Abstract
Purpose
Selective amygdalohippocampectomy (SelAH) is one of the most common surgical treatments for mesial temporal sclerosis. Microsurgical approaches are associated with the risk of cognitive and visual deficits due to damage to the cortex and white matter (WM) pathways. Our objective is to test the feasibility of an endoscopic approach through the anterior middle temporal gyrus (aMTG) to perform a SelAH.
Methods
Virtual simulation with MRI scans of ten patients (20 hemispheres) was used to identify the endoscopic trajectory through the aMTG.
A cadaveric study was performed on 22 specimens using a temporal craniotomy. The anterior part of the temporal horn was accessed using a tubular retractor through the aMTG after performing a 1.5 cm corticectomy at 1.5 cm posterior to the temporal pole. Then, an endoscope was introduced. SeIAH was performed in each specimen. The specimens underwent neuronavigation-assisted endoscopic SeIAH to confirm our surgical trajectory.
WM dissection using Klingler’s technique was performed on five specimens to assess WM integrity.
Results
This approach allowed the identification of collateral eminence, lateral ventricular sulcus, choroid plexus, inferior choroidal point, amygdala, hippocampus, and fimbria. SelAH was successfully performed on all specimens, and CT neuronavigation confirmed the planned trajectory. WM dissection confirmed the integrity of language pathways and optic radiations.
Conclusions
Endoscopic SelAH through the aMTG can be successfully performed with a corticectomy of 15 mm, presenting a reduced risk of vascular injury and damage to WM pathways. This could potentially help to reduce cognitive and visual deficits associated with SelAH.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mesial temporal lobe sclerosis is the most common pharmacoresistant disorder observed in epilepsy surgeries, accounting for 17–31% of surgical procedures for epilepsy [21, 32, 33] (Fig. 1). Anterior temporal lobectomy (ATL), developed by Falconer and Taylor in 1968 [14] and selective amygdalohippocampectomy (SelAH), introduced by Niemeyer in 1958 [29, 31] have been widely developed with similar results on seizures control [21].
Surgical approaches for SelAH can be classified into anterior (transsylvian), lateral (transcortical and subtemporal) [20], and posterior (posterior interhemispheric[37] and paramedian supracerebellar infratentorial) [38] techniques.
Described open surgical approaches are associated with risk of cognitive and language deficits on the dominant hemisphere (around 5% of patients remain with memory deficit and 2.5% with language deficit) [5, 8], and visual deficits (87% in the transsylvian approach, 54% in the temporobasal approach) [10]. The majority of cognitive deficits are caused by the resection of the mesial structures themselves. However, language and visual deficits, as well as a part of cognitive deficits, are due to cortical and white matter (WM) damage, as well as the injury of vascular structures in the transsylvian approach [2], or venous infarction due to excessive retraction of the vein of Labbé in the subtemporal approach [35] (it has been proved that 3 to 9% of patients suffer significant postoperative venous infarction due to vascular manipulation) [2, 40].
Two endoscopic approaches have been studied for SelAH on cadavers: a posterior occipital approach developed by Bahuleyan [2] and an anterior transorbital approach described by Chen [8]. These approaches have long working distances from the entry point into the cortex until the target for the SelAh.
In this study, we aim to describe and analyze an alternative approach to perform a SelAh that features minimal cortical and subcortical disruption, and that through anatomo-functional rationale, it could improve patients’ visual and cognitive outcomes. Through virtual and cadaver surgical simulations, we will evaluate the anatomic feasibility of an endoscopic approach that allows us to enter through the anterior portion of the middle temporal gyrus (aMTG) for a SelAh.
Methods
In order to analyze the feasibility of the approach applied to our clinical purposes, we designed a three steps study based on (1) anatomical planning through radiological assessment, (2) surgical simulation through cadaveric dissection, and (3) assessment of WM disruption through WM dissection and analysis. Below, we describe the particularities of each step.
-
1.
Anatomical planning through radiological assessment
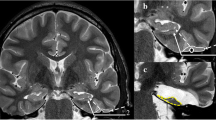
MRI scans of ten patients without evidence of intracranial pathology (n = 20 hemispheres) were used to identify the best endoscopic trajectory to enter the temporal horn of the lateral ventricle anteriorly and inferiorly.
