Abstract
Background
Intracranial multimodality monitoring (iMMM) is increasingly used in acute brain-injured patients; however, safety and reliability remain major concerns to its routine implementation.
Methods
We performed a retrospective study including all patients undergoing iMMM at a single European center between July 2016 and January 2020. Brain tissue oxygenation probe (PbtO2), alone or in combination with a microdialysis catheter and/or an 8-contact depth EEG electrode, was inserted using a triple-lumen bolt system and targeting normal-appearing at-risk brain area on the injured side, whenever possible. Surgical complications, adverse events, and technical malfunctions, directly associated with iMMM, were collected. A blinded imaging review was performed by an independent radiologist.
Results
One hundred thirteen patients with 123 iMMM insertions were included for a median monitoring time of 9 [3–14] days. Of those, 93 (76%) patients had only PbtO2 probe insertion and 30 (24%) had also microdialysis and/or iEEG monitoring. SAH was the most frequent indication for iMMM (n = 60, 53%). At least one complication was observed in 67/123 (54%) iMMM placement, corresponding to 58/113 (51%) patients. Misplacement was observed in 16/123 (13%), resulting in a total of 6/16 (38%) malfunctioning PbtO2 catheters. Intracranial hemorrhage was observed in 14 iMMM placements (11%), of which one required surgical drainage. Five placements were complicated by pneumocephalus and 4 with bone fragments; none of these requires additional surgery. No CNS infection related to iMMM was observed. Seven (6%) probes were accidentally dislodged and 2 probes (2%) were accidentally broken. Ten PbtO2 probes (8%) presented a technical malfunction after a median of 9 [ranges: 2–24] days after initiation of monitoring and 4 of them were replaced.
Conclusions
In this study, a high occurrence of complications related to iMMM was observed, although most of them did not require specific interventions and did not result in malfunctioning monitoring.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute brain injury (ABI), such as traumatic brain injury (TBI) and subarachnoid hemorrhage (SAH), is an important cause of mortality, morbidity, and economic burden worldwide [1, 2]. Monitoring of neurological function in ABI patients is mandatory to detect early neuro-worsening before irreversible damage occurs, to individualize and guide patients’ care and to assess the effects of specific therapies [3].
Clinical examination is not always reliable in this setting, as some patients might be heavily sedated [4]. As such, specific neuro-monitoring tools can help to manage secondary brain injuries in these patients. In particular, invasive intracranial pressure (ICP) and derived cerebral perfusion pressure (CPP) are the cornerstone of neuro-monitoring in severe ABI patients, as reported by current guidelines [5], although mainly based on observational studies conducted in TBI patients [6,7,8]. Indeed, a randomized control trial has failed to show any benefit of invasive ICP monitoring compared to clinical and imaging monitoring [9]. Moreover, in the era of precision medicine, a simplistic “one size fits all” strategy based only on fixed ICP/CPP target is not sufficient to optimize brain function; a complex tailored strategy for brain-injured patients based on multimodality monitoring and personalized integration of an array of data may be more adequate [10].
Intracranial multimodality monitoring (iMMM) has been implemented in many institutions, combining ICP monitoring with brain tissue oxygenation (PbtO2) probes, microdialysis catheters (i.e., to assess cerebral metabolism), and intracortical or surface electroencephalography (iEEG, to detect seizures or cortical spread depolarizations) [10, 11]. However, considering the unknown clinical significance of iMMM, concerns have been raised about its invasiveness, safety, and technical reliability [4, 12].
As such, we aimed to evaluate a single-center experience about surgical placement and related complications, management, technical malfunctions, and adverse events of an iMMM implementation in ABI patients.
Methods
Study population
All patients who underwent ICP monitoring combined with iMMM (i.e., PbtO2 ± MD and iEEG) placement at a single European academic center, accounting for a total of 35 intensive care beds, between July 2016 and January 2020 were eligible for the study.
