Abstract
Background
In tethered cord syndrome due to filum terminale pathology, the surgical approach to achieve detethering of the spinal cord may vary. Traditionally, sectioning the filum through a laminectomy at the lumbosacral level is performed.
Method
A microsurgical technique at a higher level to approach the filum below the conus tip is performed. This allows for removal of the entire distal portion of the filum through a limited interlaminar approach and dural opening.
Conclusion
We propose a technique to transect the filum terminale below the conus tip and extract the distal filum by releasing it from its intradural attachments to minimize any remnants of the filum terminale.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traditionally, sectioning the filum through a laminectomy at the lumbosacral level is performed for detethering in patients with tethered cord syndrome (TCS) [5, 9]. Some surgeons would resect a small portion of the filum for histopathological evaluation. The rationale for a lower surgical approach to the filum is to avoid being in the vicinity of the conus medullaris. Furthermore, the increased width of the thecal sac and the loss of cauda equina density in the lumbosacral space facilitate filum isolation and sectioning.
The procedure has been proposed as fairly straightforward with minimal surgical risks; however, the main challenges remain the fairly high rate of retethering of up to 8% in some studies, particularly in a thickened filum, where the residual filum may re-attach to the dura proximal to the surgical site [6, 10, 11].
The authors perform a microsurgical technique at a higher level to approach the filum below the conus tip. This minimizes any residual filum and allows for removal of the entire distal portion of the filum terminale through a limited interlaminar approach and small dural opening. We discuss ways to optimize the surgical technique and limit short- and long-term complications, such as cerebrospinal fluid (CSF) leak, pseudomeningocele, and adhesive arachnoiditis.
Relevant surgical anatomy
The conus medullaris-filum terminale transition zone typically lies at the lumbar 1–2 disc space. The conus medullaris marks the end of the spinal cord proper and the beginning of the cauda equina nerve roots. The filum terminale usually consists of fibrovascular tissue that extends from the conus medullaris and attaches to the end of the thecal sac [1]. The conus-filum interface may be determined on a preoperative magnetic resonance image (MRI) of the lumbar spine which to facilitate allows for surgical planning of the target levels for laminotomy and, therefore, filum resection. Identifying this level will guide the surgical approach and point of resection of the filum (Fig. 1).
Description of the technique
Exposure
The pediatric patient is positioned prone on gel rolls with all pressure points padded. In adults, we prefer positioning on a Wilson frame. Intraoperative neuromonitoring (IONM) with electromyography of the lower extremity is recorded including anal sphincter. An incision is marked at the interspinous level, L1-2 in the presented case, one level below the terminus of the conus medullaris, confirmed with a lateral X-ray (Fig. 1). The exposure is performed in a standard fashion of the spinous process above and below, the spinous interspace and lamina are exposed out laterally until the medial facet joint is visualized. After confirmation of the level with another X-ray, a small laminotomy is performed with a 4-mm diamond burr drill removing a sparing amount of the caudal aspect of the upper lamina and the cranial aspect of the lower lamina, with approximately a 1–2 cm wide and long exposure. Ligamentum flavum is removed with a Kerrison rongeur, and the bone edges are coagulated using monopolar electrocautery for optimal epidural hemostasis. After copious irrigation, the epidural space is gently packed with Gelfoam for hemostasis to avoid any epidural venous pooling during the intradural procedure (Fig. 2). At this point, the intradural portion of the surgery is initiated.
Resection of the filum
The microscope is brought into the field for this portion of the procedure. Using a 15-blade, the dura is incised in the rostro-caudal direction, roughly 1 cm in length—matching the size of a 1/2 in. × 1/2 in. neurosurgical pattie. The two dural leaflets are tacked up laterally to the soft tissues using a 4–0 nurolon suture (Fig. 3). Using micro scissors and a micro bayonet-shaped forceps, the arachnoid layer is dissected and excised. The filum terminale is then lifted using a nerve hook, and the stimulator is used to confirm EMG activity from the cauda equina at 0.5 milliamp (mA). The filum is stimulated up to 7 to 10 mA, and redundant signals may be obtained at higher amplitudes given the neuronal origins of the filum as previously described [7]. At this point, the filum is grabbed and lifted gently at the caudal portion using a DeBakey forceps, and the rostral end of the filum is cauterized using the bipolar cautery forceps (ISOCOOL®, Codman®, Raynham, MA, USA) on low power. Micro scissors are then utilized to sharply cut the cauterized segment of the filum. Once the filum is disconnected, the caudal end of the severed filum is then pulled rostrally, until a snap is felt, indicating that the filum has been disconnected and released at its most caudal connection point within the thecal sac, and the filum is then removed through the dural opening, paying special attention to any nerve roots that are tethered to this, in which case a Rhoton dissector is used to free up these roots from the filum. Figure 4 shows the excised filum terminale internum pathological specimen.
