Abstract
Background
An indication for selective shunting during carotid endarterectomy (CEA) is based on monitoring during a procedure. Cerebral oximetry (CO) using near-infrared spectroscopy (NIRS) may be a simple technique, but its relevance during CEA, especially with respect to cutoff values indicating shunt implantation, still needs to be elucidated.
Methods
One hundred twenty five patients underwent CEA under local anesthesia (LA) and were monitored clinically throughout the whole procedure. The patients were also monitored using bilateral NIRS probes during surgery. The NIRS values were recorded and evaluated before and after selective cross-clamping, firstly by the external carotid artery (ECA), followed by the internal carotid artery (ICA). The decrease in the ipsilateral CO values, with respect to the indication of shunting, was only analyzed after selective cross-clamping of the ICA. The decision to use an intraluminal shunt was solely based on the neurological status evaluation after ICA cross-clamping.
Results
One hundred five patients (85%) were stable throughout the CEA, while 20 patients (15%) clinically deteriorated during surgery. The mean drop in the CO after selective ICA clamping in clinically stable patients was 6%, while in patients with clinical deterioration, the NIRS decreased by 14.5% (p < 0.05). When the cutoff value for selective shunting was set as a 10% decrease of the ipsilateral CO after selective ICA clamping, the sensitivity of the technique was 100% and the specificity 83.0%.
Conclusions
Our study showed that a 10% decrease in the ipsilateral brain tissue oximetry after selective cross-clamping the ICA provides a reliable cutoff value for selective shunting during CEA. Despite the availability of a variety of monitoring tools, the NIRS may be an easy, reliable option, especially in the scenario of acute CEA in general anesthesia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carotid endarterectomy (CEA) is a well-established surgery for primary and secondary stroke prevention; however, it is associated with a low but significant risk of complications [3]. During carotid cross-clamping, some patients may suffer a stroke due to an insufficient collateral flow, which requires the implantation of an intraluminal shunt during surgery. The decision on shunt insertion is usually based on either clinical deterioration during local anesthesia or various auxiliary monitoring techniques (somatosensory evoked potentials (SEP), electroencephalography (EEG), transcranial dopplerometry (TCD), or stump pressure measurement in patients having surgery under general anesthesia (GA) [11].
Cerebral oximetry (CO) monitoring using near-infrared spectroscopy (NIRS) is a simple technique detecting oxygenated blood in a small region about 25 mm below the skin [29]. When NIRS probes are applied on the forehead, they reveal relative brain perfusion in a small area of the frontal lobe cortex. Although many studies have been performed utilizing NIRS during CEA, to date, there has not been a clear, uniformly accepted cutoff value for indicating shunt implantation using this technique [2, 17, 24, 29, 31]. This could be due to various reasons such as the availability of different technologies or minor differences in data interpretation during carotid cross-clamping, both of which could lead to differences in the final results [14].
The goal of our study was to find a cutoff value during selective internal carotid artery (ICA) cross-clamping that would provide a reliable indication for intraluminal shunt insertion during CEA. Such a cutoff would be useful for the monitoring of CEA either operated under GA or converted to GA when the patient does not tolerate awake surgery. There is a particular need for an effective monitoring method, which could be set up quickly and with the possibility of fast data interpretation, in this subgroup of patients who are converted to GA.
Materials and methods
Study design
Between November 2016 and October 2019, we conducted a prospective observational study, using bilateral CO monitoring utilizing NIRS in patients who underwent CEA under local anesthesia (LA). The study was observational and the indication to shunt insertion was solely based on clinical symptoms. The study was approved by the local ethics committee of the Masaryk Hospital in Ústí nad Labem, Czech Republic (No. 251/55).
Demographics and patient enrolment
The inclusion criteria were as follows: ICA ≥ 50% stenosis in the case of symptomatic or ≥ 60% stenosis in the case of asymptomatic stenosis, as detected by duplex ultrasound and confirmed by computed tomography angiography (CTA) with accurate stenosis measurement; indication for CEA according to criteria set by the American Heart Association; age of 30 to 90 years; and signed, informed consent [3, 33]. All the patients underwent magnetic resonance imaging (MRI) within 72 h before and after surgery according to the protocol mentioned in a previous study [27].
