Abstract
Background
The strategy for surgical treatment of tethered cord syndrome in pediatric patients is well established but still bares challenges for adult patients. This retrospective study was performed to assess the surgical outcome of adult patients with a secondary tethered cord syndrome and to evaluate the benefit of intraoperative neuromonitoring.
Methods
Clinical charts of 32 consecutive adult patients who underwent in total 38 surgical untethering procedures at our facility between 2008 and 2018 were retrospectively analyzed. Epidemiological data, MRI scans, and postoperative results were evaluated.
Results
The retethering rate in our patient cohort was 16%. Main complaints were maximal pain (82%), bladder dysfunction (79%), paresthesia (68%), and weakness in the lower extremities (68%). Forty-eight months after surgery, patients’ symptoms generally improved, with an average level of pain of 19.1% (95% CI, 5.7–32.5%), paresthesia 28.7% (95% CI, 12.6–44.8%), weakness in the lower extremities 27.7% (95% CI, 11.1–44.4%), and bladder dysfunction 60.2% (95% CI, 41.6–78.7%). The use of neuromonitoring appears to have a positive impact on patient weakness (OR = 0.07; 95% CI, 0.01–0.68) and paresthesia (OR = 0.03; 95% CI, 0.00–2.18). This benefit is less clear for the retethering rate (OR = 0.45; 95% CI, 0.06–3.26) or the overall clinical outcome (OR = 0.70; 95% CI, 0.14–3.45). The presence of a preoperative Chiari syndrome, syringomyelia, or scoliosis had no relevant influence on the retethering rate.
Conclusions
Our data confirms that untethering surgery in adult patients is relatively safe and has a reasonable chance of clinical improvement of pain, paresthesia, and weakness in the lower extremities. The use of intraoperative monitoring has a positive influence on the improvement of preoperative paralysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tethered cord syndrome (TCS) was first described in the literature in 1953 as “filum terminale syndrome” or “the cord traction syndrome”. [5] In 1976, the same symptom complex was described as “tethered spinal cord”. [8] Since 1981, “tethered cord syndrome” became the commonly used term, to describe the clinical condition including its underlying pathophysiology. [42] TCS is the result of stretch-induced damages to the spinal cord due to its adhesion with the vertebral column, mainly in the lumbosacral region. [39] Due to tension forces, the oxidative metabolism is altered by impaired microcirculation which can lead to a reduction in cytochrome A, A3, and can cause shifts in the redox ratio in the mitochondrial compartments. These changes on the cellular level can lead to a structural damage of neurons, analogous to effects of hypoxia or ischemia. The extent of damage depends on the duration and force on the spinal cord. [36, 40,41,42]
TCS is known to occur more frequently in children. It was originally described as a pediatric disease with the indication for microsurgical release of the tethered cord to prevent long-term neurological damages and secure the normal development of the child. TCS is often classified in two groups: primary and secondary TCS. As a cause for the primary TCS in children, mostly different forms of congenital spinal dysraphism can be identified. [24] These defects develop during the third and fourth week of pregnancy, if the physiological separation of the neural tube is disturbed. This can lead to the development of closed spinal dysraphism which prevents the ascension of the spinal cord by the result of tethering mesenchymal tissue components. [16, 24] The secondary form is an acquired pathology caused by previous operations, scar tissue development after open dysraphism closure, inflammation, or neoplasia. [2, 10, 16, 30, 31, 37] In the majority of patients after open dysraphism closure, TCS is associated with a low lying conus medullaris below the level of the second lumbar vertebral body. [42] In adult patients, the underlying condition causing the tethered cord is usually complex. More often a combination of primary and secondary causes can be found. The diversity and complexity of tethered cord syndrome in adults was already described by Pang et al. in 1982. [20]
The clinical presentation of TCS is heterogeneous. Main complaints include neurological, urological, and orthopedic disturbances. Neurological symptoms include pain (e.g., lumbar pain, sciatica), sensibility disorders (e.g., altered sensation of dysesthesia or paresthesia), motor weakness (spastic or floppy), and muscle atrophy. [11, 15, 37] Urological symptoms include bladder and bowel incontinence or retention. Orthopedic sequelae are deformities of the feet, knees, and hips, as well as scoliosis. All these symptoms can be present in one patient at the same time, but a patient can also present with a singular symptom, e.g., pain. [15, 33]
If clinical symptoms occur, they usually progress slowly over time, as a wide range of time to onset is well documented. [24] Usually, the diagnosis is based on a thorough neurological examination, a MRI scan of the spine and urodynamic studies. The diagnosis of a tethered cord syndrome must in advance exclude other possible causes for clinical deterioration, e.g., due to degenerative changes in the adult spine. Due to heterogeneous clinical presentation of TCS, it remains controversial when surgery is properly indicated in an adult patient. Scarce clinical data and surgical outcome measures have been published so far, especially when it comes to complex patients with multiple secondary untethering surgeries. [27, 28, 35, 37, 38]
The aim of this paper is to describe our patient collective of adults with secondary TCS and the outcome after microsurgical untethering. The influence of intraoperative neuromonitoring on the mid to long-term success and the reoperation rate are evaluated.
