Abstract
Background
Neurovascular conflict is considered a key element of classical trigeminal neuralgia (TN) and consequently, microvascular decompression (MVD) is an effective treatment. Nevertheless, failures of MVD are described by many authors. In some patients, the arachnoid membranes surrounding the trigeminal nerve and neighbouring vessels may be thickened and adhesive. Here we analyse the impact of such focal arachnoiditis on outcome after MVD for TN.
Methods
A cohort of prospectively followed patients after their MVD was reviewed for intraoperative, imaging and clinical data if findings of arachnoiditis during MVD were described. Long-term outcome assessment was the main endpoint.
Results
We reviewed data from 395 MVD procedures, performed for TN from 2001 to 2014. Intraoperative evidence of focal arachnoiditis, as described by the surgeon, has been noted in 51 patients (13%). In 35 (68.6%), neuralgia was typical and in the other 17 (31.4%) it was atypical.
As expected by definition, neurovascular conflict was found in 49 interventions (96%); it was predominantly arterial in 27 (52.9%). Accompanying arachnoiditis was encountered: mild in 20 interventions (39.2%), severe in 31 (60.8%).
A successful result (BNI I or II) was achieved in 29 patients (56.9%). The other 22 patients (43.1%) had persistence or recurrence of pain. Overall KM probability of being pain free at 15 years was 72%.
Conclusions
Intraoperative finding of arachnoiditis during MVD for classical trigeminal neuralgia is associated with poorer outcome than that of classical trigeminal neuralgia in general. This is particularly true for low grades of conflict.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurovascular conflict is considered now the main pathogenetic element of classical trigeminal neuralgia [4, 15], that is, neuralgia not secondary to tumour, vascular malformation or demyelinating disease [16]. Initially observed by Dandy and then hypothesised by Gardner to be a cause of trigeminal neuralgia [6, 7], the responsibility of the neurovascular conflict with microvascular decompression (MVD) as its effective treatment was popularized by Jannetta [1]. In fact, since the publication of retrosigmoid trigeminal root decompression from “vascular nerve entrapment” [10], many series of microvascular decompression have been published [18, 21, 22, 24, 26] leaving little doubt that vascular conflict is often at the origin of trigeminal neuralgia [14, 16]. However, in most, if not all studies, failure of MVD has been mentioned [2, 25]. Being able to identify patients that are unlikely to benefit from this type of surgery can be of great use [17].
In our own series, we have demonstrated two main outcome factors. On one hand was the degree of the conflict from the neighbouring vessels whether arterial [20] or venous [5]. On the other hand, was the ability to remove such a compression without generating a new one [23]. Other, especially negative, outcome factors may however exist.
In some patients operated on by MVD, the arachnoid membranes surrounding the nerve and neighbouring vessels may be locally thickened and adhesive creating an environment in which all passing structures are coalesced with anatomic alteration and consequent “physiological suffering” [20]. Such phenomena have been observed surrounding spinal roots and have been termed arachnoiditis [3]. This is variously described in literature in pathologic, radiologic and experimental studies.
These changes have been mentioned to occur in a generalized manner throughout the subarachnoid space in cases of clear inflammation [11] or corresponding to a non-specific inflammatory condition involving the leptomeninges and intrathecal neural elements [19]. While mostly described in the spinal segments, it has also been observed in the cranial cisterns as well [3, 19], in a focal manner.
In cases of trigeminal neuralgia, we have observed during MVD the presence of such thickened membranes disposed in a limited fashion in the trigeminal cistern [5, 20]. Not only thickened arachnoid membranes lead to difficulty in manipulating the neighbouring vessels but also, they may be compressive by themselves either focally or all along the trigeminal root thus predisposing to a negative result.
In this study, we aimed at identifying those patients that had such arachnoid thickening and adhesions, and at studying the impact on clinical presentation, outcome and on complication rates. This is a descriptive clinical cohort, since little data has been published previously—per se—on the phenomenon.
Material and methods
Patients selection
All patients having undergone MVD from 2004 to 2014 for presumed classical trigeminal neuralgia according to ICHD classification [16] were considered candidates for this study. As per hospital protocol upon retaining indication for MVD, all patients had undergone a standardized MRI [13, 14] and had what was considered on imaging a potential neurovascular conflict [17].
Patients in whom operative findings revealed thickened compressive arachnoid were included in the present study regardless of the presence of a vascular conflict of whatever nature and degree; these patients constituted the study cohort.
All patients having had prior procedures on the trigeminal nerve were excluded.