Surgical procedure
All the patients were operated under ipsilateral cervical plexus block anesthesia bupivacaine (Marcaine, AstraZeneca, Cambridge, UK) performed by an anesthesiologist; additional infiltration of the operative field with 1% trimecaine (Mesocain; Zentiva, Prague, Czech Republic) was performed by the surgeon if necessary. We used a standard surgical approach via an oblique skin dissection ventro-medial from the sternocleidomastoid muscle. After reaching the common carotid artery (CCA) bifurcation, we properly dissected all branches of the CCA: the ICA, external carotid artery—ECA, and the superior thyroid artery—STA. A dose of 5000 international units of unfractionated heparin (Heparin Léčiva; Zentiva, Prague, Czech Republic) was administered intravenously 3 min before flow arrest. The activated clotting time (ACT) was measured 3 min after injection of the heparin. In case of low ACT values (below 150), 2500 IU were added. To enhance cerebral perfusion, blood pressure was maintained at or above the level of initial pre-clamp levels during cross-clamping. Norepinephrine was administered intravenously in the case of hypotension, to optimize blood pressure.
The criteria for shunt placement were the onset of a new neurological deficit, such as hemiparesis or aphasia and/or the deterioration in level of consciousness after carotid cross-clamping. If shunting was indicated, a Bard Javid carotid shunt (Bard Inc., Murray Hill, NJ, USA) was used.
NIRS monitoring and data recording
The CO measurement was performed using the NIRS Fore-Sight oximeter (Edwards Lifesciences Corporation, USA). Two large sensors (≥ 40 kg) were applied on the forehead before surgery, and CO was recorded continually throughout the surgery. The accuracy for the sensors is ± 3.7% (1 SD) in the range rSO2 45–95% [6]. After proper dissection of the CCA and its branches, clips were applied. We first clipped the STA followed by clipping of the ECA. The NIRS values on both sides were recorded in a datasheet just before clipping of the ECA. We waited for a minimum of 60 s before clipping the ICA. The lowest NIRS values over both hemispheres were recorded. In case the decrease of the NIRS values continued over the 60-s period, we waited longer until stabilization of both values. We then instructed the patient to start counting from 1 to 20, while squeezing a squeaky rubber toy with the contralateral hand to monitor motor function. At the start of the clinical evaluation, the ipsilateral ICA was cross-clamped. In case of a stable neurological status (stable group) of the patient, the surgery continued by clipping the CCA and arteriotomy. In case of clinical deterioration (unstable group), the clip was released and we prepared for shunt insertion. In both scenarios, we recorded the lowest NIRS values above both frontal lobes.
Postoperative care and clinical evaluation
After surgery, the patients were observed for 1 night in the intensive care unit (ICU). Vital signs checking, neurological examination, and wound control were performed during admission at the ICU and then on an hourly basis during the whole stay. Three doses of 5000 IU of heparin were administered every 8 h unless the APTT reached values of 3.0 or above. In cases of an uneventful stay at the ICU, the patient was transferred to the regular neurosurgical ward. Three days after surgery, patients were usually discharged home or to their local hospitals if necessary. The patient was clinically evaluated, and a new duplex ultrasound was again assessed 3 months after surgery in the outpatient unit of the neurosurgical department.
NIRS data evaluation and statistical analysis
The data of the patients were saved and recorded in Excel Worksheets. The software STATISTICA, version 11, was used for the analytical methods. The categorical demographic characteristics between the stable and unstable patient groups were compared using the chi-square test of independency, or Fisher’s exact test when small frequencies occurred. Based on the normality test, the non-parametric two-sample Mann–Whitney test was used to compare the distribution of age between the two patient groups.
We compared the relative change in the NIRS values before and after selective clamping of the ECA and the ICA in patients with a stable clinical status during surgery and in patients with clinical deterioration. The parametric two-sample t test was applied. The p value below p < 0.05 was considered statistically significant.
Results
Patients’ demographics
The study included 125 patients. Eighty-seven (70%) patients were males and 38 (30%) were females (Table 1). The mean age of the patients was 68.4 years, and it ranged from 46 to 88 years of age. Forty-seven (38%) patients suffered from diabetes, 95 (76%) were treated for hypertension, and 5 patients (4%) had a coronary artery disease. Eighty-three (66%) patients were either current or former smokers, 29 patients had never smoked (23%), and in 13 patients (10%), we did not have this information available. While 61 (49%) patients were treated for a symptomatic carotid stenosis, 64 (51%) were treated for an asymptomatic carotid stenosis. Also, 36 (29%) patients had a significant contralateral stenosis (over 50%), and 10 (8%) patients had a contralateral carotid occlusion.
There was a significantly higher representation of women (50%) in the unstable group compared to the stable group (27%; p < 0.05). Also, 70% of patients in the unstable group had a significant contralateral ICA stenosis or occlusion, while in the stable group, it was only 30% (p < 0.001). Apart from that, there was not a statistically significant difference between the groups in any other parameter (age, smoking, diabetes, hypertension, coronary disease, smoking, and symptomatology).