Materials and methods
Study design and population
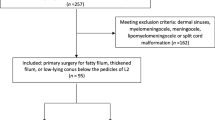
This retrospective study was conducted in our tertiary healthcare center. We reviewed clinical charts, operative records, and MRI images of all adult patients with a TCS who were surgically treated in our department between January 2008 and December 2018. The indication for surgery was clinical deterioration of symptoms in combination with an existing TCS due to previously treated spinal dysraphism. The exclusion criteria were as follows: (1) age under 18 years at the time of surgery, (2) patients with TCS caused by posttraumatic or inflammatory arachnoid scarring, and (3) adult patients with a primary TCS. Thus, all included patients were diagnosed with dysraphic malformations and treated previously. Finally, 32 patients with a total of 38 surgeries met our study criteria.
Patient data was collected using a standardized case report form. The radiological measurements were performed by two independent researchers and the mean value was used for the statistical analysis. We extracted variables related to patient characteristics (gender, age, length of hospital stay, number of previous untethering surgeries, time to the most recent untethering surgery, and preoperative complaints) and disease characteristics (type of dysraphic malformation). Analyzed complaints were pain, weakness in the lower extremities, paresthesia, and bladder dysfunction. These parameters were also assessed at all follow-up presentations as a routine procedure in our department. In addition, Odom’s criteria were calculated on the last documented outpatient visit to evaluate overall clinical outcome of the patient. [18] Evaluated surgical parameters included duration of surgery, intraoperative use of electrophysiological neuromonitoring, postoperative complications, and revision surgeries.
MRI imaging
All patients were preoperatively examined with a standard MRI of the spine. The prevalence and the extent of a Chiari malformation, a syringomyelia, just as the presence and severity of a neurogenic scoliosis were evaluated. A scoliosis was defined as a Cobb angle larger than 10°. The preoperative measurements were performed using the same imaging modalities (comparison T1 with T1 or T2 with T2, respectively).
Surgical procedure
All surgical procedures were performed under sterile conditions in the operating room with the patient under general anesthesia. The patient was operated via a midline incision in a prone position. Using microsurgical technique, the untethering of neural structures was achieved by its meticulous separation from mesenchymal tissue. Great emphasis was put on minimizing bleeding intradurally to prevent associated subarachnoiditis later on. Before the dura was finally closed, we irrigated the intradural space until the fluid was completely clear. Dura closure was done in a watertight fashion with a running suture with or without an additional duraplasty. To compensate for the loss of CSF due to the dura opening, we instilled irrigation fluid before finally putting the last stich. Based on our experience, we can hereby establish sufficient space of CSF flow around the neural structures and reconstruct the diameter of the dural sac to its maximal size. Subsequently, re-adaptation of the rectus spinal muscles and the watertight closure of the fascia was conducted. A two-layered wound closure including topical skin adhesive finished the procedure.
Intraoperative electrophysiologic neuromonitoring
Intraoperative neuromonitoring was an adjunct in 21 patients. All patients were monitored with mapping and monitoring techniques tailored to individual requirements and demands/extent of the surgical intervention (ongoing free-running EMG, evoked potentials, including EMG and motor-evoked potentials (MEP), as well as bulbocavernosus reflex (BCR)). [13] The anesthetic procedure was adapted to intraoperative neuromonitoring (IONM) requirements. The recording of baseline MEP and BCR was carried out before skin incision and the subsequent potentials that were evoked throughout the surgery at regular intervals and on demand from the surgeon were assessed by the waveform shape and amplitude changes to differentiate scar tissue from any structures suspicious of containing functional neural elements.
