Abstract
Accumulation of contrast medium in the subdural space after diagnostic intraarterial contrast administration is a rare observation. The authors report the case of a subdural contrast effusion (SCE) presenting during endovascular treatment of an intracranial dural arteriovenous fistula (DAVF) mimicking an acute subdural hematoma. Differentiation between the two by computed tomography (CT) or intraprocedural Dyna CT and early neurological examination can be crucial for patient management. We believe that repeated large-volume contrast injections via large-bore intermediate catheters into the territory of an (even partly) occluded DAVF may induce leakage of contrast medium into the extravascular subdural space thereby causing a SCE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Subdural contrast effusions (SCE) are rarely encountered but may indicate a diagnostic problem when present during endovascular therapy (EVT). The rapid evolution and hyperdense attenuation of a subdural effusion can mimic an acute subdural hematoma (SDH), and differentiation between the two can have important implications for patient treatment. SCEs have been reported on follow-up computed tomography (CT) after percutaneous coronary intervention [2, 5], endovascular treatment of subarachnoid hemorrhage [8, 10], and in 8.2% of patients with blunt trauma [1]. Only one previous report has described the formation of a SCE during an endovascular procedure [3] probably caused by a dural tear. We here present the unique case of an asymptomatic SCE, which developed during transarterial embolization of a dural arteriovenous fistula (DAVF).
Clinical presentation
Clinical presentation and diagnostic workup
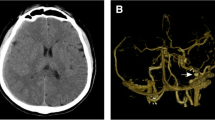
A previously healthy 52-year-old woman presented with a 4-month history of left-sided numbness of the upper lip, cheek, tongue, and forehead. Neurological examination revealed hypalgesia and dysalgesia in the dermatomes of the first and second division of the left trigeminal nerve. Digital subtraction angiography (DSA) showed a right tentorial DAVF with a venous ectasia (Fig. 1a). The DAVF was mainly supplied by the middle meningeal, occipital, and the marginal tentorial arteries and drained into the galenic venous system.
a Right external carotid artery angiogram in frontal view shows a right tentorial dural arteriovenous fistula at the level of the superior petrosal sinus. The fistula was drained by the deep venous system via the lateral mesencephalic vein (short arrow), vein of Galen, and the left transverse sinus (long arrow). A venous ectasia was present. b Right occipital artery angiogram obtained using a large-bore intermediate Sofia® catheter (catheter tip, long arrow) shows small dural arteries (short arrows) supplying the dural network of the lateral transverse sinus and tentorium (arrowheads). The SCE later developed in this area
Endovascular procedure
The patient underwent transfemoral endovascular treatment with triaxial catheterization of the right occipital artery (Fig. 1b) using a Neuron™ MAX sheath (Penumbra, CA, USA), a Sofia® 55 distal access catheter (MicroVention, CA, USA), and a 1.3-F Headway® Duo microcatheter (MicroVention). The microcatheter was placed in a pedicle close to the fistula site (Fig. 2a) and slow injection of PHIL™ 25% (MicroVention) into the small dural network feeding the arteriovenous shunt was performed and resulted in reduced flow with contrast stagnation in the venous ectasia. Surprisingly, towards the end of the procedure, some contrast stagnation was also seen on DSA and unsubtracted images along the walls of the transverse sinuses (Fig. 2b–e). Careful review of previous angiograms showed no direct contrast extravasation. A total of 260 cc of contrast medium (Omnipaque 240 mgI/mL, GE Healthcare, Brøndby, Denmark) had been administered during the procedure. A non-contrast Dyna CT revealed hyperdense areas in the posterior cranial fossa and foramen magnum, which appeared to be confined to the subdural space (Fig. 3a). Our initial suspicion was the development of an acute SDH possibly mixed with contrast. Thus, the embolic injection was stopped, and the procedure terminated.
Native fluoro-images in frontal view during the PHIL™ injection showing the tips of the Sofia® catheter (short arrow) and the Headway® Duo microcatheter (long arrow) in the right occipital artery (a, b). a No subdural contrast was visible in the beginning of the PHIL™ injection. b A subdural contrast effusion developed after 1 min of PHIL™ injection (arrowheads), but the contrast accumulation was not noted due to image subtraction. c Unsubtracted DSA image in frontal view showing extensive contrast accumulation along the right cerebellar tentorium after 5 min of PHIL™ injection (arrowheads). d At that time, the subdural contrast effusion was noted on DSA and the PHIL™ injection was terminated. No contrast extravasation was visible. e Unsubtracted radiograph in frontal view 30 min after the transarterial embolization showing contrast stagnation along the tentorium (long arrows) and cerebellar falx (short arrows)
Postoperative course
The patient woke up with no new neurological symptoms, not even headaches and had an uneventful postprocedural course. A 24-h follow-up Dyna CT showed no residual contrast in the subdural space (Fig. 3b). The diagnosis of a SCE was suggested based on rapidly evolving hyperdense attenuation and quick and complete resolution on serial images [5, 10] combined with no clinical sequelae [2, 3].