Perioperative results
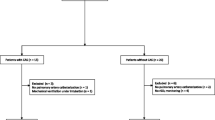
All 125 patients underwent CEA under local anesthesia, together with bilateral CO monitoring using NIRS. During surgery, 105 (85%) patients were stable after clipping the ICA, and they required no shunt insertion. The remaining 20 (16%) patients presented with clinical deterioration after clipping (Fig. 1). In 14 (11%) of these, we implanted a shunt immediately after clipping the ICA. The other 6 (5%) patients were operated without shunt implantation; in 2 of these, increasing the systemic pressure resulted in symptom improvement; in the other 2 patients, the symptoms appeared during suturing the carotid, and the surgeon decided to finish the surgery without shunt implantation; and in 2 cases, the intraluminal shunt was not implanted due to technical difficulties.
CO related to cross-clamping ECA
Overall, the mean value before clipping the ECA was 75.7 ± 0.6, and after clipping, the ECA the mean value decreased to 72.2 ± 0.6. The mean relative decrease in the NIRS values after clamping the ECA was 5.3% ± 0.8.
In the stable group of patients, the mean value before clipping the ECA was 75.7 ± 0.6, and after clipping the ECA, it decreased to72.4 ± 0.6. The mean relative decrease in the NIRS values after clamping the ECA was 4.4% ± 0.3%.
In the unstable group of patients, the mean value before clipping the ECA was 75.5 ± 1.6, and after clipping the ECA, it decreased to 71.6 (from 60 to 88). The mean relative decrease in the NIRS values after clamping the ECA was 5.7% ± 1.2%.
When comparing the change in the relative value of the NIRS after clamping the ICA, there was no statistically significant difference between the stable group and the unstable group of patients (p > 0.05).
CO related to cross-clamping ICA
Overall, the mean value of ipsilateral CO before clamping the ICA was 72.5 ± 0.6, and after clamping, it decreased to 67.7 ± 0.6. The mean relative drop was 6.6% ± 0.5%.
The mean value in the stable group before clamping the ICA was 72.7 ± 0.6, and after clamping, it decreased to 68.9 ± 0.7. The mean relative drop in the NIRS values was 5.2% ± 0.4.
In the unstable group of patients, the mean value before clamping the ICA was 71.8 (from 60 to 86), and after clamping, it decreased to 61.3 (from 52 to 70). The mean relative drop in the NIRS values was 14.5% ± 0.7.
When comparing the change in the relative value of the NIRS after clamping the ICA, there was a statistically significant difference between the stable group and the unstable group of patients (p < 0.001, Fig. 2). When we set the cutoff value at 10%, the sensitivity for selective clipping the ICA was 100% and specificity 83.0% (Table 2). When we set the cutoff value at 15%, the sensitivity for selective clipping the ICA was 45.0% and specificity 99.0%. The ROC curve shows that the 10% seems to be an optimal cutoff value for predicting clinical deterioration (Fig. 3).
CO related to cross-clamping both CCA branches (ECA and ICA)
Overall, the mean value before cross-clamping both CCA branches was 75.7 ± 0.6, and after clamping, it decreased to 67.7 ± 0.6. The mean relative drop in the NIRS values was 10.6% ± 0.6%.
In the stable group of patients, the mean value before clipping the ECA and the ICA was 75.7 ± 0.6, and after clipping, it decreased to 68.9 ± 0.7. The mean decrease in the NIRS values after clamping both the ECA and the ICA was 9.1% ± 0.5%.
In the unstable group of patients, the mean value before clipping the ECA was 75.5 ± 1.6, and after clipping, it decreased to 61.3 ± 1.2. The mean relative drop in the NIRS values after clamping both the ECA and the ICA was 19.2% ± 1.3%.
Then comparing the change in the relative value of the NIRS after clamping both ECA and ICA, there was a statistically significant difference between the stable group and the unstable group of patients (p < 0.001). When we set the cutoff at 10% of a decrease of the ipsilateral NIRS before and after clipping both the ECA and ICA, the sensitivity is again 100% but the specificity reaches only 57.8% (Table 2). When we set the cutoff value at 15%, the sensitivity is 72.2% and the specificity is 85.3%.
Clinical status results
Out of 125 patients in our study, 121 were discharged home in a stable neurological state without any deterioration, compared to the preoperative status. From these, 1 patient died several days after discharge home due to a sudden collapse. While in the stable group no patients suffered a stroke in the perioperative period, there were 4 patients in the unstable group who experienced a stroke. All 4 patients developed completed strokes during surgery, and only 1 of them improved significantly in the postoperative period.