We recorded the use of IONM and analyzed its effect on postoperative symptoms in the course of follow-up by dividing the patients into two groups: patients who received IONM and patients who were operated without. The association between the use of IONM and the frequency of repeated untethering surgery in the follow-up was analyzed.
Statistical analysis
We report absolute and relative frequencies for categorical variables, median along the interquartile range (IQR) for continuous variables by case—in total and by IONM. The course of the longitudinal data is displayed graphically in a scatterplot, using a locally weighted scatterplot smoothing (LOESS) estimate for the trend within a follow-up of 42 months after surgery—for all cases, just as by IONM group.
Mixed logistic regression models are applied to assess the impact of IONM on retethering and Odom’s criteria, introducing a random intercept by patient to control for the fact that some patients were treated more than once. Odom’s criteria are categorized into unfavorable (fair or poor) and favorable (good or excellent) outcome, and this analysis is adjusted for the time at which Odom’s criteria were evaluated, i.e., the last observation for each case.
Outcome parameters assessed at baseline and regularly in a longitudinal follow-up are bladder dysfunction, pain, paresthesia, and weakness in the lower extremities. These were all measured on an ordinal scale with 5 possible categories (0%, 25%, 50%, 75%, 100%), where higher categories indicate a worse patient condition. In the primary analysis, we use mixed ordinal regression with these four parameters as the ordinal dependent variable, taking into account the hierarchy in the data, where patients are treated more than once and potentially observed at multiple times during follow-up. Explanatory variables in these models are IONM, the respective level at baseline, age, duration of the surgery, and whether the patient already received more than one previous surgery. To account for the follow-up visits after surgery taking place at different times, we additionally adjust the models for the time of the visit. As a sensitivity analysis, we also applied mixed linear regression models, i.e., assuming that the dependent variable is measured on a continuous scale.
Results are displayed as odds ratio (OR) estimates along with 95% confidence intervals (CI) for logistic regression, just as for the ordinal regression assuming proportional odds, and for linear regression the regression coefficients are reported.
Statistical analyses were performed using R, just as additional R packages for data handling and plotting, and mixed models. [3, 4, 23, 34]
Results
A total of 32 consecutive adult patients underwent untethering surgery for secondary tethered cord syndrome in our department from January 2008 until December 2018. Due to the fact that some patients had to be re-operated in the follow-up due to a retethering episode, we evaluated 38 surgical cases in total. The analysis included 12 (37.5%) male and 20 (62.5%) female patients, with a median age of 25.7 years (IQR, 21.1–35.4; range, 18–74) at the time of surgery. The median time interval between the previous operation and the retethering episode was 11.0 years (IQR, 3.4–20.7; range, 1.5–44.9). All baseline characteristics are reported in Table 1. The initial diagnoses of the patients are summarized in Fig. 1. The median hospital stay was 8.0 days (IQR, 7.0–10.0; range, 4–36). All cases (100%) were re-untethering cases with one or multiple untethering surgeries in the past. The follow-up was conducted in our outpatient clinic. The median duration of the follow-up was 3.1 years (IQR, 1.6–5.3; range, 0.6–10.8). After 24 months, around 60% of the patients were still routinely followed up in our outpatient clinic, after 36 months 50%, and after 48 months still 40% of the patients were seen regularly. Only three patients had a follow-up below 1 year in our department due to a patient’s change of residence (follow-up rate at 12 months 91%).
The clinical symptoms leading to the diagnosis and surgical treatment were pain in 82% (31/38), bladder/bowel dysfunction in 79% (30/38), followed by paresthesia in 68% (26/12), and weakness in the lower extremities 68% (26/12). Directly after surgery, most of the cases were either neurologically stable or showed improvements (n = 37). However, neurological symptoms of one case (2.6%) worsened, as he developed a new minor weakness of the right foot and neuropathic pain. The patient improved over time, but the symptoms did not fully resolve by the time of discharge. The incidence for immediate revision surgery for surgery-related complications was 7.9% (3/38). All three patients developed a CSF fistula with cerebrospinal fluid leakage. All revision surgeries were performed successfully without any long-term sequelae.