Tractography was performed using two regions of interest and diffusions tensor imaging (DTI), with an arbitrary fractional anisotropy of 0.15 [6, 7]. The WM tracts reconstructed are the long segment of the arcuate fasciculus (L-Arc), the inferior longitudinal fasciculus (ILF), the uncinate fasciculus (UF), the inferior frontooccipital fasciculus (IFOF), the Meyer’s loop, and the optic radiations (OR).
The trajectory was defined in each hemisphere, taking into consideration the related vessels and WM tracts involved.
Virtual surgical planning was performed with the Iplannet® software (BrainLab AG, Munich, Germany) for cranial neuronavigation.
-
2.
Surgical simulation through cadaveric dissection
-
3.
Specimen preparation
Eighteen specimens were prepared using our embalmed customized formula [4], and five specimens were fixed in a 10% formalin solution and were subsequently prepared according to Klingler’s method [22].
CT scans of all the specimens were obtained for surgical planning and intraoperative neuronavigation. Surgical simulation was performed on 12 specimens for experimental study.
Each specimen was positioned in a 3-pin head clamp (Mizuho Surgical Freedom Clamp®, Mizuho Medical Co. Ltd, Tokyo, Japan) in a supine neutral position.
Specimens were registered with neuronavigation (Inav3, Stryker, Kalamazoo, USA). The surgical trajectory was planned using the landmarks obtained in the radiological images.
-
b.
Cadaveric surgical simulation
A curvilinear skin incision was made, and the layers of the scalp were dissected using a subfascial dissection to expose the zygomatic arch and the lateral wall of the orbit. Then, a transzygomatic approach with extension to the inferior lateral wall of the orbit (below the frontozygomatic suture), followed by a temporal craniotomy was performed, as it is has been previously described in our anterior MTG approach [25]. The sphenoid wing was drilled to get a good exposure of the most anterior part of the temporal lobe, and the dura was open. Then, a 15 mm corticectomy starting 15 mm posterior to the temporal pole was done.
With the aid of neuronavigation, we reached the ventricle using a needle. Then, a distal (size 12 × 50 mm) tubular retractor (VBAS, Vycor Medical, Boca Raton, USA) was used to provide a working corridor that helped to protect the surrounding parenchyma.
The 15-mm-long corticectomy started at 15 mm from the temporal pole in order to avoid Meyer’s loop and the OR, taking into consideration that the average distance from the temporal pole to the anterior edge of the optic radiations is 31.4 mm [9, 15].
Once the ventricle was cannulated, a 12-mm diameter tubular retractor was introduced to facilitate visualization and maneuverability during the intraventricular stage of the procedure. Upon fixation of the retractor, a 30° lens endoscope (1488 HD System, Stryker, Kalamazoo, USA) was introduced to the temporal horn. After accurately identifying the main structures within the ipsilateral temporal horn, we measured and assessed cortical landmarks to guide the entry into the ventricle. In addition, the transparenchymal distance and the trajectory lengths to intraventricular landmarks were measured (Fig. 2A, B).
A Endoscopic intraventricular image after performing our anterior endoscopic approach on the right temporal horn using a 30° lens, showing the head of the hippocampus HH, head of the hippocampus; CP, choroid plexus; LWV, lateral wall of the ventricle. B Endoscopic intraventricular image after performing our anterior endoscopic approach on the right temporal horn using a 30° lens, showing the body of the hippocampus. BH, body of the hippocampus; CP, choroid plexus; LWV, lateral wall of the ventricle; CE, collateral eminence; CG, choroid glomus; LVS, lateral ventricular sulcus
The surgical trajectory planned with the aid of neuronavigation was confirmed during the surgical simulation. An endoscopic entry point was observed and related to anatomical landmarks, such as the lateral surface of the temporal lobe and the temporal pole. Subsequently, an endoscopic SeIAH was performed in each specimen using an ultrasonic aspirator (Sonopet, Stryker, Kalamazoo, USA) with low values (US Power 5%, suction 10%, irrigation 5 ml/min) in order to preserve the arachnoid of the interpeduncular cistern (containing structures such as the oculomotor nerve and the first segment of the posterior cerebral artery) and the arachnoid of the crural cistern (containing the anterior second segment of the posterior cerebral artery) as described for open approaches [35, 39].