The study protocol was approved by the institutional review board (P2020/019), which waived the need for written consent. Medical charts and brain imaging were retrospectively reviewed for data collection.
Invasive multimodality monitoring
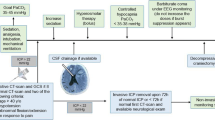
A triple-lumen bolt allowing the insertion of a PbtO2 probe (IM3.ST_EU, Integra LifeSciences Corporation, Plainsboro, NJ, USA), alone (from July 2016 to December 2018) or in association (from January 2019 to January 2020) with an 8-contact depth EEG electrode (Dixi Medical, Besançon, France) and a microdialysis catheter (M-Dialysis, Solna, Sweden), was placed in the operating room by a neurosurgeon in patients with ABI (i.e., TBI, SAH, or intracranial hemorrhage, ICH), who had indications for ICP monitoring (i.e., abnormal CT-scan findings and a Glasgow Coma Score on admission < 9). The triple-lumen bolt placement is shown in Fig. 1.
A single 1-g dose of cefazolin was administered before incision with no continuation of prophylactic antibiotic afterwards. The bolt was positioned 1.5 cm anteriorly from the Kocher’s point, targeting normal-appearing brain area of the injured side or, in case of surgical constraints (i.e., need for craniectomy, large intracranial hematoma, large hydrocephalus) in the contralateral side. In case of aneurysmal SAH, the bolt was positioned on either the ipsilateral side of the aneurysm (i.e., anterior circulation) or on the right side (i.e., no aneurysm identified or aneurysm located in the posterior circulation). For patients with delayed clinical deterioration without previous iMMM, the bolt was placed ipsilateral to the cerebral vasospasm and/or “at-risk” area, as suggested by cerebral CT-scan perfusion. Drilling was performed using a manual craniotome assembled to a 5.3-mm twist drill-bit specifically designed. External ventricular drain (EVD) or intraparenchymal ICP monitoring was inserted through an adjoining distinct burr hole located at the Kocher point.
Data collection and definitions
Demographics, indications for iMMM, severity scores on admission (Glasgow Coma Scale [13]; Acute Physiologic Assessment and Chronic Health Evaluation—APACHE—II score [14]; and Sequential Organ Failure Assessment—SOFA—score [15]), and hospital mortality were collected. Unfavorable neurological outcome was defined as a Glasgow Outcome Scale of 1–3 at hospital discharge. Working hours were defined from 8 am to 6 pm, Monday to Friday (excluding public holidays).
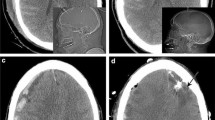
To describe iMMM-related events, surgical complications, adverse events, and technical malfunctions were retrieved from medical records. Surgical complications were defined as (a) misplacement; (b) intracranial hemorrhages (ICH); (c) bone fragments (i.e., small bone chips within the path of the device); (d) pneumocephalus; and (e) central nervous system (CNS) infections. Probe location in the subcortical anterior frontal area was considered “optimal.” Misplacement was therefore identified as non-optimally located probes, i.e., extra-axial, cortical matter, deep gray matter, paraventricular, or intraventricular locations; ICH, bone fragments, and pneumocephalus were identified on cerebral CT-scans performed after iMMM implementation, which were reviewed by a radiologist blinded to the outcome of the patient. Also, ICH were classified as grade I (i.e., \(\le\) 1 mL hemorrhage at any location along the iMMM), grade II (i.e., 1 mL hemorrhage at any location along the iMMM), or grade III (i.e., > 1 mL hemorrhage at any location along the iMMM or in the ventricle but contiguous to the catheter tip), adapting a proposed classification for EVD-related hemorrhages [16]. CNS infections were defined as meningitis, encephalitis, and/or ventriculitis with microbiological documentation on CSF samples and local skin infection and/or material culture of iMMM, as defined by the CDC/NHSN criteria [17] of hospital-acquired infections. Adverse events included accidental dislodgement or breaking of any probe or bolt device, requiring replacement or withdrawal. “Technical malfunction” was defined in the absence of any surgical complication and inconsistent PbtO2 values with the absence of PbtO2 response to the increase of the fraction of inspired oxygen to 100% for 15 min (i.e., increase < 10 mmHg).