DWI results
Out of the total 125 patients in our study, 120 had a DWMRI examination before and after CEA. Five patients could not undergo MRI mainly due to contraindications to MRI examination, such as the presence of a cardio-stimulator or metal implants. Out of these 120, we observed no new ischemic lesion in 100 patients and new ischemic lesion in 20 patients. The vast majority were small asymptomatic ischemic lesions. In the group of stable patients (n = 100), we observed a new lesion in 8 (8%). In the patients with clinical deterioration (n = 20), we observed a new ischemic lesion in 11 (55.0%) patients (p < 0.001).
Discussion
The perioperative use of a shunt is an integral part of CEA, but the indication for shunt implantation is still controversial [28]. While some surgeons prefer the routine use of shunting, many advocate selective insertion based on monitoring [7]. The use of selective shunting has been reported between 7 and 12% [5, 13, 23, 26, 30]. This is in agreement with our recent study, when selective shunting in patients undergoing CEA under LA was 12% [26]. On the other hand, we have also shown that carotid shunting in itself is associated with an increased risk of developing DWI-positive ischemic lesions [26]. Although a vast majority of these lesions were clinically asymptomatic, the routine use of shunts during CEA still puts many patients at unnecessary risk of developing brain ischemia. Also, the use of shunt is associated with increased risk of surgical revision due to an intimal flap, ICA stenosis, kinking, etc. [17]. Therefore, selective shunting seems to be a rational approach to avoid a hypoperfusion stroke during carotid cross-clamping.
The use of shunt insertion is based either on clinical evaluation during awake surgery or on various supplementary monitoring methods during CEA under GA; these include SEP, EEG, TCD, stump pressure (SP), or less often also CO utilizing NIRS technology [4, 24]. None of these methods is considered superior, and they are often used based on local preferences and experience, but NIRS is most likely the least established one. NIRS measures light absorbance to calculate oxyhemoglobin and deoxyhemoglobin, which provides an indirect measure of brain activity, particularly in the frontal cortex. In the past 20 years, there have been several studies validating the use of NIRS during CEA under LA (Table 3); however, there still lacks a general consensus regarding the cutoff value indicating an intraluminal shunt insertion.
Cutoff values of NIRS
In our study, we demonstrate that a 10% decrease in the ipsilateral CO after carotid cross-clamping provides a reliable cutoff value for indication of selective shunting. The sensitivity of the cutoff value is 100% in our series, meaning that none of the patients who deteriorated would be missed. Even more, in 4 patients, the clinical deterioration appeared later during the study despite the fact that they were stable at the time of clinical testing. In all these patients, the ipsilateral CO still dropped by more than 10%. It seems that the change in the ipsilateral CO shows the change earlier compared to clinical testing which may manifest later after cross-clamping. This may be of advantage as implanting the shunt right from the beginning may prevent unnecessary stress and rush if the clinical deterioration manifests later when the carotid artery is already open.
Currently, there has been no generally accepted cutoff value for indicating an intraluminal shunt implantation during CEA based on NIRS in the literature yet. One of the main reasons for this is that there are 3 different companies distributing NIRS sensors and monitors, with each company using slightly different technologies (INVOS 5100C, EQUANOX Classic 7600, and Fore-Sight) [25]. They differ, for example, in the depth of light penetration and collection, partly due to various spatial differences between the sensors and detectors in each device, which may influence the resultant values of CO. Hyttel-Sorensen et al. showed in a study on peripheral tissue oximetry that all 3 oximeters presented different results, concluding that these three oximeters cannot be used interchangeably [14] (Table 3). Table 3 shows that the values have ranged from 10 to 20%. While in most studies implementing the INVOS the cutoff values were around 20%, in two studies implementing the Fore-Sight (including ours), the cutoff values were 9 and 10% respectively [15]. Also, the sensitivity and specificity values in both studies are relatively high compared to other monitoring techniques such as the SEPs, EEG, or stump pressure [12, 24]. The lower values associated with the use of Fore-Sight could be a possible result of a slightly different technology, allowing monitoring of tissue 25 mm below skin compared to INVOS, which provides monitoring of tissue 20 mm below skin and could therefore be “contaminated” by the signal from soft tissues under the sensor. Because of different methodologies, no universal cutoff value can be probably drawn for NIRS in CEA, but it would need to be specified for each NIRS technology.