A SelAH was performed, using an anterior transtemporal endoscopic approach through the aMTG (ATE-SelAH), removing the hippocampus (body, head, and tail), parahippocampal gyrus, and amygdala. Resection ended posteriorly at the level of the quadrigeminal cistern, medially at the arachnoid of the ambient cistern, and anteriorly at the uncus (Fig. 3). Once these landmarks were exposed, the complete SelAH was considered to be achieved [23, 35, 39] and confirmed with the aid of neuronavigation.
-
3.
Assessment of WM disruption through WM dissection and analysis
Intraventricular image after performing the ATE-SelAH on a right temporal horn. The arachnoid of the ambient cistern has been removed for a good exposure of the main structures CP, choroid plexus; BVR, basal vein of Rosenthal; HV, hippocampal vessels; M, midbrain; P2A, P2A segment of the posterior cerebral artery; ET, edge of the tentorium; ITA, inferior temporal arteries; white asterisk: quadrigeminal cistern; white dot: crural cistern
WM dissection was performed on five specimens prepared with Klingler’s method after surgical simulation of the ATE-SelAh. All dissected specimens underwent a preoperative CT scan for navigation. We wanted to observe our endoscopic trajectory and examine WM integrity and transgression of long ventral and dorsolateral surface association fibers. The following WM tracts were identified during dissection, and their relationship to the surgical corridor created was analyzed.
The arcuate fasciculus (AF) connects frontal, parietal, and temporal regions around the Sylvian fissure. We studied the long segment of the arcuate fasciculus (L-Arc), which runs from the posterior part of the middle temporal gyrus to the frontal operculum [28]. It is associated with lexical and semantic language processing [41].
We identified the inferior longitudinal fasciculus (ILF) within the inferior temporal gyrus that connects the temporal pole to the dorsolateral occipital cortex [41]. It has been suggested that the ILF is involved in object identification and recognition [15].
The uncinate fasciculus (UF) connects the inferior frontal gyrus with the temporal pole forming a hook shape, more profound to the inferior half of the anterior limiting sulcus, and it is considered to be part of the ventral limbic pathway [27, 41].
The inferior frontooccipital fasciculus (IFOF), a frontooccipital association ventral semantic fiber pathway that connects the inferior and middle frontal gyrus to the parietal and occipital lobes [26, 41], was studied as it passes through the insula and temporal isthmus [41].
The anterior band of the optic radiations (OR) was also studied, which courses in an anterolateral direction and reach anterior as the tip of the temporal horn of the lateral ventricle [41]. At this point, the optic radiations turn backward in Meyer’s loop.
At this level, a subcortical window is observed with the following boundaries: the posterior margin at a superficial layer is the termination of the L-Arc at the middle temporal gyrus. At a deep layer, the posterior margin is the anterior part of Meyer’s loop and inferior border of the IFOF. The anterior margin is the posterior border of the UF, and the inferior margin is the ILF [3, 17, 41].
Results
We successfully performed a SelAH using the described approach. For both virtual and cadaveric simulation, the ATE-SelAH demonstrated to be feasible in all cases. Also, we were able to identify the subcortical tracts in the vicinity of our entry point in our five WM dissected specimens. We identified the main landmarks necessary to perform SelAH using the proposed approach. Identification of the amygdala, hippocampus (head, body, and tail), inferior choroidal point, choroid plexus, fimbria, crus fornix, lateral ventricular sulcus, and collateral eminence was possible in all specimens.
CT neuronavigation confirmed this trajectory
The SelAH, including resection of the hippocampus (body, head, and tail), amygdala, and parahippocampal gyrus was done. The anterior limit of the resection was the uncus, the medial limit was the arachnoid of the ambient cistern, and the posterior limit was at the level of the quadrigeminal cistern.
Table 1 shows the relevant landmarks and measurements of the ATE-SelAH.
The distance from the endoscope entry point into the ventricle to the inferior choroidal point was 10 (10.5–8) mm. The length of resection from anterior to posterior was 55 (57.75–54) mm.
Our entry point into the ventricle through the aMTG to reach the amygdala was located between the UF and anterior to the Meyer’s loop, optic radiations, and IFOF. Our approach went through a subcortical window located in the aMTG and formed anteriorly by the posterior part of the UF, posteriorly by the L-Arc, Meyer’s loop, and the IFOF, and inferiorly by the ILF (Fig. 4).
WM dissection on neuronavigated specimens confirmed the integrity of Meyer’s loop, UF, L-Arc, the IFOF, and ILF using our approach (Fig. 5).