Statistical analysis
Descriptive statistics were computed for all study variables. A Kolmogorov–Smirnov test was used and histograms and normal-quantile plots were examined to verify the normality of the distribution of continuous variables. Discrete variables were expressed as counts (percentage) and continuous variables as means ± SD or median [25th–75th percentiles]. Demographics and clinical differences between subgroups of patients (TBI vs non-TBI patients; iMMM with complications vs. no complication; iMMM placement in non-working hours vs. working hours; iMMM placement under antiaggregant/anticoagulant therapy vs. others; PbtO2 alone vs PbtO2 + iEEG/MD) were assessed using chi-square, Fisher’s exact test, Student’s t-test, or Mann–Whitney U test, as appropriate. A p < 0.05 was considered statistically significant. All descriptive statistics and statistical analyses were performed using “R” version 3.6.1.
Results
Study population
A total of 305 patients with an acute brain injury patients were admitted over the study period, of whom 135 had a GCS < 9 on admission and 113 patients (median age 52 [41–62] years; 57/113, 50% male sex) underwent 123 iMMM placements during the study period; of those, 93 (76%) patients had only PbtO2 probe insertion and 30 (24%) had also microdialysis and/or iEEG monitoring. ICP was monitored in all patients. SAH was the most frequent indication for iMMM (n = 56, 50%); 35 (31%) patients had TBI. Median GCS on admission was 7 [1, 2, 5, 7,8,9, 11, 12, 18]. The main characteristics of the study population are shown in Table 1.
iMMM and complications
The median duration of iMMM was 9 [3-14] days. Among the 10 patients that required a second iMMM (i.e., due to complications/malfunctioning in 9 and due to delayed new deterioration in 1), the placement occurred after 7 [3-9] days and numerically more frequent in SAH patients (n = 6) than others (ICH, n = 2; TBI, n = 2). At least one complication was observed in 67/123 (54%) iMMM placement, corresponding to 58/113 (51%) patients. No significant differences were observed between patients showing at least one iMMM-related complication when compared to others (Table 1).
Misplacement was observed in 16/123 (13%) iMMM placements, consisting of 5 extra-axial (i.e., 4 subdural and 1 subarachnoid), 7 intra-axial (i.e., 6 paraventricular and one in deep gray matter), and 4 intraventricular probes. The 5 extra-axial probes and the single probe placed deeply in the gray matter were malfunctioning; all 6 paraventricular probes were functioning and gave consistent PbtO2 values. The 4 intraventricular probes were successfully repositioned (with reliable PbtO2 values) by unscrewing the bolt and at the bedside without complications, resulting in a total of 6/16 (38%) malfunctioning PbtO2 catheters in case of misplacement.
ICH was observed in 14 iMMM placements (11%), including 7 tract hemorrhages (6%, all grade I; 3 of those PbtO2 catheters were also malfunctioning), 5 extra-dural hematomas (4%, all grade II; of which one required surgical drainage—one PbtO2 catheters was malfunctioning), and 2 distal probe tip hematomas (2%, all grade I; no PbtO2 catheter was malfunctioning). Only 2/14 (14%) patients who experienced ICH were under antiaggregant/anticoagulant therapy (OR for increased risk of ICH 0.74 [0.15–3.58], p = 0.7 vs. patients without antiaggregant/anticoagulant therapy).