Contribution of ECA to CO
In our study, we evaluated the change in signal with respect to the contribution of selective ECA and ICA cross-clamping. The cerebral oximeters currently on the market have 2 receptors. They use the process of spatial resolution in which the increasing distance between them allows for the assessment of deeper tissue and should prevent the signal from extracranial contamination. However, a simple experiment using a pneumatic band around the forehead has shown that extracranial contamination was demonstrated in all 3 NIRS devices ranging between 6.8 and 16.6% [8]. A selective evaluation of the change in CO values after selective clipping of the ICA should eliminate this “contamination signal” providing more precise decision-making in the indication of an intraluminal shunt insertion.
In none of the studies utilizing NIRS during CEA summarized in Table 3 would the authors present the exact procedure of the cross-clamping algorithm during surgery. Was the clip put on the CCA or selectively on the ICA? Naftalovich et al. presented a case in which the rSO2 signal decreased by 8% after selective shunting the ECA, while there was no change after cross-clamping the ICA [25]. We found that cross-clamping the ECA leads to a mean relative decrease in the ipsilateral NIRS by an average of 5.3%. Furthermore, there was a decrease in the ipsilateral NIRS value in the stable group and the unstable group by 4.4% and 5.7%, respectively. Therefore, excluding the influence of the ECA may be an advantage to improve the differentiation of patients at risk of shunting compared to the stable patients. The question still remains whether the signal contributed by the ECA is only a result of signal contamination from the soft tissue under the probes or as a result of collaterals feeding the brain [25].
By first cross-clamping the ECA, we could increase the specificity in the cutoff value from 58 to 83% (Table 2) while keeping high sensitivity of 100%. If we used the cutoff value of 10% without eliminating the influence of the ECA, we could still reach 100% sensitivity, but only for the price of significantly decreasing the specificity to 58%. This may be a step forward as compared to the largest study implementing CO using Fore-Sight during CEA under LA [15]. In that study, the authors set a cutoff value at 9% with a sensitivity of 95% and specificity 81%. Evaluating the change of ipsilateral CO after selective clamping, the ICA may increase the sensitivity and specificity of this method. This could be one of the possible steps leading to improving the technology as suggested by Kakkos and Tsolakis in their commentary to the article of Jonsson et al. [16].
CO and shunt patency
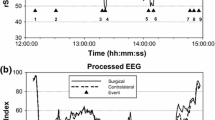
NIRS monitoring may serve not only as guidance to selective shunting during CEA but also possibly as an indicator of shunt function during surgery as noticed in one previous study [15]. In our own study, we observed 2 cases of inadequate function after shunt insertion (Fig. 4). In both patients, the values after shunt implantation increased by less than 50% of the original, pre-cross-clamping value. While in one patient, it was associated with functional deterioration (loss of consciousness); the other patient was clinically stable. In both cases, we adjusted the shunt position by pulling them down to the CCA, which eventually led to improved shunt function and almost complete restoration of the NIRS values. In the symptomatic patient, this resulted in restoration of the symptoms. We speculate that the reason for shunt insufficiency was the distal end of the shunt which most probably adhered to the wall of the ICA; in both cases, the preoperative CTA showed coiling of the ICA under the skull base (Fig. 4 insertion).
Bilateral CO monitoring during a left CEA in an unstable patient with an insufficient intraluminal shunt function (blue line—left side). After cross-clamping the left ICA and shunt insertion, the values did not restore to less than 50% of the original values and the patient remained symptomatic. After several adjustments, the shunt was finally pulled downwards and the NIRS values restored to almost pre-clipping values. The neurological status of the patient improved. The inserted CTA shows the most possible reason for shunt malfunction—coiling of the distal ICA
Clinical use of NIRS in CEA
Today, we have several available monitoring tools for cerebral ischemia during CEA [22, 24]. Still, the advantage of NIRS is that it provides continuous monitoring during surgery (opposite to stump pressure). The probes can be readily applied on the forehead and thus may be also used in cases when patients in whom we need to convert them to GA due to intolerance. According to our study, the data collection was very stable in all the patients. Furthermore, the reading and interpretation are simple, requiring no further highly qualified personnel, which is otherwise necessary for interpretation of SEP or EEG. Moreover, the technique is not dependent on a temporal window as opposed to TCD.
Patients following a minor stroke or TIA have a significantly increased risk of repeated stroke, even within hours after the initial event, and should be treated early [10, 32]. Such surgeries may then be performed outside the regular operating hours with limited personnel available. Also, some patients may not tolerate LA and need to be converted to GA during surgery. NIRS seems to be the most convenient monitoring tool for these situations. The other option would be to proceed with routine shunting. However, shunt insertion carries a risk of developing an ischemic lesion and should be avoided if unnecessary [26]. In our current study, we also found an increased number of ischemic lesions in unstable patients (50%), most of them being embolic in nature most probably resulting from inserting the shunt into a lumen of an atherosclerotic vessel.