Picture showing the entry point of the endoscope into the ventricular system through the subcortical window. The subcortical window (yellow) is located in the anterior middle temporal gyrus, formed anteriorly by the posterior part of the UF (blue), posteriorly by Meyer’s loop, optic radiations (red) and IFOF, posteriorly and superiorly by the L-Arc and inferiorly by the ILF. Our trajectory was confirmed by neuronavigation UF, uncinate fasciculus; ML, Meyer’s loop; OR, optic radiations; AC, anterior commissure
Discussion
Anatomo-functional impact of the temporal cortex and the temporal stem
We present a novel technique to perform SelAH with minimal disruption to eloquent WM tracts. We assessed the feasibility of an endoscopic SelAH through the most anterior part of the middle temporal gyrus, thereby minimizing the risk of damage to the anterior bundle of optic radiations and Meyer’s loop, L-Arc, IFOF, ILF, and UF. This approach is possible with a 15 mm corticectomy, which is smaller than the corticectomy usually required in open approaches (around 20–30 mm) [5, 35, 39]. Furthermore, the anterior location of the corticectomy allowed us to achieve the mesial structures without transgression of the temporal stem. This anterior and smaller incision could be especially important in the dominant hemisphere.
In the dominant hemisphere, eloquent cortex location has high interindividual variability. However, we can take into consideration that the aMTG is considered a place with a low probability of eloquence. The studied approach offers a potentially safer corticectomy step, as passes through the anterior part of the middle temporal gyrus avoiding transecting eloquent cortex [11, 24, 30]. Nevertheless, it is essential to remember that cortical eloquence is, in essence, an expression of subcortical connectivity.
We followed an anatomo-functional rationale to plan the entry point and surgical corridor following the three-dimensional location of WM pathways, which could play an essential role in language and optic radiations and could be preserved with our approach. With our endoscopic SelAH, we avoid damage to the UF, Meyer’s loop, OR, L-Arc, IFOF, and ILF.
The resection length from anterior to posterior was 55 (57.75–54) mm, resection that includes the uncus and the hippocampus, based on other studies. Schmeiser’s retrospective study measured the extent of hippocampal resection achieved using the SelAH via the transsylvian approach. The resection on postoperative MRI of 70 cases treated in the Epilepsy Center Freiburg had a length ranging from 2.8 to 4.5 cm [36].
On the other hand, the lateral mesencephalic sulcus is also used as a guiding landmark at the end of the resection of a SelAH [31]. On average, the lateral mesencephalic sulcus is located 5.02 ± 0.2 cm in the anteroposterior axis from the temporal pole [39]. These studies support that the length of the resection from anterior to posterior in the axial axis achieved by our technique includes the complete extension of the amygdala and the hippocampus.
The Meyer’s loop damage often determines the appearance of homonymous superior quadrantanopsia that occurs in around 70–80% of cases of anterior temporal lobectomy [1, 19]. The rate of visual field deficit in a transcortical selective amygdalohippocampectomy was found to be quite similar, around 70% [1].
Because our approach is based on a corticectomy that is shorter and more anterior than the conventional microsurgical procedures, it would be expected that Meyer’s loop and the anterior bundle of the optic radiations would suffer less direct or indirect damage, carrying a lower risk of postoperative visual field deficit.
Our approach goes through a subcortical window between the posterior part of the UF, Meyer’s loop, and the anterior bundle of optic radiations, L-Arc, and IFOF. Taking into consideration the location of our entry point, from DTI data and white matter dissection, we can consider that L-Arc and IFOF are preserved, as the L-Arc is more posterior and superficial and the IFOF is more posterior to our entry point. We use this subcortical window as a safe corridor to perform our approach. As we have said, this more anterior and smaller corticectomy could provide us a safer corridor to minimize the risk of the transgression of Meyer’s loop and the anterior bundle of the optic radiations on both hemispheres, also trying to preserve the integrity of language WM pathways on the dominant hemisphere.
As previously mentioned, the potential decreased risk of damaging the temporal cortex and temporal stem pathways with the ATE-SelAH could be a critical point that may be of paramount importance for the quality of life in epileptic patients, as not only mesial structures contribute to recall and recognition but also the lateral temporal cortex and temporal stem fibers [18].