Clinical outcome is presented based on a smoothed estimate to see the trend over time (Fig. 2). At 48 months after surgery, patients generally showed improvements of the preoperative complaints. On average, pain was at 19.1% (95% CI, 5.7–32.5%) compared with 81% at baseline, paresthesia at 28.7% (95% CI, 12.6–44.8%) compared with 68%, weakness in the lower extremities at 27.7% (95% CI, 11.1–44.4%) compared with 68%, and bladder dysfunction at 60.2% (95% CI, 41.6–78.7%) compared with the initial 79%. Overall, there was an improvement in the clinical symptoms in the course of the postoperative period; this improvement trend was least pronounced with regard to bladder dysfunction.
Overall course of symptoms of all patients over time (pain, bowel and bladder dysfunction, paresthesia, and weakness in the lower extremities). The lines show the extent of the symptoms in percent, estimated with locally weighted scatterplot smoothing. Each dot represents one clinical evaluation of a patient during a follow-up visit, varied around measured categories for better visualization. The 95% confidence intervals are indicated with gray shading
Five patients experienced one and one patient experienced two retethering episodes with a total retethering rate of 19%. All patients were untethered again in our department as new symptoms developed during follow-up. The median time until the retethering was 22.5 months (IQR, 7.0–44.8; range, 4.0–53.0). New symptoms leading to a re-untethering surgery in all cases were a combination of symptoms, where weakness, pain, and bladder dysfunction were the most prominent. Overall, outcome assessment of all patients was done by applying Odom’s criteria on the last outpatient visit. One patient (3%) had an excellent outcome, 20 patients (63%) showed a good outcome, 8 patients (25%) had a fair outcome, and 3 patients (9%) were rated as poor. In the mixed logistic regression analysis, only a slight potential benefit of IONM with respect to Odom’s criteria (OR 0.70; 95% CI, 0.14–3.45) was seen (Fig. 3). All patients who had to be re-untethered during our follow-up showed either a good or excellent outcome measured by Odom’s criteria.
Forest plot displaying the effect of the use of intraoperative neuromonitoring on the retethering rate and Odom’s criteria. Results are displayed as odds ratio estimates along with 95% confidence intervals, based on mixed logistic regression models, controlling for last time seen for Odom’s criteria
MRI analysis
All patients were preoperatively analyzed with an MRI scan of the spine. The presence and extent of a Chiari malformation, a syringomyelia, and a scoliosis were evaluated. In 39% of the patients, a Chiari malformation was detected with a mean tonsillar herniation of 15.6 ± 9.1 mm. A syringomyelia with a width of 2.8 ± 2.4 mm and a scoliosis with an average Cobb angle of 19.5 ± 9.5° were evident on the imaging studies in 29% of the cases. A Chiari malformation was present in 33% of patients with a retethering, in comparison with 41% of patients without a retethering episode. Seventeen percent of patients with a retethering episode had both a syringomyelia and a scoliosis. In comparison, 31% of the patients without a retethering had a syringomyelia and scoliosis.
Intraoperative neuromonitoring
Intraoperative neuromonitoring (IONM) was applied during surgery in 25 of the 38 cases (65.8%). It became standard of care in 2013 and was therefore subsequently applied to all cases, prior to 2013 only if available. The median duration of surgery using IONM was 221 min (IQR, 187–247; range, 78–303), compared with 190 min (IQR, 155–207; range, 90–231) for surgeries without IONM. The clinical outcome is again presented based on a smoothed estimate, separated by IONM groups (Fig. 4).
Comparison of the course of symptoms over time according to whether IONM was used (solid lines) or IONM was not used (dotted lines) according different symptom categories as pain, bowel and bladder dysfunction, paresthesia, and weakness in the lower extremities, respectively. The lines show the extent of the symptoms in percent, estimated with locally weighted scatterplot smoothing. Each dot represents one clinical evaluation of a patient during a follow-up visit, varied around measured categories for better visualization. The 95% confidence intervals are indicated with gray shading
Based on a mixed logistic regression, IONM was somewhat related to a lower retethering rate (OR = 0.45; 95% CI, 0.06–3.26); a definite advantage of IONM can however not be postulated due to large imprecision in the effect (Fig. 3).