Five placements were complicated by pneumocephalus (4%, one PbtO2 catheter was dysfunctional) and 4 with bone fragments (3%, one PbtO2 catheter was dysfunctional); none of these complications required additional surgery. No CNS infection related to iMMM was observed. Seven (6%) probes were accidentally dislodged, and in 1 patient, the probe was replaced; 2 probes (2%) were accidentally broken and no replacement was performed.
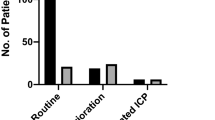
Ten PbtO2 probes (8%) presented a technical malfunction (i.e., not attributable to surgical complications/adverse events) after a median of 9 [ranges: 2–24] days after initiation of monitoring and 4 of them were replaced. All complications are summarized in Table 2. Table 2 also shows a comparison between normally functioning probes (n = 101) and dysfunctional probes (n = 22). The association of several complications is depicted in Fig. 2.
iMMM and complications in subgroups
The distribution of complications was similar in TBI and in non-TBI patients. Similarly, there were no differences in the rate of complications according to the time of iMMM placement (non-working hours vs. working hours). There were no significant differences in the rate of complications between iMMM with only PbtO2 monitoring compared to iMMM including PbtO2, microdialysis, and/or iEEG (47/93, 51% vs. 20/30, 65%, p = 0.22). These data are shown in Table 3.
Discussion
In this study, we observed that surgical complications and adverse events were common after iMMM placement, in particular probe misplacement and small-volume intracranial hemorrhage. No infection related to iMMM was observed. The only misplacement was significantly associated with malfunctioning probes. No significant association of iMMM-related complications was found with underlying ABI indication, timing of placement, antithrombotic regimens, and modalities of iMMM. Also, the neurological outcome was worse in patients without complications.
The most investigated surgical complications related to iMMM are probe misplacement and intracranial hemorrhage. Placement of PbtO2 probes outside the white matter, within a lesion, or within a ventricle may yield irrelevant brain oxygen measurement and would result in additional costs (i.e., for probe replacement and/or because of lack of an adequate monitoring tool to guide interventions). Misplacement rates ranged from 0 to 14.3% across different studies [4, 12, 18,19,20] and varied depending on the targeted area of the brain (i.e., higher when the target was contusional than healthy brain area). In our cohort, 13% of iMMM placement was associated with probe misplacement. Although this is quite higher than in most previous reports, we investigated a heterogeneous cohort of patients with different forms of ABI, while most of the other studies focused on TBI patients. Moreover, potential differences in baseline population characteristics and neurosurgical approach to iMMM implementation would provide significant bias to adequately compare these data to previous studies. Of note, our results compared for the first time traumatic and non-traumatic brain injury. Also, subgroup analyses of risk factors for the occurrence of iMMM-related complications were performed. It still remains difficult to put these findings in perspective and compare iMMM-related complications with those occurring during an ICP monitoring placement, as these may depend on the type of catheter inserted (i.e., intraparenchymal vs. EVD) as well as the experience of the operator, and range between 8 and 45% [21].
ICH rates due to iMMM placement ranged between 0 and 40.5% in different series [4, 12, 18,19,20]. Of note, imaging review, time from insertion to complication, and definition of ICH were often not comprehensively described. In our series, the ICH rate of 11% was relatively low, using an independent radiologist who blindly reviewed systematic postoperative imaging, including immediate post-placement but also delayed imaging studies. Only one case required surgical drainage (i.e., a compressive right parasagittal extra-dural hematoma). Dings et al. [18] described a similar case of ICH due to placement of the PbtO2 probes close to the sagittal sinus, which should require particular attention from physicians placing iMMM. Foreman et al. [12], after reviewing 42 post-placement CT-scans of quadruple-lumen bolts, presented the highest ICH rate (40.5%); this rate dropped to 7.1% when tract hemorrhages were excluded. Large prospective multicentric registries according to predefined guidelines and definitions of complications, including different types of ABI populations, are required to better describe iMMM-related complications, to evaluate the potential role of the underlying ABI disease, and to compare the occurrence of these complications with the ones related to the use of ICP monitoring.