Anatomical study of surgical techniques for SelAH
With the ATE-SelAH, we suggest that there is less risk of damage to the Sylvian vasculature than with the anterior SelAH open approach (transsylvian approach) [1, 31].
Furthermore, in the transsylvian approach, it is needed to perform a corticectomy through the temporal stem. It has been shown that a 20-mm incision starting at the limen of the insula and extending backward would cross the UF, the anterior fibers of the IFOF and the anterior bundle of the optic radiations, carrying a risk of visual field deficit in either hemisphere or damage to language pathways in the dominant hemisphere [34].
Taking into consideration the lateral transcortical open approaches for SelAH, we believe that the ATE-SelAH offers a decreased risk of damaging language and visual tracts. The lateral transcortical procedures involve performing an incision along the superior border of the middle temporal gyrus and subpial extension of this line along the superior temporal sulcus. This means, by definition, crossing through the temporal white matter pathways to reach the temporal horn of the lateral ventricle [31].
The risk of damage to the vein of Labbé is one of the potential complications in the subtemporal approach due to cortical retraction [35]. Therefore, brain ischemia, and edema have been demonstrated after surgical retraction. Postoperative magnetic resonance cranial studies after SelAH have shown Wallerian degeneration of axons in the anterior temporal lobe and the optic radiations due to subtemporal retraction [2]. In order to preserve the temporal veins, the drainage pathways of the superficial Sylvian vein and bridging veins, including the vein of Labbé, should be preoperative carefully evaluated. The new proposed approach could be considered whether the study of the temporal veins shows unfavorable conditions.
On the other hand, in the endoscopic posterior occipital approach [2], the authors had difficulties reaching the mesial aspect of the amygdala and the uncus, which requires an additional lateral burr hole to overcome the lack of medial exposure [2]. Besides, from this posterior approach, the choroid plexus obscured the correct visualization of the hippocampus, where it turned superomedially [2]. The endoscopic anterior transorbital approach [8] has the risk of potential injury to intraorbital neurovascular structures and postoperative diplopia. Furthermore, the ocular globe retraction that can be tolerated by patients is estimated in less than the median of 6 mm that they used in their study (usually diplopia in this kind of procedure is 19% with less retraction) [8].
Study limitations
There is some risk of the transgression of the OR at the level of the entry point of the endoscope into the ventricle due to interindividual variability (the anterior edge of Meyer’s loop from the pole of the temporal lobe has been ranged between 28 and 34 mm, average 31.4 mm)[9]. Subsequently, it is possible that some patients could experience a visual field deficit with the clinical application of this approach.
There is also some potential risk of disrupting the most posterior part of the UF.
The UF is considered to be part of the ventral limbic pathway [41]. However, this implicates little clinic repercussions based on previous studies of the UF function [12]. Albeit, it is also damaged on the transsylvian [1], and transmiddle temporal gyrus approaches [5], both with limited reported deficits.
To minimize these risks, we recommend performing a diffusion tensor imaging MRI with tractography reconstruction of language pathways and optic radiations. Although there is a partial depiction of white matter pathways with DTI techniques, they are a tool that can help to individualize the trajectory in each patient using our main landmarks prior to surgery.
This is a surgical simulation study on fixed cadaveric specimens to prove anatomical feasibility. Therefore, we cannot take into consideration the cerebrospinal fluid and blood dynamic, as well as the use of anesthetics and physiological modifiers.
To study the feasibility of this approach, we have worked with fixed cadaveric specimens that do not have the real brain retractability. As a result, we needed to perform the modified orbitozygomatic craniotomy in order to achieve all the angles of work needed to perform the endoscopic SelAH, especially at the posterior limit.
We suggest that, in a real patient, our proposed approach to perform a SelAh, with a corticectomy more anterior and shorter than the one used in open approaches, as well as the working corridor through the described surgical window, could be done without removing the zygoma and the lateral wall of the orbit. As it has been previously published, a pterional craniotomy with an interfascial temporalis dissection, followed by reflection of the temporalis muscle posteriorly and inferiorly, increases the exposure of the anterior temporal lobe and the anterioposterior-lateral angle of work [13, 16]. Therefore, we propose that working through our surgical window, a safe corridor anterior to Meyer’s loop and optic radiations, avoiding transgression to language WM pathways, could be done in real patients using a smaller craniotomy. Further studies would be necessary to confirm it.
