Abstract
Background
Aneurysmal subarachnoid hemorrhage (aSAH) patients admitted to primary stroke centers are often transferred to neurosurgical and endovascular services at tertiary centers. The effect on microsurgical outcomes of the resultant delay in treatment is unknown. We evaluated microsurgical aSAH treatment > 72 h after the ictus.
Methods
All aSAH patients treated at a single tertiary center between August 1, 2007, and July 31, 2019, were retrospectively reviewed. The additional inclusion criterion was the availability of treatment data relative to time of bleed. Patients were grouped based on bleed-to-treatment time as having acute treatment (on or before postbleed day [PBD] 3) or delayed treatment (on or after PBD 4). Propensity adjustments were used to correct for statistically significant confounding covariables.
Results
Among 956 aSAH patients, 92 (10%) received delayed surgical treatment (delayed group), and 864 (90%) received acute endovascular or surgical treatment (acute group). Reruptures occurred in 3% (26/864) of the acute group and 1% (1/92) of the delayed group (p = 0.51). After propensity adjustments, the odds of residual aneurysm (OR = 0.09; 95% CI = 0.04–0.17; p < 0.001) or retreatment (OR = 0.14; 95% CI = 0.06–0.29; p < 0.001) was significantly lower among the delayed group. The OR was 0.50 for rerupture, after propensity adjustments, in the delayed setting (p = 0.03). Mean Glasgow Coma Scale scores at admission in the acute and delayed groups were 11.5 and 13.2, respectively (p < 0.001).
Conclusions
Delayed microsurgical management of aSAH, if required for definitive treatment, appeared to be noninferior with respect to retreatment, residual, and rerupture events in our cohort after adjusting for initial disease severity and significant confounding variables.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Patients with aneurysmal subarachnoid hemorrhage (aSAH) are often admitted to primary stroke centers before being transferred to tertiary care centers or comprehensive stroke centers (CSCs) for neurosurgical or endovascular treatment. This triage is common and built into the stroke care system infrastructure. This triage process was developed for a more common pathology (i.e., ischemic stroke), and it does not take into account the added complexities of managing patients with ruptured aneurysms, who may require expertise beyond that offered at some CSCs. Although advanced endovascular capabilities are becoming more common, microsurgical expertise is increasingly centralized to tertiary or quaternary care centers [25]. When patients with ruptured aneurysms are transferred to specialized centers, they may experience brief delays in treatment due to delayed presentation, diagnostic angiography, transfer time, and attempted endovascular treatment [1, 21, 23]. Furthermore, more time may be required to plan advanced microsurgical management of complex cases, such as a cerebral bypass, than to treat “simple” aneurysms. The typical practice for patients with ruptured aneurysms is treatment within 72 h of presentation [8]. In this study, we evaluate the impact of microsurgically treating patients with ruptured aneurysms more than 72 h after their ictus to determine whether delays due to transferring patients to tertiary care centers have a negative effect on patient outcomes.
Methods
This retrospective cohort study was approved by the St. Joseph’s Hospital and Medical Center Institutional Review Board, Phoenix, Arizona. The requirement for informed consent was waived due to the low risk to patients and the retrospective nature of the study. All aSAH patients treated between August 1, 2007, and July 31, 2019, at a single tertiary care center were retrospectively reviewed using a prospectively maintained research database.
A second inclusion criterion was the availability of treatment data time relative to time of bleed (per patient or family history). Patients were grouped into 2 cohorts on the basis of bleed-to-treatment time: the acute-treatment group (acute group) comprised those treated on postbleed day (PBD) 3 or earlier (i.e., ≤ 72 h), and the delayed-treatment group (delayed group) comprised those treated on PBD 4 or later (> 72 h). Information analyzed included patient age and race, Hunt and Hess score, Fisher grade, admission Glasgow Coma Scale (GCS) score at patient presentation, and aneurysm treatment. The primary outcome was worsened modified Rankin Scale (mRS) score after treatment. Secondary outcomes included neurologic outcomes, with a poor neurologic outcome defined as an mRS score > 2.