Discussion
Effusion of contrast medium into the subdural space is a rare event related to intraarterial and intravenous contrast administration which may occur after high doses of contrast medium above 200 mL [1, 5]. It presents early after contrast administration and often shows complete resolution on follow-up CT within 2 days [1, 2, 5, 8, 10]. It may reoccur after repeated contrast infusions [10]. While the patient’s course is often self-limiting and without new neurological symptoms, it is important to exclude a subdural hematoma as a possible complication of endovascular therapy [6]. SCEs may occur in critically ill patients with severe neurological symptoms, but in these patients, symptoms have previously been caused by the underlying condition rather than the SCE [5, 10].
The diagnosis of a SCE has previously been established on conventional CT imaging with attenuation values above 100 HU [5], since extravasated contrast medium (mean 155 HU; range 91–274 HU) has significantly higher attenuation values than hematoma (mean 54 HU; range 28–82 HU) [9]. However, subdural effusions with lower attenuation values have been reported after intraarterial (35 HU) and intravenous (median 35 HU; interquartile range 16 HU) contrast administration, because the contrast medium may lose some [1, 10].
Recent studies advocate dual-energy CT as a valuable diagnostic tool to differentiate between SCEs, hematomas, and hematomas mixed with contrast [1, 4, 8]. Although dual-energy CT may provide optimal discrimination between iodine and blood, we believe an immediate Dyna CT is sufficient and should be performed in the angiosuite to exclude an acute SDH, when a hyperdense accumulation is noted during EVT. Periprocedural monitoring of heartrate and blood pressure and early postoperative neurological examination should be performed to identify signs of increased intracranial pressure. Clinical suspicion of a hematoma mixed with contrast should prompt acute neurosurgical management.
SCEs may occur due to the disruption of the vascular integrity of dural vessels but have also been reported in patients with no intracranial abnormalities [1]. In general, SCEs are caused by changes in contrast permeability [5, 10] and hydrostatic pressure in the dural vessels [1, 3]. Contrast medium may diffuse into the dural extracellular space through fenestrated dural vessels [1, 5].
Rennert and Hamer [5] reported a SCE after cardiac arrest. They suggested that increased contrast permeability was caused by transient disruption of the dural vessels due to the neurotoxic effect of hyperosmolar contrast medium or by vessel damage from hypertension, ischemia or hypoxia. In our view, the extensive SCE in their patient may be explained by damage to the dural vessels due to hypoxia possibly worsened by ischemia-reperfusion injury. Furthermore, microcirculatory dysfunction and impaired cerebral autoregulation may have led to an increased hydrostatic pressure–related leakage of contrast medium [7]. Zamora and Lin [10] proposed that neuroinflammation may increase extravasation of contrast in dural vessels after subarachnoid hemorrhage and interestingly several cases have subsequently been reported [1, 8]. Furthermore, neovascularization in chronic subdural hematomas have been mentioned as another explanation of increased contrast permeability [10].
Hydrostatic pressure–related SCEs can be categorized based on the administration of contrast medium into the systemic circulation or intracerebral vasculature. Bodanapally et al. [1] reported a high proportion of 8.2% of SCEs after systemic intravenous contrast administration in patients with blunt head traumas. These SCEs mainly involved the frontal, parietal, and temporal lobes and were thought to be caused by age-related cerebral volume loss and intracranial hypotension [1]. Fujiwara et al. [3] reported a SCE located to the posterior cranial fossa occurring during transfemoral cavernous sinus venography after bilateral contrast injections into the inferior petrosal sinuses. Although the exact mechanism remained uncertain, the authors discussed dural tearing by catheter manipulation as a possible cause. However, it may also be considered that contrast injection itself could have caused the localized SCE in their patient due to increased hydrostatic pressure in small dural vessels.
In our case, the contrast accumulation was not immediately noted due to image subtraction used for the DSA runs and frequent road map resets during the embolization. The SCE initially followed the walls of the right and then the left transverse sinuses, then the cerebellar tentorium, and eventually the spinal subdural space. Dyna CT images revealed bilateral contrast effusions with more extensive accumulation on the right side (Fig. 3a) corresponding to the location of the DAVF and side of repeated contrast injections. As the patient was placed in supine position throughout the procedure, it seems likely that contrast medium extravasated on the right side and migrated to the dependent portion as has been previously reported [3].
Assessment of unsubtracted angiograms obtained during the injection of PHIL™ showed that the SCE developed over a period of about 5 min (Fig. 2b, c). No contrast accumulation was present before the injection of PHIL™ (Fig. 2a) and the position of both the Sofia® catheter and Headway® Duo microcatheter did not change during the development of the SCE. As the catheter tip was located far from the SCE, we believe that it is unlikely that the SCE was caused by direct catheter or wire manipulations.