As with all endoscopic approaches, bleeding control could present a challenge [2]. However, our approach is anterior, allowing for proximal control of the anterior choroidal artery and hippocampal vessels. Therefore, this provides a better alternative for the control achieved in posterior endoscopic approaches.
As this is a surgical simulation study, there are no reported clinical cases to assess the outcome of this approach. Therefore, outcome measures such as visual, cognitive, and language deficits, hospital stay, safety, morbidity, mortality, and control of seizures are currently lacking. Further clinical studies will be necessary to evaluate the clinical outcomes of our proposed approach.
This technique does not pretend to replace the conventional open approaches but to offer the possibility of a new approach that could be potentially beneficial to a selected group of patients. This is a surgical simulation study, so clinical aspects as safety and efficacy need further confirmation.
Conclusions
The ATE-SelAH through the anterior MTG can be successfully performed with an anterior corticectomy of 15 mm long, and potential decreased risk of damaging the temporal cortex and the temporal stem pathways. The anterior trajectory of our approach offers early access to the vasculature of the amygdala and hippocampus, which could help in early bleeding control.
The trajectory of the ATE-SelAH is performed through a subcortical window in the aMTG, with an entry point into the ventricle located posterior to the UF and anterior to the OR, Meyer’s loop, and IFOF. This could potentially reduce visual, language, and a part of cognitive deficits associated with the procedure, which is crucial in the dominant hemisphere. Further clinical and radiological studies will be necessary to assess the usefulness and limitations of the described technique.
References
Adada B (2008) Selective amygdalohippocampectomy via the transsylvian approach. Neurosurg Focus 25(3):E5. https://doi.org/10.3171/FOC/2008/25/9/E5
Bahuleyan B, Fisher W, Robinson S, Cohen AR (2013) Endoscopic transventricular selective amygdalohippocampectomy: cadaveric demonstration of a new operative approach. World Neurosurg 80:178–182. https://doi.org/10.1016/j.wneu.2012.10.005
Baydin S, Gungor A, Tanriover N, Baran O, Middlebrooks EH, Rhoton AL Jr (2016) Fiber tracts of the medial and inferior surfaces of the cerebrum. World Neurosurg. https://doi.org/10.1016/j.wneu.2016.05.016
Benet A, Rincon-Torroella J, Lawton MT, Gonzalez Sanchez JJ (2014) Novel embalming solution for neurosurgical simulation in cadavers. J Neurosurg 120:1229–1237. https://doi.org/10.3171/2014.1.jns131857
Bozkurt B, da Silva CR, Chaddad-Neto F, da Costa MD, Goiri MA, Karadag A, Tugcu B, Ovalioglu TC, Tanriover N, Kaya S, Yagmurlu K, Grande A (2016) Transcortical selective amygdalohippocampectomy technique through the middle temporal gyrus revisited: An anatomical study laboratory investigation. J Clin Neurosci 34:237–245. https://doi.org/10.1016/j.jocn.2016.05.035
Catani M, Howard RJ, Pajevic S, Jones DK (2002) Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage 17:77–94. https://doi.org/10.1006/nimg.2002.1136
Catani M, Thiebaut de Schotten M (2008) A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 44:1105–1132. https://doi.org/10.1016/j.cortex.2008.05.004
Chen HI, Bohman LE, Loevner LA, Lucas TH (2014) Transorbital endoscopic amygdalohippocampectomy: a feasibility investigation. J Neurosurg 120:1428–1436. https://doi.org/10.3171/2014.2.jns131060
Choi C, Rubino PA, Fernandez-Miranda JC, Abe H, Rhoton AL Jr (2006) Meyer’s loop and the optic radiations in the transsylvian approach to the mediobasal temporal lobe. Neurosurgery 59:228-ONS235. https://doi.org/10.1227/01.neu.0000223374.69144.81 (discussion ONS235–226)