A rerupture event was defined as a rupture occurring in the same vascular territory of the initial SAH either during the same hospital stay or as a reason for readmission after discharge. Retreatment was therefore defined as either rerupture or residual aneurysm as evident on follow-up imaging requiring an additional intervention, either through microsurgery or endovascular treatment. Computed tomography angiography is routinely performed immediately after microsurgical intervention. In addition, most patients undergo angiography for evaluation later in their hospital stay while still in the intensive care unit [7].
Patients were treated using either open or endovascular techniques, with the decision regarding the most appropriate procedure decided by a team consisting of neurosurgeons experienced in open procedures and neurosurgeons experienced in endovascular procedures. Alternating preference was given to microsurgery or endovascular treatment in cases of equipoise. Tranexamic acid was given to patients to protect against rerupture if treatment was to be delayed more than 24 h. Patients who were treated endovascularly underwent either magnetic resonance angiography or conventional angiography at 3 months, depending on the degree of concern at final treatment. There is no standardized algorithm for treating residual aneurysms; such aneurysms are discussed in multidisciplinary conference when identified. Vasospasm was defined as spasm of the vessel in the same territory of the lesion as evident on angiography.
Data were calculated as mean (standard deviation [SD]) or number and percentage. Statistical analyses performed included data aggregation, exploratory analysis, and multivariate analysis using R, version 4.0.1 (R Foundation for Statistical Computing). Demographic and clinical characteristics of patients were analyzed with an independent 2-sample t test for interval variables and a χ2 test for categorical variables. A p value of < 0.05 was defined as significant. Subsequently, propensity-adjusted regression modeling was used to compare the acute and delayed groups, controlling for variables found to be significant confounders to the primary outcome below a p threshold of 0.20.
Results
Treatment groups
Of the 956 aSAH patients treated during the 12-year study period, 92 (10%) were classified as having delayed treatment (≥ PBD 4), and 864 (90%) were classified as having acute treatment (≤ PBD 3). Patient, aneurysm, and treatment characteristics are shown in Table 1. The delayed group included 29 patients who had planned endovascular treatment but crossed over to open surgical treatment; thus, all 92 patients with delayed treatment underwent surgery. Patients in the acute group were treated both surgically (502/864, 58%) and endovascularly (362/864, 42%). A representative case example is presented in Fig. 1 [30].
Case example of a complex aneurysm treated in a delayed fashion. A woman in her late 40s presented with the worst headache of her life and was found to have diffuse aSAH. A Coronally oriented 3-dimensional reconstruction of computed tomography angiography (CTA) and B sagittal CTA showing the complex, bilobed aneurysm involving the efferent A2 segments. Given the complex fusiform nature of the aneurysm, it was decided that the patient required a bypass. She presented shortly after her aSAH, but for optimal scheduling purposes, surgery was performed on PBD 4. She underwent a right modified orbitozygomatic craniotomy with bifrontal craniotomy for interhemispheric approach and a right A3 to left A3 side-to-side continuous intraluminal bypass. C Intraoperative photograph of the R A3 (S–S*c) L A3 in situ bypass [30] followed by D trapping of the aneurysm. E Posteroanterior projection digital subtraction angiography (DSA) via the left internal carotid artery (ICA) shows filling of the bilateral distal ACA territories via the bypass. F Posteroanterior projection DSA via the right ICA showing successful exclusion of the aneurysm. The patient did well postoperatively, and was neurologically intact. She was discharged on postoperative day 10. Used with permission from Barrow Neurological Institute, Phoenix, Arizona
Female patients accounted for the majority in both treatment groups (acute: 601/864, 70%; delayed: 60/92, 65%; p = 0.46). The mean (SD) age of patients was similar in both treatment groups (acute: 56 [14] years; delayed: 55 [14] years; p = 0.70). The mean (SD) time from aSAH to surgery was significantly shorter in the acute group (0.94 [0.76] days) than the delayed group (8.65 [7.47] days) (p < 0.001). Aneurysm type varied significantly between the 2 groups, with saccular aneurysms being more prevalent in the acute group (695/864, 80%) than in the delayed group (58/92, 63%) and fusiform, dissecting, and blister aneurysms being more prevalent in the delayed group (32/92, 35%) than in the acute group (165/864, 19%) (p = 0.001). Mean (SD) aneurysm size also varied significantly between the acute and delayed groups (6.4 [3.7] vs. 5.7 [4.3] mm, p < 0.001). The mean (SD) GCS score at admission also differed significantly between the acute (11.5 [3.9]) and delayed (13.2 [3.2]) groups (p < 0.001). Fisher grades and Hunt and Hess scores at admission also differed significantly between treatment groups (p ≤ 0.002).