We observed no infection related to the iMMM placement. In fact, infections due to this type of intraparenchymal monitoring appear to be rare: in the largest series available including 501 patients, only one infection was reported [4].
Accidental dislodgement and probe breaking are usually reported, ranging from 5.9 to 7.4% and from 2.5 to 4.5%, respectively [4, 12, 18,19,20]. Whether these complications occur because of agitation or discomfort, during daily standard interventions (i.e., changes in bed positioning, physical therapy) or during transport (i.e., to perform brain imaging or other diagnostic/therapeutic procedures) remains unclear from our retrospective data. Neurocritical patients often require great manipulation and transportation which may make them vulnerable to these adverse events [12]. An interesting dynamic approach to adverse events was proposed by Foreman et al. [12], who quantified sudden stops of data recording as discontinuations, resulting either from dislodgement or unplugging by time tracking them as one-off events rather than only reporting their occurrence. During a median monitoring time of 97 h, device discontinuation was noted in 25/43 (58%) patients and concerned the combined ICP/PbtO2 probe in 7 (16%) cases, with a total of 4% of the recorded data considered unusable. They also found a significant association between discontinuation and the number of patient trips for procedures or imaging.
Finally, despite complications and technical malfunctioning, iMMM appears to be safe, as only one additional surgery with no subsequent short-term neurological sequelae was observed. Moreover, hospital mortality was similar between patients with and without complications. Interestingly, patients with complications present a significantly better neurological outcome. As most of these complications are not clinically relevant (i.e., a non-functional catheter does not influence per se patients’ outcome) or quite minimal (small ICH or bone fragments may not result in significant brain damage), this incidental statistical finding may not be of importance. Furthermore, potential biases and uncontrolled confounding factors may contribute to it. For instance, ICU length of stay was significantly shorter in the group of patients without complications—mortality was not significantly higher. It can be hypothesized that these patients had not sufficient time to develop such events and be diagnosed (e.g., immortal time or survivorship biases). Finally, potential interactions between the diagnosis of complications and the neurocritical management of patients may be missed in this retrospective analysis. The data collection did not include additional relevant information, which could have been helpful to further characterize the outcome difference, although this was beyond the scope of the study. Importantly, 10 (8%) probes presented a technical malfunction independently from other complications and, in some cases, this occurred within the first week of monitoring. The additional costs for probe replacement as well as the immediate loss of relevant monitoring data in these patients should also be further evaluated in larger cohorts, as it might be important information for centers who would decide to implement iMMM in their routine practice.
This study has some limitations due to its monocentric retrospective design, which limits the generalization of the main findings and implies that some complications might have been underdiagnosed. Also, we did not specifically investigate the long-term effects of the reported complications. Moreover, the role of neurosurgeons’ skills was not considered in the analysis as iMMM placement was performed by rotating residents supervised by senior neurosurgeons with no main operator clearly identified into medical charts. Second, we used a triple-lumen bolt device placed into the operating room and our findings do not apply to other possible solutions (i.e., double-lumen bolts or subcutaneous tunelization without a bolt) or setting (i.e., placement into the ICU and/or by a non-neurosurgeon). Third, the PbtO2 was the sole modality concerned by technical malfunction report, as being the only one used in clinical routine (i.e., microdialysis and iEEG data were only collected for research purposes).
Conclusions
iMMM placement including PbtO2 monitoring was associated with several mild complications but had an acceptable short-term safety profile in our institution. These data underline the need to standardize the reporting of technical and surgical iMMM complications to better evaluate its risk/benefit ratio. International collaboration through prospective multicentric data collection according to well-defined complications would promote the optimal use of iMMM.
Availability of data and material
All the data are available on demand at mejdi.albarajraji@gmail.com.
Code availability
Not applicable.