Risk factors for shunting
The need for shunt implantation was higher in patients with a significant contralateral stenosis or occlusion. This is a consistent finding in many other studies which have shown that contralateral occlusion is probably the strongest predictor of shunt placement [9, 21]. Some authors advocate routine shunting or endovascular treatment for patients with a contralateral occlusion [18, 34]. Despite the higher incidence of neurological instability in patients with contralateral carotid occlusion in our series (60%), the majority of patients (70%) with contralateral stenosis or occlusion still did not require shunt during CEA. We thus still agree with Kretz et al. that the status of the contralateral carotid artery should not be considered a high risk for surgery, and we do not recommend either routine shunting or even primary endovascular treatment for such patients [20]. We even tend to be more active in this subgroup of patients as they carry a higher risk of ischemic stroke [1]. At the same time, we recommend a routine evaluation of the cerebral vasculature with specific focus on the collaterals in the Circle of Willis using preoperative CT angiography to better forecast the need for shunt placement.
Less is known about the influence of gender on perioperative shunting during CEA. Only a few other studies aside from ours have shown that females had a higher risk of perioperative hypoperfusion requiring shunt implantation [9, 21]. Some speculations regarding the differences in vascular anatomy compared to men have been suggested, but overall, the issue is still rather unclear and needs further studies [9, 19].
Study limitations
The study has some limitations. Most importantly, the patients were operated under LA, and thus we cannot directly extrapolate the results to CEA in GA. Interestingly, a recent study utilizing NIRS in patients undergoing CEA under GA used a cutoff value of 15% (using the INVOS technology) with very good results [17]. We have also not used any monitoring methods other than clinical testing and NIRS. The use of more techniques may provide more robust data.
Conclusions
The 10% relative decrease of the ipsilateral CO after selective cross-clamping the ICA provides a reliable cutoff with 100% sensitivity and 83% specificity in predicting cerebral hypoperfusion during CEA in patients operated under LA. NIRS is a simple non-invasive technique enabling continuous monitoring throughout the whole procedure, which may be additionally used in proper shunt positioning in cases of complicated vascular anatomy. The reliability of CO monitoring using NIRS during CEA in patients operated under GA still needs to be confirmed.
References
AbuRahma AF, Metz MJ, Robinson PA (2003) Natural history of > or =60% asymptomatic carotid stenosis in patients with contralateral carotid occlusion. Ann Surg 238:551–561 discussion 561-552
Ali AM, Green D, Zayed H, Halawa M, El-Sakka K, Rashid HI (2011) Cerebral monitoring in patients undergoing carotid endarterectomy using a triple assessment technique. Interact Cardiovasc Thorac Surg 12:454–457
American College of Cardiology Foundation/American Heart Association Task F, American Stroke A, American Association of Neuroscience N, American Association of Neurological S, American College of R, American Society of N, Congress of Neurological S, Society of Atherosclerosis I, Prevention, Society for Cardiovascular A, Interventions et al (2011) 2011 asa/accf/aha/aann/aans/acr/asnr/cns/saip/scai/sir/snis/svm/svs guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. J Neurointerv Surg 3:100–130
Bond R, Rerkasem K, Counsell C, Salinas R, Naylor R, Warlow CP, Rothwell PM (2002) Routine or selective carotid artery shunting for carotid endarterectomy (and different methods of monitoring in selective shunting). Cochrane Database Syst Rev CD000190
Calligaro KD, Dougherty MJ (2005) Correlation of carotid artery stump pressure and neurologic changes during 474 carotid endarterectomies performed in awake patients. J Vasc Surg 42:684–689
CASMED Field service manual. Fore-sight_mc-2000 series cerebral oximeter. https://www.manualslib.com/manual/1221626/Casmed-Fore-Sight-Elite.html (accessed Apr 28, 2020)
Congruksut W, Vaniyapong T, Rerkasem K (2014) Routine or selective carotid artery shunting for carotid endarterectomy (and different methods of monitoring in selective shunting). Cochrane Database Syst Rev CD000190
Davie SN, Grocott HP (2012) Impact of extracranial contamination on regional cerebral oxygen saturation: a comparison of three cerebral oximetry technologies. Anesthesiology 116:834–840