Outcomes
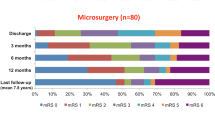
Cerebrovascular bypasses were performed in 6 cases in the delayed group, with 2 cases of partial occlusion or trapping. The mean (SD) length of follow-up was similar between the acute and delayed groups (732 [1201] vs. 794 [1238], p = 0.25). In the acute group, 26 of 864 (3%) patients had rerupture events, whereas in the delayed group, 1 of 92 (1%) had rerupture (p = 0.51). The percentage of patients requiring retreatment was 5% (41/864) for the acute group and 2% (2/92) for the delayed group (p = 0.42), whereas the percentage of patients with residual aneurysms was 8% (66/864) in the acute group and 2% (2/92) in the delayed group (p = 0.08) (Table 2).
After propensity adjustment for significantly confounding variables (race, GCS score at admission, Fisher grade, and aneurysm type), the odds of a poor outcome (defined as an mRS score > 2) were similar between groups (odds ratio [OR] = 0.96, 95% CI = 0.80–1.15, p = 0.70). After the propensity adjustment, the presence of a residual aneurysm (OR = 0.08, 95% CI = 0.03–0.17, p < 0.001) and retreatment frequency (OR = 0.13, 95% CI = 0.05–0.28, p < 0.001) were significantly less in the delayed setting (Table 3). The OR (95% CI) for rerupture, after propensity adjustment, in the delayed versus acute setting was 0.50 (0.26–0.93) (p = 0.03).
Delays in treatment
The reason for the delay was available for 79 of the 92 patients who received delayed treatment (Table 4); patients could have more than one reason for delayed treatment. The most common reason for delay was crossover from endovascular treatment to surgery or the need for angiography (34/79, 43%), which can occur if the anatomy cannot be discerned with initial computed tomography angiography. Another 38% (30/79) of treatments were delayed because the patient presented in a way that did not warrant immediate attention; therefore, the patient came to medical attention late. Only 3% (2/79) of these patients had treatment delays specifically due to a weekend presentation (late Friday through Sunday). Three of 79 (4%) had treatment delayed for necessary medical stabilization or treatment of preoperative complications, whereas 2 of 79 (3%) had treatment delays due to other reasons (initially negative findings on imaging and initially refusing treatment).
A transfer from an outside hospital delayed treatment in 20% (16/79) of patients in the delayed group, which suggests that patients may benefit from an earlier transfer to a major neurosurgical center instead of first presenting to an outside hospital and then waiting for a transfer to receive definitive care. Early treatment of patients transferred from an outside hospital was a standard practice at our institution during the study period. Of the 16 delayed group patients transferred from outside hospitals, 13 (81%) were treated within 24 h of arrival to our center, which was still on or after PBD 4. The mean (SD) mRS in this subgroup was 1.92 (1.50), and the mRS score was > 2 at last follow-up in 4 of 13 (31%). These 13 patients, who were not endovascularly or surgically treated for their aSAHs at an outside hospital but were treated surgically within 24 h of transfer to our center, experienced an outcome similar to our acute group in terms of final mRS score and mRS score > 2 (p = 0.17, p = 0.24, respectively). However, this subgroup had a significantly higher mean (SD) GCS score at admission relative to the acute group (13.9 [1.8] vs. 11.5 [3.9], respectively, p = 0.02). Vasospasm was experienced by 612 of 864 (71%) patients in the acute group and 66 of 92 (72%) patients in the delayed group.