Occlusion of a DAVF with liquid embolics, even if only partial, can cause profound hemodynamic changes of the dural blood circulation with reduced flow and increased vascular resistance. During embolization, control runs were performed using the intermediate catheter (5-F Sofia®, internal diameter 0.055 in.) placed distal in the occipital artery. The larger inner catheter lumen allows for more forceful injections of large amounts of contrast medium. During the embolization with stepwise occlusion of the low-resistance AV shunting, an increasing amount of contrast gets rerouted into the remaining high-resistance normal dural vascular territory. This may have led to an increased pressure, which possibly triggered an opening of the smallest dural vessels and induced radiographically invisible micro-leakages from the smallest dural vessels. A control DSA obtained right before injection of PHIL™ using the Sofia® catheter (Fig. 1b) supports this hypothesis, because small arteries were visible supplying the right lateral tentorium corresponding to the area where the SCE initially developed (Fig. 2b). We believe the contrast leakage occurred in the smallest dural arteries, since no direct arterial extravasation or vessel perforation was visible throughout the procedure.
Although our patient did not develop symptoms, SCE occurring during endovascular procedures can have implications when mimicking a SDH and possibly changing treatment strategy or leading to premature termination of a treatment session. Benign subdural contrast effusions are extremely rare but should be considered in cases with subdural contrast accumulations during or after intraarterial and intravenous contrast administration. While patients with SCE often remain asymptomatic, exclusion of a subdural hematoma by CT and early neurological examination is crucial. We believe that in our case, repeated contrast injections via a large-bore intermediate catheter into an occluding dural AV shunt may have overwhelmed the remaining non-target territory with subsequent leakage of contrast medium into the subdural space. The distinction between a benign SCE and a SDH is of clinical relevance when performing EVT in patients with DAVFs.
Abbreviations
- CT:
-
computed tomography
- DAVF:
-
dural arteriovenous fistula
- DSA:
-
digital subtraction angiography
- EVT:
-
endovascular therapy
- HU:
-
Hounsfield unit
- SCE:
-
subdural contrast effusion
- SDH:
-
subdural hematoma
References
Bodanapally UK, Dreizin XD, Issa G, Archer-Arroyo KL, Sudini K, Fleiter TR (2017) Dual-energy CT in enhancing subdural effusions that masquerade as subdural hematomas: diagnosis with virtual high-monochromatic (190-keV) images. Am J Neuroradiol 38(10):1946–1952. https://doi.org/10.3174/ajnr.A5318
Chattopadhyay S, Srinisavan M, Thomas P (2008) Postangiographic contrast enhancement mimicking acute subdural hemorrhage in a patient with severe occipital headache and neurological symptoms: a case report. J Med Case Rep 2:119. https://doi.org/10.1186/1752-1947-2-119
Fujiwara T, Tanohata K, Onishi T, Matsui K (1992) Extravasation of contrast medium into the subdural space during cavernous sinus venography: a rare complication. Neuroradiology 34(4):355–357. https://doi.org/10.1007/BF00588204
Phan CM, Yoo AJ, Hirsch JA, Nogueira RG, Gupta R (2012) Differentiation of hemorrhage from iodinated contrast in different intracranial compartments using dual-energy head CT. AJNR Am J Neuroradiol 33(6):1088–1094. https://doi.org/10.3174/ajnr.A2909
Rennert J, Hamer OW (2010) Large subdural effusions after angiography mimicking acute subdural hematoma. J Comput Assist Tomogr 34(2):249–250. https://doi.org/10.1097/RCT.0b013e3181c6e704
Sato K, Matsumoto Y, Endo H, Tominaga T (2017) A hemorrhagic complication after Onyx embolization of a tentorial dural arteriovenous fistula: a caution about subdural extension with pial arterial supply. Interv Neuroradiol 23(3):307–312. https://doi.org/10.1177/1591019917694839
Sekhon MS, Ainslie PN, Griesdale DE (2017) Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: a “two-hit” model. Crit Care 21(1):90. https://doi.org/10.1186/s13054-017-1670-9
Tan LA, Chen M, Muñoz LF (2016) Letter to the Editor: Utility of dual-energy CT in differentiating contrast extravasation from intracranial hematoma. J Neurosurg 124(1):279–280. https://doi.org/10.3171/2015.5.JNS15953
Willmann JK, Roos JE, Platz A, Pfammatter T, Hilfiker PR, Marincek B, Weishaupt D (2002) Multidetector CT: detection of active hemorrhage in patients with blunt abdominal trauma. AJR Am J Roentgenol 179(2):437–444. https://doi.org/10.2214/ajr.179.2.1790437
Zamora CA, Lin DD (2015) Enhancing subdural effusions mimicking acute subdural hematomas following angiography and endovascular procedures: report of 2 cases. J Neurosurg 123(5):1184–1187. https://doi.org/10.3171/2014.10.JNS142172
Author information
Authors and Affiliations
Contributions
Conception and design: Dahl and Benndorf. Acquisition of data: Dahl and Benndorf. Analysis and interpretation of data: Dahl and Benndorf. Drafting the article: all authors. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Benndorf. Administrative/technical/material support: Dahl and Benndorf. Study supervision: Benndorf.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Patient consent
The patient has consented to the submission of the case report to the journal.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Vascular Neurosurgery - Arteriovenous malformation
Rights and permissions
About this article
Cite this article
Dahl, R.H., Eskesen, V. & Benndorf, G. Subdural contrast effusion during endovascular therapy: case report. Acta Neurochir 161, 2403–2407 (2019). https://doi.org/10.1007/s00701-019-04049-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-019-04049-2