Based on mixed ordinal regression models adjusting for time of measurement after surgery, the use of neuromonitoring had a significant positive impact on the weakness in the lower extremities for the whole follow-up (OR = 0.07; 95% CI, 0.01–0.68). The use of IONM reduced the odds of an increased level of weakness by 93%. Patients may benefit from the use of IONM regarding a lower level of paresthesia (OR = 0.03; 95% CI, 0.00–2.18); however, this effect cannot be estimated with a satisfying level of precision, as indicated by a wide range of the CI. The benefit of IONM on pain (OR = 0.77; 95% CI, 0.16–3.72) and bladder dysfunction (OR = 0.54; 95% CI, 0.02–19.22) is even less clear (Fig. 5).
Additionally, we also introduced the following potentially explanatory variables to the model, due to problems in model estimation only where feasible: the respective baseline assessment, age (in 10-year increments), duration of surgery (in 1-h increments), and the number of previous untethering surgeries (Fig. 6). Patients with pain as a symptom preoperatively were more likely to have a worse pain in the long-term follow-up. Older age seems to be of benefit overall, but especially concerning improvements in paresthesia. The duration of surgery did not relevantly influence any of the symptoms in the long-term follow-up. Repeated untethering surgeries seem to have a positive effect on the patient’s weakness and a negative effect on pain. The longer the follow-up, the more likely are improvements in pain, weakness, and paresthesia. Bladder dysfunction is not expected to improve over time. The results based on the additionally carried out mixed linear regression as a sensitivity analysis widely confirm the results derived from the ordinal model (Supplementary Figure 1).
Forest plot displaying the effect of several explanatory variables (IONM, baseline assessment, age in 10-year increments, duration of surgery in 1-h increments) on clinical outcome parameters: weakness, paresthesia, pain, and bladder dysfunction. Not all explanatory variables could be included in all models. Results are displayed as odds ratio estimates along with 95% confidence intervals, based on mixed ordinal regression models, controlling for time of measurement
Discussion
The main findings of this study are the following ones:
-
1)
The retethering rate in our patient cohort was almost 20%.
-
2)
Microsurgical untethering surgery for tethered cord syndrome in adult patients can be performed with a low risk of complications, and with satisfying degree of improvement in pain, weakness in the lower extremities, and paresthesia.
-
3)
The risk to develop a retethering episode may be reduced by the use of IONM.
-
4)
The application of IONM leads to an improvement in the motor function of the patients in the clinical course without significantly extending the operating time.
Different approaches have been suggested to understand the mechanism behind the fact that the time of symptom onset and the type of complaint shows such large heterogeneity in adults compared with pediatric patients, among whom a deterioration of the bladder dysfunction is most often the prevalent sign for a retethering. [19] Since the main trigger of symptoms in pediatric patients may be growth, other viable causes for a symptom onset in adult patients are discussed. Pang et al. suggested that sudden movements like bending or trauma may cause further deterioration due to intermittent traction on the cord. [20] Gupta et al. indicated that natural head flexion with time could injure the conus medullaris. [6] Shukla et al. were able to verify these causes, as seven of their patients had a very active lifestyle. [27] Pathophysiologically, a clinical deterioration may be caused by a limited plasticity of neurons in slowly progressing chronically stretched spinal cord, which at some point is not any longer able to compensate for the existing traction. [40] A combination of these factors and a progression of arachnoid scarring over time from previous surgeries could explain the difference of symptomatology in adult TCS.
We know that especially adult patients experience a high rate of retethering with the indication for repeated untethering surgeries. [20, 24, 29, 37] Rates for relevant retethering episodes in adult patients reach up to 50%. [29] The large variability in the published figures may be related to the differences in the follow-up duration. A longer follow-up seems to correlate with a higher number of recurrences. Due to the disappointing results, other treatment options have been studied. In the recent years, the number of publications describing spinal column shortening procedures as an effective treatment option has increased. [1, 9, 12] Other authors recommend a more conservative approach with omnidirectional care or spinal cord stimulation as viable treatment options. [17, 29] However, we are convinced that a repetition of the untethering procedure should remain the standard therapy for these patients. Our results show that a meticulous re-untethering can result in an acceptable to good outcome in most patients. We could also demonstrate that older age should not be a reason not to perform a re-untethering operation in a symptomatic patient. Surprisingly, higher age at the time of surgery was related with higher chances of symptom improvement in our cohort. Except for the symptom pain, the number of previously performed untethering procedures seems to have no negative consequences for the recovery of a weakness in the legs or the improvement of the sensitivity in the legs.