Weekend effect analysis
Day of admission data were available for 955 of the 956 treated aSAH patients; 679 (71%) were admitted on a weekday (Monday–Friday afternoon), and 276 (29%) were admitted on a weekend (late Friday–Sunday). Mean (SD) time to treatment between weekday and weekend admission was not significantly different (0.79 [1.10] vs. 0.84 [1.24] days, p = 0.54). The proportion of patients with poor outcomes (mRS score > 2) was also not significantly different between weekday and weekend admissions (50% [338 of 679] vs. 51% [140 of 276], p = 0.79). Weekend admission was determined as the rationale for delayed treatment in only 2 cases.
Discussion
Several studies established a strong volume-outcome relationship with regard to managing aSAH [2, 25], including for microsurgical management [28]. Compared to acute ischemic stroke, the need for definitive treatment of ruptured aneurysms is measured on a scale of hours to days rather than minutes [14]. Furthermore, the treatment of some ruptured aneurysms, especially those not amenable to endovascular embolization, requires a level of microneurosurgical expertise that may not be available at all CSCs. For such patients, the question then arises of whether treatment delay is acceptable to facilitate treatment by an expert. The American Stroke Association has recommended that low-volume hospitals (e.g., < 10 aSAH cases per year) should consider early transfer of patients with aSAH to high-volume centers (e.g., > 35 aSAH cases per year) with an experienced team [8]. In a Japanese series assessing CSC outcomes among 27,490 patients, Kurogi et al. [20] found that, in the modern endovascular era, better surgical outcomes were achieved at facilities with “high” CSC capabilities. Therefore, obtaining definitive treatment for aSAH at a high-volume center may yield better patient outcomes despite the inherent delay of care associated with patient transfer. In the current study, we assess the outcomes of patients who underwent surgical treatment in a delayed fashion (≥ PBD 4) compared with those who underwent acute endovascular or surgical treatment between PBD 0 and PBD 3.
Historically, aneurysms were often treated surgically in a delayed fashion, after the period of peak vasospasm (PBD 11–14). In the International Cooperative Study on the Timing of Aneurysm Surgery, the challenges of mid-phase aneurysm surgery (PBD 3–11) were thought to be significant brain swelling and the potential risk of clinical worsening. In the early 1980s and 1990s, groups began to challenge this notion of delayed treatment, reporting similar outcomes with early surgical intervention of aSAH patients [16, 24]. It is now standard to treat aneurysms within 24 h after admission at most centers because of the potential of rehemorrhage [17]. Buscot et al. [4] retrospectively reviewed 482 cases of aSAH-treated endovascularly or microsurgically and found that early intervention had more favorable outcomes at discharge and at 12-month follow-up. Time to treatment was not associated with any complications, but favorable outcomes were associated with treatment within 12.5 h of symptom onset. Phillips et al. [26] had similar findings, reporting improved clinical outcomes of aSAH patients treated within 24 h of rupture. However, our findings indicate that, in some cases, it may be suitable to engage in a transfer process with inherent treatment delay to obtain definitive surgical management. Such delayed surgical treatment was uncommon and occurred in only 92 cases among 956 patients treated in this study cohort. The 2 most common reasons for delayed treatment (relative to time of hemorrhage) were crossover from endovascular to surgical treatment or time to angiography (34/79 patients, 43%) and late patient presentation after symptom onset (30 of 79 patients, 38%). There is a high risk of rebleeding within the first few hours of aSAH, which can deter treatment centers from conducting early catheter angiography, ultimately delaying definitive treatment [1, 21]. After propensity correction for race, GCS score at admission, Fisher grade, and aneurysm type, a significant difference was found in neurologic worsening or rerupture rates between the acute and delayed groups. The higher mean GCS score observed in the delayed group was most likely due to patients with more serious clinical presentations (i.e., coma) presenting earlier and necessitating earlier treatment. In fact, several factors were favorable in the delayed-surgery group, specifically significantly lower rates of residual aneurysm and the need for retreatment. Similarly, Gittins et al. [11] examined delayed treatment in poor-grade aSAH in a cohort of 111 patients and found that immediate and delayed treatment resulted in similar morbidity and mortality at 12-month follow-up.
