Design in Traumatic Brain Injury) Study Group, CRASH (Corticosteroid Randomisation After Significant Head Injury) Trial Collaborators (2011) The added value of ordinal analysis in clinical trials: an example in traumatic brain injury. Crit Care 15(3):R127
Rordorf G, Ogilvy CS, Gress DR, Crowell RM, Choi IS (1997) Patients in poor neurological condition after subarachnoid hemorrhage: early management and long-term outcome. Acta Neurochir (Wien) 139(12):1143–1151
Said M, Gümüs M, Rodemerk J et al (2022) Systematic review and meta-analysis of outcome-relevant anemia in patients with subarachnoid hemorrhage. Sci Rep 12(1):20738
Schatlo B, Fung C, Stienen MN et al (2021) Incidence and outcome of aneurysmal subarachnoid hemorrhage: the Swiss study on subarachnoid hemorrhage (Swiss SOS). Stroke 52(1):344–347
Sonne A, Bækgaard ES, Banner J, Rasmussen LS (2020) Spontaneous subarachnoid haemorrhage as a cause of out-of-hospital death. J Stroke Cerebrovasc Dis 29(11):105239
Sweeney K, Silver N, Javadpour M (2016) Subarachnoid haemorrhage (spontaneous aneurysmal). BMJ Clin Evid 2016:1213
Tasiou A, Brotis AG, Paschalis T, Tzerefos C, Kapsalaki EZ, Giannis T, Tzannis A, Fountas KN (2022) Intermediate surgical outcome in patients suffering poor-grade aneurysmal subarachnoid hemorrhage. A single center experience. Int J Neurosci 132(1):38–50
Teo M, Guilfoyle MR, Turner C, Kirkpatrick PJ, Collaborators STASH (2017) What factors determine treatment outcome in aneurysmal subarachnoid hemorrhage in the modern era? A post hoc STASH analysis. World Neurosurg 105:270–281
Turner CL, Budohoski K, Smith C, Hutchinson PJ, Kirkpatrick PJ, Murray GD, STASH collaborators, (2015) Elevated baseline C-reactive protein as a predictor of outcome after aneurysmal subarachnoid hemorrhage: data from the simvastatin in aneurysmal subarachnoid hemorrhage (STASH) trial. Neurosurgery 77(5):786–792 (discussion 792-793)
Välimäki V, Luostarinen T, Satopää J, Raj R, Virta JJ (2021) Neurointensive care results and risk factors for unfavorable outcome in aneurysmatic SAH: a comparison of two age groups. Acta Neurochir (Wien) 163(5):1469–1478
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, Initiative STROBE (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370(9596):1453–1457
Wang X, Han C, Xing D, Wang C, Ding X (2019) Early management of poor-grade aneurysmal subarachnoid hemorrhage: a prognostic analysis of 104 patients. Clin Neurol Neurosurg 179:4–8
Weir RU, Marcellus ML, Do HM, Steinberg GK, Marks MP (2003) Aneurysmal subarachnoid hemorrhage in patients with Hunt and Hess grade 4 or 5: treatment using the Guglielmi detachable coil system. AJNR Am J Neuroradiol 24(4):585–590
White P, Gregson B, McColl E, et al (2021) Emergent aneurysm treatment compared with treatment on neurological improvement in patients with ruptured poor-grade aneurysmal subarachnoid haemorrhage: the TOPSAT2 RCT. NIHR J Libr, Southampton (UK)
Whitfield PC, Kirkpatrick PJ (2001) Timing of surgery for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev 2:CD001697
Wolfert C, Maurer CJ, Berlis A, Schneider H, Steininger K, Motov S, Krauss P, Sommer B, Shiban E (2022) Hydrocephalus, cerebral vasospasm, and delayed cerebral ischemia following non-aneurysmatic spontaneous subarachnoid hemorrhages: an underestimated problem. Neurosurg Rev 46(1):23
Wostrack M, Sandow N, Vajkoczy P et al (2013) Subarachnoid haemorrhage WFNS grade V: is maximal treatment worthwhile? Acta Neurochir (Wien) 155(4):579–586
Zhao B, Zhao Y, Tan X, Cao Y, Wu J, Zhong M, Wang S (2015) Factors and outcomes associated with ultra-early surgery for poor-grade aneurysmal subarachnoid haemorrhage: a multicentre retrospective analysis. BMJ Open 5(4):e007410
Zheng K, Zhao B, Tan X-X, Li Z-Q, Xiong Y, Zhong M, Chen S-Y (2018) Comparison of aggressive surgical treatment and palliative treatment in elderly patients with poor-grade intracranial aneurysmal subarachnoid hemorrhage. Biomed Res Int 2018:5818937
Funding
The study was partially funded by Friends of A, a charitable organization.
Author information
Authors and Affiliations
Contributions
Concept and design: JH, MOD, MJ.
Acquisition of data: MOD, DK, PC, DN, DC, JT, AOH, SP, DR.
Interpretation of data: JH, MOD, MJ, JT, AOH, SP, DR.
Statistical analysis: JH.
Drafting of the manuscript: JH, MJ.
Critical revision of the manuscript for important intellectual content: all authors.
Supervision: MJ.
All authors reviewed the manuscript prior to final submission.
Corresponding authors
Ethics declarations
Ethical approval
This study was approved by the local institutional review board (CA248) and performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All participants provided informed consent to all treatment.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Henry, J., Dablouk, M.O., Kapoor, D. et al. Outcomes following poor-grade aneurysmal subarachnoid haemorrhage: a prospective observational study. Acta Neurochir 165, 3651–3664 (2023). https://doi.org/10.1007/s00701-023-05884-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-023-05884-0