Pain was the most common symptom in adult patients with secondary TCS. It was also the complaint that improved the most in our follow-up. Shukla et al. described similar results in their study of 20 adult patients (age > 16) of whom 13 (65%) presented with pain, and in 11 (84.6%) patients, the pain improved after surgery. [27] Lee et al. mentioned pain as the chief complaint in their study of 64 patients. Forty-three patients presented with back pain and 33 with leg pain, with an improvement after surgery in 78% and 83% of all cases. [15] Our results concerning paresthesia and weakness of the lower extremities are also in line with previously published articles reviewing the surgical outcome for TCS. [6, 10, 11, 20, 32, 37] Our results on bladder dysfunction do not suggest a relevant improvement of the patients in the follow-up compared with the preoperative symptom severity, which is in line with other published results. [29]
Although surgical untethering in children is well established as a treatment, surgery in adults, especially with complicated retethering cases, is still a subject of discussion. By including only secondary TCS patients with spinal dysraphism as a precondition, we showed that surgery can be performed safely and with long-term success in the majority of patients, particularly if IONM is used. Just one patient experienced a deterioration of symptoms as a direct consequence of the operation. Krassioukov et al. similarly reported a permanent neurological complication rate of 1.6%. [14] Haro et al. described the advantage of evoked EMG to better determine neural tissue from lipomatous structures. [7] The beneficial use of multimodal intraoperative neuromonitoring with SSEPs (high specificity and low sensitivity) and continuous EMG (low specificity and high sensitivity) has also been demonstrated. [21] Due to the use of IONM, a better identification of anatomical structures, as well as a proof of functionality, is possible. [22] In particular the motor nerve roots can be easily identified and protected. This may explain why the motor outcome of our patients operated under IONM was better in the long-term follow-up. Our hopes that IONM allows a more aggressive approach that directly translates into a lower reoperation rate due to a more complete untethering were not fulfilled, although there was a weak tendency towards a lower retethering rate in our statistical analyses. An increase in safety of untethering procedures with a minimization of the short to mid-term morbidity due to the use of IONM has already been shown elsewhere. [25, 26] MRI imaging plays an important role as an adjunct tool in the diagnosis of tethered cord syndrome in adults.
Limitations
Due to the single center, retrospective nature of this study and the long-time span of inclusion, certain limitations have to be mentioned. This study is the non-randomized patient allocation to IONM. However, the decision on the use of IONM was not linked to patient characteristics. It rather resulted from historical differing treatment protocol, since IONM was not available for every surgery before 2013. There was no selection bias concerning the inclusion of patients, as we analyzed all consecutive adult patients with a secondary TCS in a predefined period of time. Concerning outpatient follow-up, we cannot exclude a bias since there were differences in the total length and the frequency the patients were seen in our outpatient clinic. These limitations resulted in non-standardized neurological examination data acquisition as well as in varying sample sizes during different follow-up time points. Patients with a very satisfactory postoperative result may have avoided a long-term follow-up as they may have been less affected by relevant symptoms. On the other hand, patients with subjectively less satisfactory result may have asked for a second opinion in another department as well. Both effects are known in retrospective studies and cannot be entirely excluded. The comparably low number of included patients due to the rare nature of the disease is another limitation that must be taken into account when interpreting results of statistical analyses and deducing generalized conclusions. However, any prospective controlled study on the topic appears to be more than challenging.
Conclusion
Even though TCS in adults is a rather rare disease and only few clinical centers treat this syndrome frequently, we believe that the decision to operatively treat those patients is solidly founded on the data assessed in the present study. Especially in patients needing multiple untethering surgeries, good outcome results can be achieved. For this reason, we see no restrictions towards repeated untethering procedures in clinically deteriorating adult patients with TCS, independently of the number of previous surgeries. We clearly recommend using IONM in each adult patient during untethering surgery. Our data shows that long-term motor outcome in particular benefits from the use of intraoperative monitoring.