Microsurgical treatment is thought to be increasing in complexity in the endovascular era, as the patients who crossover from the endovascular suite to the operating theater often have large aneurysms or aneurysms with other complex features. This phenomenon of the disappearing “simple posterior communicating artery aneurysm” was quantified in a study of 218 patients by Sanai et al., as more aneurysms are requiring anterior clinoidectomy, dissection of an adherent anterior choroidal artery, and other technical maneuvers [27]. In the experience of the senior author (M.T.L.), bypass was performed in approximately 5% of all aneurysm treatments (240/5000) [3]. A patient requiring a complex bypass for definitive treatment of a ruptured aneurysm may require transfer to a center with surgical expertise or may have delayed treatment for other reasons, as was found in 8 patients in this series. Aneurysms requiring bypass are usually not amenable to endovascular treatment without devices requiring antiplatelet treatment, and, as such, surgery becomes potentially a safer and necessary option. Occasionally, complex microsurgical treatment may require patients with aSAH to be medically stabilized first; this appears to be a reasonable compromise based on the results of our study. The treatment of aneurysms in patients in our recent cohort has also previously been described to be more complex in other ways; even the need for complex clip reconstruction or associated medical comorbidities may have supported the need for such a transfer [6].
Challenge with vasospasm and cerebral edema
The mean (SD) time from aSAH to surgery in this cohort was 2.07 (2.74) days. This timing encroaches upon the classic period of PBD 4–14 for peak vasospasm or delayed cerebral ischemia risk. However, our results did not find an increased risk of vasospasm in our cohort. Despite these findings, it remains our practice (in accordance with established guidelines) to attempt definitive aneurysm treatment as soon as feasible following clinical presentation and avoid operation within the PBD 4–14 time window.
Weekend effect and work flow in aSAH treatment
Our analysis of patients presenting on weekdays versus weekends yielded no difference in time to treatment or clinical outcome at last follow-up. Although our analysis is underpowered to assess the weekend effect, literature is conflicting on whether worse outcomes occur for patients admitted over the weekend who have a delay in scheduling treatment. One single-center retrospective review of 413 patients by Mikhail et al. [22] found the weekend effect to be an independent risk factor for death within 12 weeks following the initial hemorrhage, whereas another large multicenter review of 5667 cases found weekend presentation not to be a significant predictor of death [9]. A different multicenter retrospective review of 1482 cases by Kim and Jwa [19] found that patients with SAH with “off-hour” presentations to the hospital did not experience worse outcomes or higher mortality than those with “on-hour” presentation. Because of the variability in the capacity between institutions to definitively treat aSAHs, there is bias in comparing single-center experiences. However, the weekend effect may vary across institutions even when time from aSAH presentation to treatment does not vary significantly [10, 12, 15]. Morbidity due to the weekend effect should be evaluated, and the degree of clinical relevance should be taken into account. Because the main reasons for delayed surgery were endovascular crossover, delayed presentation, and delayed transfer, the time from aSAH to surgery can potentially be reduced with efforts to make transfers of care more efficient and increase symptom awareness.
At our center, the neurovascular on-call team consists of neurosurgeons who practice either open microsurgery or endovascular surgery. In a given month, 1 to 2 neurosurgeons who practice open surgery and 2 neurosurgeons who practice endovascular surgery cover calls. As a result, either type of neurosurgical procedure can be covered at all times, including advanced ones. The decision-making process is shared between open and endovascular surgeons, with alternating preference given to microsurgery or endovascular treatment in cases of equipoise, as was performed in the Barrow Ruptured Aneurysm Trial [29]. Patients undergoing treatment more than 24 h after transfer to our center are given tranexamic acid to protect against rerupture [13].