Attention should be paid to the rostral portion of the opening to search for the remnant portion of the filum attached to the conus, and using a Rhoton micro dissector, this is gently released from arachnoid layer and moved within the cauda equina to avoid having the filum exposed to the dura and the site of the dural closure in the future.
Dural closure
After adequate irrigation and ensuring good intradural hemostasis, the dura is closed using a 5–0 prolene suture in running watertight fashion. A local fat autograft harvested from the subcutaneous area or the epifascial fat and dural sealant is utilized (Tisseel, Baxter, Deerfield, IL, USA) as well as a Duraform patch (Natus Medical Inc, Pleasanton, CA, USA). The layers are then closed in standard fashion using 0-vicryl for the muscle and fascial layers, 3–0 vicryl for a deep dermal layer, 3–0 monocryl in a subcuticular running technique for the skin, and occlusive Dermabond dressing with Prineo overlay (Dermabond Prineo®, Ethicon Inc., Raritan, USA).
Indications
Case vignette
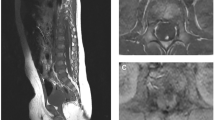
A 5-year-old female presents with progressive low back pain, left leg pain, and urinary retention. On examination, her strength was intact, but she was noted to have increased lower extremity reflexes and tone in her left leg indicative of leg spasticity. Magnetic resonance imaging (MRI) of the entire spine did not reveal any pathologies other than a thickened filum terminale and a conus-filum transition zone at the mid to low L2 vertebrae consistent with radiographic evidence of tethered cord in the setting of spina bifida occulta (Figs. 5a, b). Given the above symptom triad suggestive of tethered cord syndrome, she is considered a candidate for surgical intervention and underwent an L1-2 laminectomy for microsurgical resection of the filum. She remained flat for 24 h post-operatively and worked with physical therapy afterwards and was discharged home on post-operative day 3. At 6-month-follow up, her pain was completely resolved, and she is back to normal physical functioning. Her urinary retention issues had also resolved. An MRI at this point showed appropriate post-surgical changes from detethering with a regular appearance of the cauda equina and no irregularities in the surgical level (Figs. 5c, d).
a, b Pre-operative lumbar MRI with identification of conus-filum terminale transition zone (arrow). T2-weighted sagittal and axial cuts are shown with cut lines. c, d 6-month post-operative lumbar MRI showing detethered cord status post filum resection, with no evidence of cauda equina tethering or arachnoiditis
Discussion
The proposed microsurgical technique aims to minimize remnants of the filum terminale. Given the fatty as well as the fibroconnective nature of the pathological filum, it has the potential to adhere to the dura and/or the arachnoid layer involving cauda equina nerve roots causing re-tethering including cauda equina tethering [11]. The rostral and caudal end of the filum once sectioned have this potential. As such, we see the rationale of removing the entire distal filum.
Limitations
We demonstrate that this technique is safe without any hemorrhagic complications from the distal release, which may potentially result in arachnoiditis. This is ruled out in the post-op MRI in our case example. However, apprehension may face the surgeon regarding resection of the filum especially when thin nerve roots are incorporated and tethered to the filum. However, these are often redundant or vestigial nerves which may be sacrificed without any effects on neurological function [7].
Finally, one can also speculate that a shorter filum stump reduces any biomechanical stretch forces that might still be present as compared to when the full length pathological filum remnant is attached to the conus [2, 8]. It should be mentioned that the sectioning of the filum terminale anatomically at level of the conus has historically been performed and implemented by William J. Gardner as the terminal ventriculostomy in the treatment of syringomyelia [4].
How to avoid complications
To avoid any stimulating elements that might cause inflammation to the arachnoid and cauda equina resulting in arachnoiditis, care must be taken to ensure hemostasis is satisfactory as well as deep anesthesia prior to opening of the dura to avoid any potential unintended Valsalva events during the intradural portion of the procedure. In this regard, the use of a diamond burr drill minimizes bleeding from the bone while performing the laminotomy. The use of a subcutaneous autologous fat graft with the dural sealant which covers the entire dural incision minimizes CSF leak. Autologous fat has a long tradition in the management of complex CSF leaks in skull base and spine surgery [3].