References
Aldave G, Hansen D, Hwang SW, Moreno A, Briceno V, Jea A (2017) Spinal column shortening for tethered cord syndrome associated with myelomeningocele, lumbosacral lipoma, and lipomyelomeningocele in children and young adults. J Neurosurg Pediatr 19:703–710
Bowman RM, Mohan A, Ito J, Seibly JM, McLone DG (2009) Tethered cord release: a long-term study in 114 patients. J Neurosurg Pediatr 3:181–187
Christensen RHB (2019) Ordinal - regression models for ordinal data
Douglas Bates MM, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Garceau GJ (1953) The filum terminale syndrome (the cord-traction syndrome). J Bone Joint Surg Am 35-A:711–716
Gupta SK, Khosla VK, Sharma BS, Mathuriya SN, Pathak A, Tewari MK (1999) Tethered cord syndrome in adults. Surg Neurol 52:362–369 discussion 370
Haro H, Komori H, Okawa A, Kawabata S, Shinomiya K (2004) Long-term outcomes of surgical treatment for tethered cord syndrome. J Spinal Disord Tech 17:16–20
Hoffman HJ, Hendrick EB, Humphreys RP (1976) The tethered spinal cord: its protean manifestations, diagnosis and surgical correction. Childs Brain 2:145–155
Hsieh PC, Stapleton CJ, Moldavskiy P, Koski TR, Ondra SL, Gokaslan ZL, Kuntz C (2010) Posterior vertebral column subtraction osteotomy for the treatment of tethered cord syndrome: review of the literature and clinical outcomes of all cases reported to date. Neurosurg Focus 29:E6
Huttmann S, Krauss J, Collmann H, Sorensen N, Roosen K (2001) Surgical management of tethered spinal cord in adults: report of 54 cases. J Neurosurg 95:173–178
Iskandar BJ, Fulmer BB, Hadley MN, Oakes WJ (2001) Congenital tethered spinal cord syndrome in adults. Neurosurg Focus 10:e7
Kokubun S, Ozawa H, Aizawa T, Ly NM, Tanaka Y (2011) Spine-shortening osteotomy for patients with tethered cord syndrome caused by lipomyelomeningocele. J Neurosurg Spine 15:21–27
Kothbauer KF, Deletis V (2010) Intraoperative neurophysiology of the conus medullaris and cauda equina. Childs Nerv Syst 26:247–253
Krassioukov AV, Sarjeant R, Arkia H, Fehlings MG (2004) Multimodality intraoperative monitoring during complex lumbosacral procedures: indications, techniques, and long-term follow-up review of 61 consecutive cases. J Neurosurg Spine 1:243–253
Lee GY, Paradiso G, Tator CH, Gentili F, Massicotte EM, Fehlings MG (2006) Surgical management of tethered cord syndrome in adults: indications, techniques, and long-term outcomes in 60 patients. J Neurosurg Spine 4:123–131
Lew SM, Kothbauer KF (2007) Tethered cord syndrome: an updated review. Pediatr Neurosurg 43:236–248
Novik Y, Vassiliev D, Tomycz ND (2019) Spinal cord stimulation in adult tethered cord syndrome: case report and review of the literature. World Neurosurg 122:278–281
Odom GL, Finney W, Woodhall B (1958) Cervical disk lesions. J Am Med Assoc 166:23–28
Ogiwara H, Lyszczarz A, Alden TD, Bowman RM, McLone DG, Tomita T (2011) Retethering of transected fatty filum terminales. J Neurosurg Pediatr 7:42–46
Pang D, Wilberger JE Jr (1982) Tethered cord syndrome in adults. J Neurosurg 57:32–47
Paradiso G, Lee GY, Sarjeant R, Hoang L, Massicotte EM, Fehlings MG (2006) Multimodality intraoperative neurophysiologic monitoring findings during surgery for adult tethered cord syndrome: analysis of a series of 44 patients with long-term follow-up. Spine (Phila Pa 1976) 31:2095–2102
Pouratian N, Elias WJ, Jane JA Jr, Phillips LH 2nd, Jane JA Sr (2010) Electrophysiologically guided untethering of secondary tethered spinal cord syndrome. Neurosurg Focus 29:E3