Although the regionalization of aSAH care to centers of excellence that have both microsurgical and neuroendovascular expertise was not directly evaluated in this study, our findings support this practice. Treatment of aSAH in a minimally delayed fashion, due to transfer or optimal scheduling issues, still yielded good outcomes. The delayed treatment of these aneurysms by surgery was definitive, with a lower frequency of residual aneurysms or rehemorrhages than in the acute group. These results support the notion that aneurysms need not be temporized by partial coiling or other such nondefinitive treatment before transfer to a center of excellence. Rather, it is appropriate for aneurysms requiring microsurgical expertise to be transferred to an experienced center, even if this transfer causes slight delays in treatment.
Limitations
Due to the retrospective nature of the study, limitations are inherent in the analysis. No analysis was performed on medical comorbidities or complications during the hospitalization. Additionally, it is impossible to know the actual timing of rupture. The determination of rupture time in our data was based on the patient's or family’s description of the ictus. Additionally, in lieu of having admission timing data and in-hospital delays, we used data for the recorded bleed day and treatment day. Patients who died of rerupture before they could be transferred or while in transport would not be captured in these data due to the inherent limitation of our database, which may lead to potential selection bias. The degree of this bias is difficult to ascertain, but is theoretically estimated by the proportion of patients who suffer early rerupture in other series (as high as 7% in a Japanese study of patients transferred from remote islands [18] and in a study of patients in a major US city [5]). In addition, a selection bias exists toward patients who have slightly better clinical grade who are considered appropriate surgical candidates, both by the treating team as well as by surrogate decision makers. Patients with delayed treatment in this series were biased toward positive outcomes by their better clinical grade. Thus, we state strongly that these conclusions should not support intentionally delayed treatment after presentation to a capable tertiary or quaternary center. Future research is needed to determine criteria for patient-specific factors that can guide physicians in their decision-making regarding the timing of surgery.
Conclusion
Delayed microsurgical management of ruptured aneurysms, if required for definitive treatment by an experienced neurosurgical team, appears to be noninferior with respect to retreatment, residual, and rerupture events. We found that the delay in treatment in navigating definitive microsurgical care for patients with ruptured aneurysms did not negatively affect outcomes, with the potential for improved long-term outcomes. This finding suggests that it may be appropriate and safe to delay treatment for complex ruptured aneurysms to facilitate the transfer of patients for treatment at high-volume centers.
Abbreviations
- aSAH:
-
Aneurysmal subarachnoid hemorrhage
- CI:
-
Confidence interval
- CSC:
-
Comprehensive stroke center
- GCS:
-
Glasgow Coma Scale
- mRS:
-
Modified Rankin Scale
- OR:
-
Odds ratio
- PBD:
-
Postbleed day
- SD:
-
Standard deviation
References
An H, Park J, Kang DH, Son W, Lee YS, Kwak Y, Ohk B (2019) Should cerebral angiography be avoided within three hours after subarachnoid hemorrhage? J Korean Neurosurg Soc 62:526–535
Berman MF, Solomon RA, Mayer SA, Johnston SC, Yung PP (2003) Impact of hospital-related factors on outcome after treatment of cerebral aneurysms. Stroke 34:2200–2207