Delev D, Wabbels B, Schramm J, Nelles M, Elger CE, von Lehe M, Clusmann H, Grote A (2016) Vision after trans-sylvian or temporobasal selective amygdalohippocampectomy: a prospective randomised trial. Acta Neurochir (Wien) 158:1757–1765. https://doi.org/10.1007/s00701-016-2860-y
Duffau H (2017) A two-level model of interindividual anatomo-functional variability of the brain and its implications for neurosurgery. Cortex 86:303–313. https://doi.org/10.1016/j.cortex.2015.12.009
Duffau H, Gatignol P, Mandonnet E, Peruzzi P, Tzourio-Mazoyer N, Capelle L (2005) New insights into the anatomo-functional connectivity of the semantic system: a study using cortico-subcortical electrostimulations. Brain 128:797–810. https://doi.org/10.1093/brain/awh423
Effendi ST, Momin EN, Basma J, Michael LM, Duckworth EAM (2020) The ultimate skull base maneuver does not involve removing bone: quantifying the benefits of the interfascial dissection. J Neurol Surg B Skull Base 81:62–67. https://doi.org/10.1055/s-0039-1679886
Falconer MA, Taylor DC (1968) Surgical treatment of drug-resistant epilepsy due to mesial temporal sclerosis. Etiology and significance Arch Neurol 19:353–361
Fernandez-Miranda JC, Rhoton AL Jr, Alvarez-Linera J, Kakizawa Y, Choi C, de Oliveira EP (2008) Three-dimensional microsurgical and tractographic anatomy of the white matter of the human brain. Neurosurgery 62:989–1026. https://doi.org/10.1227/01.neu.0000333767.05328.49 (discussion 1026–1028)
Goel A (1997) Splitting of temporalis muscle for basal exposure–technical note. Neurol Med Chir (Tokyo) 37:59–62. https://doi.org/10.2176/nmc.37.59
Goga C, Ture U (2015) The anatomy of Meyer’s loop revisited: changing the anatomical paradigm of the temporal loop based on evidence from fiber microdissection. J Neurosurg 122:1253–1262. https://doi.org/10.3171/2014.12.jns14281
Helmstaedter C, Grunwald T, Lehnertz K, Gleissner U, Elger CE (1997) Differential involvement of left temporolateral and temporomesial structures in verbal declarative learning and memory: evidence from temporal lobe epilepsy. Brain Cogn 35:110–131. https://doi.org/10.1006/brcg.1997.0930
Hervas-Navidad R, Altuzarra-Corral A, Lucena-Martin JA, Castaneda-Guerrero M, Vela-Yebra R, AlJC S (2002) Defects in the visual field in resective surgery for temporal lobe epilepsy. Rev Neurol 34:1025–1030
Hori T, Tabuchi S, Kurosaki M, Kondo S, Takenobu A, Watanabe T (1993) Subtemporal amygdalohippocampectomy for treating medically intractable temporal lobe epilepsy. Neurosurgery 33:50–56 (discussion 56–57)
Hu WH, Zhang C, Zhang K, Meng FG, Chen N, Zhang JG (2013) Selective amygdalohippocampectomy versus anterior temporal lobectomy in the management of mesial temporal lobe epilepsy: a meta-analysis of comparative studies. J Neurosurg 119:1089–1097. https://doi.org/10.3171/2013.8.jns121854
Klingler J (1935) Erleichterung der makroskopischen Präparation des Gehirn durch den Gefrierprozess. Schweiz Arch Neurol Psychiat 36:247–256
Kovanda TJ, Tubbs RS, Cohen-Gadol AA (2014) Transsylvian selective amygdalohippocampectomy for treatment of medial temporal lobe epilepsy: Surgical technique and operative nuances to avoid complications. Surg Neurol Int 5:133. https://doi.org/10.4103/2152-7806.140651
Krolak-Salmon P, Guenot M, Tiliket C, Isnard J, Sindou M, Mauguiere F, Vighetto A (2000) Anatomy of optic nerve radiations as assessed by static perimetry and MRI after tailored temporal lobectomy. Br J Ophthalmol 84:884–889
Lau R, Rodriguez Rubio R, Martino J, Sanmillan JL, Benet A, Tayebi Meybodi A, Gandhi S, Kournoutas I, Gabarros A (2019) Endoscopic transanterior middle temporal approach to the atrium-an anatomical feasibility study. World Neurosurg 128:e98–e106. https://doi.org/10.1016/j.wneu.2019.04.034
Martino J, Brogna C, Robles SG, Vergani F, Duffau H (2010) Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex 46:691–699. https://doi.org/10.1016/j.cortex.2009.07.015
Martino J, De Lucas EM (2014) Subcortical anatomy of the lateral association fascicles of the brain: A review. Clin Anat 27:563–569. https://doi.org/10.1002/ca.22321