References
Chin JH, Vora N (2014) The global burden of neurologic diseases. Neurology 83(4):349–351. https://doi.org/10.1212/WNL.0000000000000610
Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung YC, Punchak M, Agrawal A, Adeleye AO, Shrime MG, Rubiano AM, Rosenfeld JV, Park KB (2018) Estimating the global incidence of traumatic brain injury. Journal of neurosurgery, 1–18. Advance online publication. https://doi.org/10.3171/2017.10.JNS17352
Le Roux P, Menon DK, Citerio G, Vespa P, Bader MK, Brophy GM, Diringer MN, Stocchett N, Videtta W, Armonda R, Badjatia N, Böesel J, Chesnut R, Chou S, Claassen J, Czosnyka M, De Georgia M, Figaji A, Fugate J, Helbok R, Horowitz D, Hutchinson P, Kumar M, McNett M, Miller C, Naidech A, Oddo M, Olson D, O’Phelan K, Provencio JJ, Puppo C, Riker R, Robertson C, Schmidt M, Taccone F, Neurocritical Care Society; European Society of Intensive Care Medicine (2014) Consensus summary statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Intensive care medicine 40(9):1189–1209. https://doi.org/10.1007/s00134-014-3369-6
Bailey RL, Quattrone F, Curtin C, Frangos S, Maloney-Wilensky E, Levine JM, LeRoux PD (2019) The safety of multimodality monitoring using a triple-lumen bolt in severe acute brain injury. World neurosurgery 130:e62–e67. https://doi.org/10.1016/j.wneu.2019.05.195
Hawryluk G, Aguilera S, Buki A, Bulger E, Citerio G, Cooper DJ, Arrastia RD, Diringer M, Figaji A, Gao G, Geocadin R, Ghajar J, Harris O, Hoffer A, Hutchinson P, Joseph M, Kitagawa R, Manley G, Mayer S, Menon DK, Meyfroidt G, Michael DB, Oddo M, Okonkwo D, Patel M, Robertson C, Rosenfeld JV, Rubiano AM, Sahuquillo J, Servadei F, Shutter L, Stein D, Stocchetti N, Taccone FS, Timmons S, Tsai E, Ullman JS, Vespa P, Videtta W, Wright DW, Zammit C, Chesnut RM (2019) A management algorithm for patients with intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med 45(12):1783–1794. https://doi.org/10.1007/s00134-019-05805-9
Alali AS, Fowler RA, Mainprize TG, Scales DC, Kiss A, de Mestral C, Ray JG, Nathens AB (2013) Intracranial pressure monitoring in severe traumatic brain injury: results from the American College of Surgeons Trauma Quality Improvement Program. J Neurotrauma 30(20):1737–1746. https://doi.org/10.1089/neu.2012.2802
Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GW, Bell MJ, Bratton SL, Chesnut R, Harris OA, Kissoon N, Rubiano AM, Shutter L, Tasker RC, Vavilala MS, Wilberger J, Wright DW, Ghajar J (2017) Guidelines for the management of severe traumatic brain injury. Fourth Edition Neurosurgery 80(1):6–15. https://doi.org/10.1227/NEU.0000000000001432
Farahvar A, Gerber LM, Chiu YL, Carney N, Härtl R, Ghajar J (2012) Increased mortality in patients with severe traumatic brain injury treated without intracranial pressure monitoring. J Neurosurg 117(4):729–734. https://doi.org/10.3171/2012.7.JNS111816
Chesnut RM, Temkin N, Carney N, Dikmen S, Rondina C, Videtta W, Petroni G, Lujan S, Pridgeon J, Barber J, Machamer J, Chaddock K, Celix JM, Cherner M, Hendrix T, Global Neurotrauma Research Group (2012) A trial of intracranial-pressure monitoring in traumatic brain injury. The New England journal of medicine 367(26):2471–2481. https://doi.org/10.1056/NEJMoa1207363
Roh D, Park S (2016) Brain multimodality monitoring: updated perspectives. Curr Neurol Neurosci Rep 16(6):56. https://doi.org/10.1007/s11910-016-0659-0
Citerio G, Oddo M, Taccone FS (2015) Recommendations for the use of multimodal monitoring in the neurointensive care unit. Curr Opin Crit Care 21(2):113–119. https://doi.org/10.1097/MCC.0000000000000179