Burkhardt JK, Lawton MT (2019) Practice trends in intracranial bypass surgery in a 21-year experience. World Neurosurg 125:e717–e722
Buscot MJ, Chandra RV, Maingard J, Nichols L, Blizzard L, Stirling C, Smith K, Lai L, Asadi H, Froelich J, Reeves MJ, Thani N, Thrift A, Gall S (2022) Association of onset-to-treatment time with discharge destination, mortality, and complications among patients with aneurysmal subarachnoid hemorrhage. JAMA Netw Open 5:e2144039. https://doi.org/10.1001/jamanetworkopen.2021.44039
Catalano AR, Winn HR, Gordon E, Frontera JA (2012) Impact of interhospital transfer on complications and outcome after intracranial hemorrhage. Neurocrit Care 17:324–333
Catapano JS, Srinivasan VM, Labib MA, Rumalla K, Nguyen CL, Rahmani R, Baranoski JF, Cole TS, Rutledge C, Jadhav AP, Ducruet AF, Albuquerque FC, Zabramski JM, Lawton MT (2022) The times they are a-changin’: increasing complexity of aneurysmal subarachnoid hemorrhages in patients treated from 2004 to 2018. World Neurosurg 161:e168–e173
Catapano JS, Srinivasan VM, Rumalla K, Labib MA, Nguyen CL, Cole TS, Baranoski JF, Rutledge C, Rahmani R, Lawton MT, Ducruet AF, Albuquerque FC (2022) Length of hospital stay in aneurysmal subarachnoid hemorrhage patients without vasospasm on angiography: potential for a fast-track discharge cohort. J Neurointerv Surg 14:376–379
Connolly ES Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, Hoh BL, Kirkness CJ, Naidech AM, Ogilvy CS, Patel AB, Thompson BG, Vespa P (2012) Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 43:1711–1737
Crowley RW, Yeoh HK, Stukenborg GJ, Ionescu AA, Kassell NF, Dumont AS (2009) Influence of weekend versus weekday hospital admission on mortality following subarachnoid hemorrhage. Clinical article. J Neurosurg 111:60–66
Deshmukh H, Hinkley M, Dulhanty L, Patel HC, Galea JP (2016) Effect of weekend admission on in-hospital mortality and functional outcomes for patients with acute subarachnoid haemorrhage (SAH). Acta Neurochir (Wien) 158:829–835
Gittins A, Talbott N, Gilani AA, Packer G, Browne R, Mullhi R, Khan Z, Whitehouse T, Belli A, Mehta RL, Gao-Smith F, Veenith T (2021) Outcomes following acute poor-grade aneurysmal subarachnoid bleed—is early definitive treatment better than delayed management? J Intensive Care Soc 22:198–203
Goertz L, Kabbasch C, Pflaeging M, Pennig L, Laukamp KR, Timmer M, Styczen H, Brinker G, Goldbrunner R, Krischek B (2021) Impact of the weekend effect on outcome after microsurgical clipping of ruptured intracranial aneurysms. Acta Neurochir (Wien) 163:783–791
Hillman J, Fridriksson S, Nilsson O, Yu Z, Saveland H, Jakobsson KE (2002) Immediate administration of tranexamic acid and reduced incidence of early rebleeding after aneurysmal subarachnoid hemorrhage: a prospective randomized study. J Neurosurg 97:771–778
Hopkins LN, Holmes DR Jr (2017) Public health urgency created by the success of mechanical thrombectomy studies in stroke. Circulation 135:1188–1190
Johnson WC, Morton-Gonzaba NA, Lacci JV, Godoy D, Mirahmadizadeh A, Seifi A (2019) Re-evaluating the weekend effect on SAH: a nationwide analysis of the association between mortality and weekend admission. Neurocrit Care 30:293–300
Kassell NF, Boarini DJ, Adams HP Jr, Sahs AL, Graf CJ, Torner JC, Gerk MK (1981) Overall management of ruptured aneurysm: comparison of early and late operation. Neurosurgery 9:120–128
Kassell NF, Torner JC (1983) Aneurysmal rebleeding: a preliminary report from the Cooperative Aneurysm Study. Neurosurgery 13:479–481
Kawahara I, Matsunaga Y, Tsutsumi K, Takahata H, Ono T, Toda K, Baba H (2014) Timing of helicopter transportation for patients presenting with subarachnoid hemorrhage on isolated islands. No Shinkei Geka 42:537–543