Domenick Sridharan N, Thirumala P, Chaer R, Balzer J, Long B, Crammond D, Makaroun M, Avgerinos E (2018) Predictors of cross-clamp-induced intraoperative monitoring changes during carotid endarterectomy using both electroencephalography and somatosensory evoked potentials. J Vasc Surg 67:191–198
Giles MF, Rothwell PM (2005) The need for emergency treatment of transient ischemic attack and minor stroke. Expert Rev Neurother 5:203–210
Group GTC, Lewis SC, Warlow CP, Bodenham AR, Colam B, Rothwell PM, Torgerson D, Dellagrammaticas D, Horrocks M, Liapis C, Banning AP et al (2008) General anaesthesia versus local anaesthesia for carotid surgery (gala): a multicentre, randomised controlled trial. Lancet 372:2132–2142
Guay J, Kopp S (2013) Cerebral monitors versus regional anesthesia to detect cerebral ischemia in patients undergoing carotid endarterectomy: a meta-analysis. Can J Anaesth 60:266–279
Hans SS, Jareunpoon O (2007) Prospective evaluation of electroencephalography, carotid artery stump pressure, and neurologic changes during 314 consecutive carotid endarterectomies performed in awake patients. J Vasc Surg 45:511–515
Hyttel-Sorensen S, Hessel TW, Greisen G (2014) Peripheral tissue oximetry: comparing three commercial near-infrared spectroscopy oximeters on the forearm. J Clin Monit Comput 28:149–155
Jonsson M, Lindstrom D, Wanhainen A, Djavani Gidlund K, Gillgren P (2017) Near infrared spectroscopy as a predictor for shunt requirement during carotid endarterectomy. Eur J Vasc Endovasc Surg 53:783–791
Kakkos SK, Tsolakis IA (2017) Commentary on “near infrared spectroscopy as a predictor for shunt requirement during carotid endarterectomy”. Eur J Vasc Endovasc Surg 53:792
Kondov S, Beyersdorf F, Schollhorn J, Benk C, Rylski B, Czerny M, Harloff A, Siepe M (2019) Outcome of near-infrared spectroscopy-guided selective shunting during carotid endarterectomy in general anesthesia. Ann Vasc Surg 61:170–177
Kong J, Li J, Ye Z, Fan X, Wen J, Zhang J, Liu P (2017) Carotid endarterectomy with routine shunt for patients with contralateral carotid occlusion. Ann Thorac Cardiovasc Surg 23:227–232
Krejza J, Arkuszewski M, Kasner SE, Weigele J, Ustymowicz A, Hurst RW, Cucchiara BL, Messe SR (2006) Carotid artery diameter in men and women and the relation to body and neck size. Stroke 37:1103–1105
Kretz B, Abello N, Astruc K, Terriat B, Favier C, Bouchot O, Brenot R, Steinmetz E (2012) Influence of the contralateral carotid artery on carotid surgery outcome. Ann Vasc Surg 26:766–774
Kretz B, Abello N, Bouchot O, Kazandjian C, Beaumont M, Terriat B, Bernard A, Brenot R, Steinmetz E (2014) Risk index for predicting shunt in carotid endarterectomy. Ann Vasc Surg 28:1204–1212
Li J, Shalabi A, Ji F, Meng L (2017) Monitoring cerebral ischemia during carotid endarterectomy and stenting. J Biomed Res:31
McCarthy RJ, McCabe AE, Walker R, Horrocks M (2001) The value of transcranial doppler in predicting cerebral ischaemia during carotid endarterectomy. Eur J Vasc Endovasc Surg 21:408–412
Moritz S, Kasprzak P, Arlt M, Taeger K, Metz C (2007) Accuracy of cerebral monitoring in detecting cerebral ischemia during carotid endarterectomy: a comparison of transcranial doppler sonography, near-infrared spectroscopy, stump pressure, and somatosensory evoked potentials. Anesthesiology 107:563–569
Naftalovich R, Pantin EJ, Denny JT (2015) Cerebral oximetry decrease after external carotid clamping with normal electroencephalography and no change after internal carotid clamping. A A Case Rep 5:216–218
Orlicky M, Vachata P, Bartos R, Waldauf P, Sames M (2015) A selective carotid artery shunting for carotid endarterectomy: prospective MR DWI monitoring of embolization in a group of 754 patients. J Neurol Surg A Cent Eur Neurosurg 76:89–92
Orlicky M, Hrbac T, Sames M, Vachata P, Hejcl A, Otahal D, Havelka J, Netuka D, Herzig R, Langova K, Skoloudik D (2019) Anesthesia type determines risk of cerebral infarction after carotid endarterectomy. J Vasc Surg 70:138–147
Rerkasem K, Rothwell PM (2009) Routine or selective carotid artery shunting for carotid endarterectomy (and different methods of monitoring in selective shunting). Cochrane Database Syst Rev CD000190
Rigamonti A, Scandroglio M, Minicucci F, Magrin S, Carozzo A, Casati A (2005) A clinical evaluation of near-infrared cerebral oximetry in the awake patient to monitor cerebral perfusion during carotid endarterectomy. J Clin Anesth 17:426–430
Ritter JC, Green D, Slim H, Tiwari A, Brown J, Rashid H (2011) The role of cerebral oximetry in combination with awake testing in patients undergoing carotid endarterectomy under local anaesthesia. Eur J Vasc Endovasc Surg 41:599–605