Martino J, De Witt Hamer PC, Berger MS, Lawton MT, Arnold CM, de Lucas EM, Duffau H (2013) Analysis of the subcomponents and cortical terminations of the perisylvian superior longitudinal fasciculus: a fiber dissection and DTI tractography study. Brain Struct Funct 218:105–121. https://doi.org/10.1007/s00429-012-0386-5
Niemeyer P (1958) The transventricular amygdala-hippocampectomy in temporal lobe epilepsy. In: Baldwin M, Bailey P (eds) Temporal lobe epilepsy. Charles Thomas, Springfield, Mass, USA, pp 461–482
Ojemann G, Ojemann J, Lettich E, Berger M (2008) Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. 1989. J Neurosurg 108:411–421. https://doi.org/10.3171/JNS/2008/108/2/0411
Olivier A (2000) Transcortical selective amygdalohippocampectomy in temporal lobe epilepsy. Can J Neurol Sci 27(Suppl 1):S68-76. https://doi.org/10.1017/s031716710000069x (discussion S92–66)
Pasquier B, Peoc HM, Fabre-Bocquentin B, Bensaadi L, Pasquier D, Hoffmann D, Kahane P, Tassi L, Le Bas JF, Benabid AL (2002) Surgical pathology of drug-resistant partial epilepsy. A 10-year-experience with a series of 327 consecutive resections. Epileptic Disord 4:99–119
Piao YS, Lu DH, Chen L, Liu J, Wang W, Liu L, Yu T, Wang YP, Li YJ (2010) Neuropathological findings in intractable epilepsy: 435 Chinese cases. Brain Pathol 20:902–908. https://doi.org/10.1111/j.1750-3639.2010.00386.x
Ribas EC, Yagmurlu K, Wen HT, Rhoton AL Jr (2015) Microsurgical anatomy of the inferior limiting insular sulcus and the temporal stem. J Neurosurg 122:1263–1273. https://doi.org/10.3171/2014.10.JNS141194
Sajko T, Skoro I, Rotim K (2013) How I do it - selective amygdalohippocampectomy via subtemporal approach. Acta Neurochir (Wien) 155:2381–2387. https://doi.org/10.1007/s00701-013-1846-2
Schmeiser B, Wagner K, Schulze-Bonhage A, Elger CE, Steinhoff BJ, Wendling AS, Mader I, Prinz M, Scheiwe C, Zentner J (2017) Transsylvian selective amygdalohippocampectomy for mesiotemporal epilepsy: experience with 162 procedures. Neurosurgery 80:454–464. https://doi.org/10.1093/neuros/nyw089
Smith KA, Spetzler RF (1995) Supratentorial-infraoccipital approach for posteromedial temporal lobe lesions. J Neurosurg 82:940–944. https://doi.org/10.3171/jns.1995.82.6.0940
Ture U, Harput MV, Kaya AH, Baimedi P, Firat Z, Ture H, Bingol CA (2012) The paramedian supracerebellar-transtentorial approach to the entire length of the mediobasal temporal region: an anatomical and clinical study. Laboratory investigation J Neurosurg 116:773–791. https://doi.org/10.3171/2011.12.JNS11791
Wheatley BM (2008) Selective amygdalohippocampectomy: the trans-middle temporal gyrus approach. Neurosurg Focus 25:E4. https://doi.org/10.3171/FOC/2008/25/9/E4
Wise BL (1994) A review of brain retraction and recommendations for minimizing intraoperative brain injury. Neurosurgery 35:172–173. https://doi.org/10.1097/00006123-199407000-00034
Yagmurlu K, Vlasak AL, Rhoton AL Jr (2015) Three-dimensional topographic fiber tract anatomy of the cerebrum. Neurosurgery 11(Suppl 2):274–305. https://doi.org/10.1227/neu.0000000000000704 (discussion 305)
Acknowledgements
We want to express our gratitude to the generosity of the body donors and their families, who helped to make possible this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
This project was done with the approval of the Ethics Committee of both institutions, University of California San Francisco and Bellvitge University Hospital.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurosurgical Anatomy
Rights and permissions
About this article
Cite this article
Lau, R., Gabarros, A., Martino, J. et al. Anterior transtemporal endoscopic selective amygdalohippocampectomy: a virtual and cadaveric feasibility study. Acta Neurochir 164, 2841–2849 (2022). https://doi.org/10.1007/s00701-022-05295-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-022-05295-7