Kim T, Jwa C (2021) Impact of off-hour hospital presentation on mortality in different subtypes of acute stroke in Korea: National Emergency Department Information System Data. J Korean Neurosurg Soc 64:51–59
Kurogi R, Kada A, Ogasawara K, Kitazono T, Sakai N, Hashimoto Y, Shiokawa Y, Miyachi S, Matsumaru Y, Iwama T, Tominaga T, Onozuka D, Nishimura A, Arimura K, Kurogi A, Ren N, Hagihara A, Nakaoku Y, Arai H, Miyamoto S, Nishimura K, Iihara K (2020) Effects of case volume and comprehensive stroke center capabilities on patient outcomes of clipping and coiling for subarachnoid hemorrhage. J Neurosurg 134:929–939
Kusumi M, Yamada M, Kitahara T, Endo M, Kan S, Iida H, Sagiuchi T, Fujii K (2005) Rerupture of cerebral aneurysms during angiography—a retrospective study of 13 patients with subarachnoid hemorrhage. Acta Neurochir (Wien) 147:831–837
Mikhail M, Ayling OGS, Eagles ME, Ibrahim GM, Macdonald RL (2019) Association between weekend admissions and mortality after aneurysmal subarachnoid hemorrhage: the “weekend effect” revisited. J Neurosurg 132:1167–1173
Nichols L, Stirling C, Stankovich J, Gall S (2020) Time to treatment following an aneurysmal subarachnoid hemorrhage, rural place of residence and inter-hospital transfers. Australas Emerg Care 23:225–232
Ohman J, Heiskanen O (1989) Timing of operation for ruptured supratentorial aneurysms: a prospective randomized study. J Neurosurg 70:55–60
Pandey AS, Gemmete JJ, Wilson TJ, Chaudhary N, Thompson BG, Morgenstern LB, Burke JF (2015) High subarachnoid hemorrhage patient volume associated with lower mortality and better outcomes. Neurosurgery 77:462–470; discussion 470
Phillips TJ, Dowling RJ, Yan B, Laidlaw JD, Mitchell PJ (2011) Does treatment of ruptured intracranial aneurysms within 24 hours improve clinical outcome? Stroke 42:1936–1945
Sanai N, Caldwell N, Englot DJ, Lawton MT (2012) Advanced technical skills are required for microsurgical clipping of posterior communicating artery aneurysms in the endovascular era. Neurosurgery 71:285–294; discussion 294–285
Solomon RA, Mayer SA, Tarmey JJ (1996) Relationship between the volume of craniotomies for cerebral aneurysm performed at New York state hospitals and in-hospital mortality. Stroke 27:13–17
Spetzler RF, McDougall CG, Zabramski JM, Albuquerque FC, Hills NK, Russin JJ, Partovi S, Nakaji P, Wallace RC (2015) The Barrow Ruptured Aneurysm Trial: 6-year results. J Neurosurg 123:609–617
Tayebi Meybodi A, Gadhyia A, Borba Moreira L, Lawton MT (2021) Coding cerebral bypasses: a proposed nomenclature to better describe bypass constructs and revascularization techniques. J Neurosurg 136:163–174
Acknowledgements
We thank the staff of Neuroscience Publications at Barrow Neurological Institute for assistance with manuscript preparation.
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Stefan W. Koester and Candice L. Nguyen. The first draft of the manuscript was written by Stefan W. Koester and Visish M. Srinivasan and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This retrospective study was approved by the Institutional Review Board and St. Joseph’s Hospital and Medical Center in Phoenix, Arizona.
Informed consent
Informed consent was waived due to the low risk to patients in this IRB-approved, retrospective study.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Vascular Neurosurgery – Aneurysm
Rights and permissions
About this article
Cite this article
Srinivasan, V.M., Koester, S.W., Karahalios, K. et al. Microsurgical treatment of ruptured aneurysms beyond 72 hours after rupture: implications for advanced management. Acta Neurochir 164, 2431–2439 (2022). https://doi.org/10.1007/s00701-022-05283-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-022-05283-x