Foreman B, Ngwenya LB, Stoddard E, Hinzman JM, Andaluz N, Hartings JA (2018) Safety and reliability of bedside, single burr hole technique for intracranial multimodality monitoring in severe traumatic brain injury. Neurocrit Care 29(3):469–480. https://doi.org/10.1007/s12028-018-0551-7
Teasdale G, Maas A, Lecky F, Manley G, Stocchetti N, Murray G (2014) The Glasgow Coma Scale at 40 years: standing the test of time. The Lancet Neurology 13(8):844–854. https://doi.org/10.1016/S1474-4422(14)70120-6
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13(10):818–829
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive care medicine 22(7):707–710. https://doi.org/10.1007/BF01709751
Samaniego EA, Hasan DM (2019) In Reply: External ventricular drain and hemorrhage in aneurysmal subarachnoid hemorrhage patients on dual antiplatelet therapy: a retrospective cohort study. Neurosurgery 84(1):E99–E100. https://doi.org/10.1093/neuros/nyy489
Horan TC, Andrus M, Dudeck MA (2008) CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 36(5):309–332. https://doi.org/10.1016/j.ajic.2008.03.002
Dings J, Meixensberger J, Jäger A, Roosen K (1998) Clinical experience with 118 brain tissue oxygen partial pressure catheter probes. Neurosurgery 43(5):1082–1095. https://doi.org/10.1097/00006123-199811000-00045
Stuart RM, Schmidt M, Kurtz P, Waziri A, Helbok R, Mayer SA, Lee K, Badjatia N, Hirsch LJ, Connolly ES, Claassen J (2010) Intracranial multimodal monitoring for acute brain injury: a single institution review of current practices. Neurocrit Care 12(2):188–198. https://doi.org/10.1007/s12028-010-9330-9
van Santbrink H, Maas AI, Avezaat CJ (1996) Continuous monitoring of partial pressure of brain tissue oxygen in patients with severe head injury. Neurosurgery 38(1):21–31. https://doi.org/10.1097/00006123-199601000-00007
Tavakoli S, Peitz G, Ares W, Hafeez S, Grandh R (2017) Complications of invasive intracranial pressure monitoring devices in neurocritical care. Neurosurg Focus 43(5):E6. https://doi.org/10.3171/2017.8.FOCUS17450
Funding
The research was not supported by any company. The devices were bought by the intensive care unit and the neurosurgery department to be used in daily clinical practice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Ethical approval was obtained from ethics committees of the Erasme Hospital, Free University of Brussels, Brussels, Belgium: file EC P2020/019.
Additional declarations for articles in life science journals that report the results of studies involving humans and/or animals
Not applicable.
Consent to participate
For this type of study, formal consent is not required.
Consent for publication
Consent to submit has been received from all co-authors and responsible authorities at the institute/organization where the work has been carried out before the work is submitted.
Competing interests
Fabio Silvio Taccone received lecture fees from Integra Lifescience. All other authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mejdeddine Al Barajraji and Elisa Bogossian equally contributed as first authors.
Sophie Schuind and Sami Barrit equally contributed as senior authors.
This article is part of the Topical Collection on Neurosurgical intensive care
Rights and permissions
About this article
Cite this article
Al Barajraji, M., Bogossian, E., Dewitte, O. et al. Safety profile of an intracranial multimodal monitoring bolt system for neurocritical care: a single-center experience. Acta Neurochir 163, 3259–3266 (2021). https://doi.org/10.1007/s00701-021-04992-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-021-04992-z