Samra SK, Dy EA, Welch K, Dorje P, Zelenock GB, Stanley JC (2000) Evaluation of a cerebral oximeter as a monitor of cerebral ischemia during carotid endarterectomy. Anesthesiology 93:964–970
Sehatzadeh S (2015) Is transient ischemic attack a medical emergency? An evidence-based analysis. Ont Health Technol Assess Ser 15:1–45
von Reutern GM, Goertler MW, Bornstein NM, Del Sette M, Evans DH, Hetzel A, Kaps M, Perren F, Razumovky A, von Reutern M, Shiogai T et al (2012) Grading carotid stenosis using ultrasonic methods. Stroke 43:916–921
Ward A, Ferraris V, Saha S (2012) Carotid endarterectomy with contralateral carotid occlusion: is shunting necessary? Int J Angiol 21:135–138
Funding
The Internal Grant Agency of the Krajská zdravotní provided financial support in the form of a grant (IGA-KZ-2017-1-13). The Health Research Council of the Czech Republic provided a grant NV19-04-00270. The sponsors had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee (Ethical Committee of the Krajská zdravotní) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Comments
We have here an outstanding clinical investigation by an experienced carotid surgery group. The study goal was to validate bilateral NIRS CO decreases with neurologic deterioration in awake patients, and thereby to establish a critical NIRS value to indicate the need for indwelling shunt.
The method was well conceived and elegant. Patients were monitored for CO with forehead NIRS monitors, and were also operated under loco-regional anesthesia. In this way the accuracy of the NIRS could be validated, but the shunting decision was actually made by direct observation of the patient for neurological changes.
One hundred and five patients (85%) were stable throughout the CEA, while 20 patients (15%) clinically deteriorated during surgery. The mean drop in the cerebral oximetry after selective ICA clamping in clinically stable patients was 6%, while in patients with clinical deterioration the NIRS decreased by 14.5% (p < 0.05). When the cut-off value for selective shunting was set as a 10% decrease of the ipsilateral cerebral oximetry after selective ICA clamping, the sensitivity of the technique was 100% and the specificity 83.0%.
As side observations, female sex and contralateral stenosis/occlusion were significant risk factors for shunt placement.
The series is not completely pure, since for various reasons 5% of the patients were not shunted despite a neurological change suggesting that shunt placement would be beneficial (the unstable group). Four patients (3.2% of the series) in the unstable group suffered strokes; it is unclear whether these patients were the non-shunted patients or shunted patients, but this is of concern.
DWI MRI studies were done postop in 120/125 patents and the results are fascinating. In the group of stable patients (n = 100), they observed a new lesion in 8 (8%). In the patients with clinical deterioration (n = 20), they observed a new ischemic lesion in 11 (55.0%) patients (p < 0.001). Clearly even most patients with DWI lesions were clinically well, but it is interesting and worrisome that patients showed DWI lesions despite successful shunt placement.
My personal technique is somewhat different, and my shunt placement rate is a little higher. I use combined EEG/SSEP monitoring and general anesthesia for CEA. We shunt for any change at all in either EEG or SSEP, or both. With this technique I shunt 15% of patients, and 25% if there is contralateral occlusion, strictly by following the monitoring (1).
The authors conclude that a 10% decrease in the ipsilateral brain tissue oximetry after selective cross-clamping of the ICA provides a reliable cut-off value for selective shunting during CEA. I agree with this, and I believe their study is a true contribution to our knowledge of selective shunting and patient protection for best outcomes.
Christopher Miranda Loftus
PA, USA
1. Loftus CM: Carotid Artery Surgery: Principles and Technique. 2nd edition. New York, Informa Publishing 2006.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Vascular Neurosurgery - Ischemia
Rights and permissions
About this article
Cite this article
Hejčl, A., Jiránková, K., Malucelli, A. et al. Selective internal carotid artery cross-clamping increases the specificity of cerebral oximetry for indication of shunting during carotid endarterectomy. Acta Neurochir 163, 1807–1817 (2021). https://doi.org/10.1007/s00701-020-04621-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-020-04621-1