R Core Team (2019) A language and environment for statistical computing. In: R Foundation for Statistical Computing V, Austria (ed)
Rajpal S, Tubbs RS, George T, Oakes WJ, Fuchs HE, Hadley MN, Iskandar BJ (2007) Tethered cord due to spina bifida occulta presenting in adulthood: a tricenter review of 61 patients. J Neurosurg Spine 6:210–215
Sala F, Squintani G, Tramontano V, Arcaro C, Faccioli F, Mazza C (2013) Intraoperative neurophysiology in tethered cord surgery: techniques and results. Childs Nerv Syst 29:1611–1624
Sala F, Tramontano V, Squintani G, Arcaro C, Tot E, Pinna G, Meglio M (2014) Neurophysiology of complex spinal cord untethering. J Clin Neurophysiol 31:326–336
Shukla M, Sardhara J, Sahu RN, Sharma P, Behari S, Jaiswal AK, Srivastava AK, Mehrotra A, Das KK, Bhaisora KS (2018) Adult versus pediatric tethered cord syndrome: clinicoradiological differences and its management. Asian J Neurosurg 13:264–270
Stetler WR Jr, Park P, Sullivan S (2010) Pathophysiology of adult tethered cord syndrome: review of the literature. Neurosurg Focus 29:E2
Sun J, Zhang Y, Wang H, Wang Y, Yang Y, Kong Q, Xu X, Shi J (2018) Clinical outcomes of primary and revision untethering surgery in patients with tethered cord syndrome and spinal bifida. World Neurosurg 116:e66–e70
Tani S, Yamada S, Fuse T, Nakamura N (1991) Changes in lumbosacral canal length during flexion and extension--dynamic effect on the elongated spinal cord in the tethered spinal cord. No To Shinkei 43:1121–1125
Tani S, Yamada S, Knighton RS (1987) Extensibility of the lumbar and sacral cord. Pathophysiology of the tethered spinal cord in cats. J Neurosurg 66:116–123
van Leeuwen R, Notermans NC, Vandertop WP (2001) Surgery in adults with tethered cord syndrome: outcome study with independent clinical review. J Neurosurg 94:205–209
Warder DE, Oakes WJ (1994) Tethered cord syndrome: the low-lying and normally positioned conus. Neurosurgery 34:597–600 discussion 600
Wickham et al (2019) Welcome to the tidyverse. J Open Source Softw 4:1686
Yamada S (2004) Tethered cord syndrome in adults and children. Neurol Res 26:717–718
Yamada S, Knerium DS, Mandybur GM, Schultz RL, Yamada BS (2004) Pathophysiology of tethered cord syndrome and other complex factors. Neurol Res 26:722–726
Yamada S, Lonser RR (2000) Adult tethered cord syndrome. J Spinal Disord 13:319–323
Yamada S, Siddiqi J, Won DJ, Kido DK, Hadden A, Spitalieri J, Everett BA, Obasi CG, Goldenberg TM, Giles LG, Yamada SM (2004) Symptomatic protocols for adult tethered cord syndrome. Neurol Res 26:741–744
Yamada S, Won DJ (2007) What is the true tethered cord syndrome? Childs Nerv Syst 23:371–375
Yamada S, Won DJ, Pezeshkpour G, Yamada BS, Yamada SM, Siddiqi J, Zouros A, Colohan AR (2007) Pathophysiology of tethered cord syndrome and similar complex disorders. Neurosurg Focus 23:E6
Yamada S, Won DJ, Siddiqi J, Yamada SM (2004) Tethered cord syndrome: overview of diagnosis and treatment. Neurol Res 26:719–721
Yamada S, Zinke DE, Sanders D (1981) Pathophysiology of “tethered cord syndrome”. J Neurosurg 54:494–503
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Spine - Other
Electronic supplementary material
ESM 1
(TIFF 31640 kb)
Rights and permissions
About this article
Cite this article
Finger, T., Aigner, A., Depperich, L. et al. Secondary tethered cord syndrome in adult patients: retethering rates, long-term clinical outcome, and the effect of intraoperative neuromonitoring. Acta Neurochir 162, 2087–2096 (2020). https://doi.org/10.1007/s00701-020-04464-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-020-04464-w