Specific perioperative considerations
The patient is placed on bed rest for 24 h post-operatively to minimize the risk of CSF leak. A Foley is placed intraoperatively and remains in place while the patient is flat for comfort.
Specific information to give to the patient about surgery and potential risks
CSF leaks and adhesive arachnoiditis, despite reported rates ranging from 0.6 to 6% according to the most recent literature, are not trivial complications. A CSF leak may encourage cauda equina tethering to the surgical site, and repeated surgical correction is needed with the potential of cauda equina irritation and arachnoid adhesions and its long-term sequalae. Though rare, adhesive arachnoiditis and the resulting chronic pain syndrome defeat the purpose of the entire surgery.
Conclusions
Traditionally, sectioning of the filum through a laminectomy or interlaminar approach at the lumbosacral level is performed. A prospective study to compare the “high” vs. the “low” filum approach would be beneficial to compare the retethering rate and surgical outcomes between both techniques.
References
Sassack B, Carrier JD (2022) Anatomy, back, lumbar spine. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan–
De Vloo P, Monea AG, Sciot R, van Loon J, Van Calenbergh F (2016) The filum terminale: a cadaver study of anatomy, histology, and elastic properties. World Neurosurg 90:565-573.e1
Gao X, Du P, Xu J, Sun J, Ding W, Yang D-L (2022) Repair of cerebrospinal fluid leak during posterior thoracolumbar surgery using paraspinal muscle flap combined with fat graft. Front Surg 9:969954
Gardner WJ, Bell HS, Poolos PN, Dohn DF, Steinberg M (1977) Terminal ventriculostomy for syringomyelia. J Neurosurg 46(5):609–617
Hayashi T, Kimiwada T, Kohama M, Shirane R, Tominaga T (2018) Minimally invasive surgical approach to filum lipoma. Neurol Med Chir (Tokyo) 58(3):132–137
Kestle JRW (2012) Retethering after filum cutting. World Neurosurg 77(1):82
Klinge PM, McElroy A, Leary OP, Donahue JE, Mumford A, Brinker T, Gokaslan ZL (2022) Not just an anchor: the human filum terminale contains stretch sensitive and nociceptive nerve endings and responds to electrical stimulation with paraspinal muscle activation. Neurosurgery 91(4):618–624
Klinge PM, Srivastava V, McElroy A, Leary OP, Ahmed Z, Donahue JE, Brinker T, De Vloo P, Gokaslan ZL (2022) Diseased filum terminale as a cause of tethered cord syndrome in Ehlers-Danlos syndrome: histopathology, biomechanics, clinical presentation, and outcome of filum excision. World Neurosurg 162:e492–e502
Romagna A, Suchorska B, Schwartz C, Tonn J-C, Zausinger S (2013) Detethering of a congenital tethered cord in adult patients: an outcome analysis. Acta Neurochir (Wien) 155(5):793–800
Vassilyadi M, Tataryn Z, Merziotis M (2012) Retethering in children after sectioning of the filum terminale. Pediatr Neurosurg 48(6):335–341
Yong RL, Habrock-Bach T, Vaughan M, Kestle JR, Steinbok P (2011) Symptomatic retethering of the spinal cord after section of a tight filum terminale. Neurosurgery 68(6):1594–601 (discussion 1601-1602)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This article did not require IRB approval.
Informed consent
Due to its retrospective and deidentified nature, no patient identifiers were included.
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
10 Key Points
1. Tethered cord release classically involves sectioning the filum.
2. Retethering and adhesive arachnoiditis are significant concerns post-operatively.
3. Approaching the filum closer to the conus minimizes the remnant filum.
4. Resection of the filum can be done safely by detaching it from intradural attachments.
5. Positioning the proximal filum stump within the cauda nerve roots limits retethering.
6. Meticulous hemostasis is critical to avoid developing arachnoiditis.
7. Use of a diamond burr drill and monopolar electrocautery for the bone edges helps with hemostasis.
8. A local autologous fat graft technique is demonstrated which limits CSF leak rates.
9. Follow-up MRI at 6 months reveals no signs of retethering or arachnoiditis.
10. A limited interlaminar approach and small dural opening are sufficient to achieve the goals of the surgery.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 178633 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdulrazeq, H., Shao, B., Sastry, R.A. et al. Microsurgical approach for resection of the filum terminale internum in tethered cord syndrome—a case demonstration of technical nuances and vignettes. Acta Neurochir 165, 3505–3509 (2023). https://doi.org/10.1007/s00701-023-05568-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-023-05568-9