Preoperative assessment
In these patients, clinical diagnosis of trigeminal neuralgia was confirmed using predefined criteria from the patients’ history and examination [4, 16]. Definite diagnosis of trigeminal neuralgia was retained solely if at some point (at least in the beginning of the disease) the patient presented a clear component of paroxysmal pain and effectiveness of anticonvulsants, at least transient. Neuralgia was considered typical when described as purely paroxysmal in nature and atypical when the paroxysmal pain was associated with a continuous background of pain. This corresponds to Sweet’s classical description and the ICHD definition of classical trigeminal neuralgia or classical trigeminal neuralgia associated with background of continuous pain [16]. Patients were all clinically assessed prior to retaining surgical indication by an ophthalmologist, otorhinolaryngologist and stomatologist.
All patients underwent preoperative MRI using our standardized protocol (high-resolution 3D hyper T2 focalised on the trigeminal nerve, high-resolution 3D TOF angio and high-resolution 3D T1 with gadolinium). They all presented a potential conflict which was considered a criterion to retain surgical indication.
Assessment of outcome
Post-operative visits were performed systematically in all patients at 3–4 months after the surgery, point at which they were consulted by the operating surgeon (MS) and by an ophthalmologist and otorhinolaryngologist. Patients were then followed on a regular basis. Patients with recurrence were followed as per patient requirement and at least once a year. Patients without recurrence were seen in consultation at 3 months and 1 year and were instructed to contact in case of recurrence or change of contact details. At last follow-up, patients were questioned according to an internal standardized questionnaire. The questionnaire was filled by one of the authors not directly involved in the surgery (EM). No patients were lost to follow up.
Patients were questioned about the immediate persistence of pain after the surgery and in cases of delayed relief when did such relief occur. Relief was considered early when occurring before the third month and delayed if relief occurred after the third month outpatient visit and before the 1-year term. Any use of medication was noted.
The main outcome measure was the Barrow Neurological Institute (BNI) score. Patients not requiring any medication who were completely pain free were classed as BNI I, whereas those who were medication free but had some minor symptoms (i.e. not requiring treatment) were classed as BNI II. These two classes were considered a successful result. Patients still under medication were classed as BNI III, if the medication controlled the pain. This was divided into IIIa if the patient had no pain but continued the medication and IIIb if the patient still had symptoms present, but adequately controlled by medication. Patients in whom the pain was inadequately controlled or not improved at all were classed as BNI IV or V respectively. Classes IIIa to V were considered failures of MVD. The date of reappearance of symptoms was noted and in all cases classed as BNI IIIb-V.
Patients were considered as having a recurrence if after a period of relief of at least 3 months neuralgia came back.
Some patients benefited from a second procedure such as a RF-Thermolesion, radiosurgery or hospitalization for pain treatment with continuous drug infusions (in particular tricyclic antidepressants). These were considered failures of MVD. Patients having received a second surgical procedure were classed as BNI V, whereas patients having been hospitalized for drug infusions only were classed as BNI IV.
Surgical procedure
Microvascular decompression was performed using the microsurgical technique previously described in detail elsewhere [21]. In short, an infratentorial supracerebellar approach through a “key-hole” retrosigmoid craniectomy was used to access the nerve. After opening the trigeminal cistern, the superior petrosal vein was identified and dissected free. Sacrifice of veins was avoided. Compressive vessels were searched for all along the trigeminal root from the brainstem and the TREZ to the porus of Meckel’s cave and the conflicting vessels were dissected, dislodged and ideally maintained at a distance using small Teflon pieces not contacting the nerve [23]. Particular attention was made not to generate a “neo-compression” between the prosthetic material and the nerve root [23]. In the case of compressive veins, they were dissected free and detached from the root [5]. When this was insufficient to achieve decompression, the vein was coagulated and divided to release the root. Venous coagulation was avoided in all other circumstance to decrease operative complications [5].
Intraoperative findings
The denomination used for identification of vessels was described elsewhere [5]. In brief, the responsible arteries were the superior cerebellar artery (SCA), the anterior-inferior cerebellar artery (AICA) and the vertebro-basilar arteries (VBA), whereas the veins found were from the superficial and deep superior petrosal venous systems (sSPVS and dSPVS). Each conflict was carefully graded for its specific degree of compression according to previously described grading scale: grade I adhesive contact between the vessel and the nerve, grade II displacement or distortion of the nerve and grade III engrooving (indentation) of the nerve [20, 21]. Nerves were considered atrophic if their diameter was significantly diminished from what was expected [8] and the nerve had a greyish aspect.
Anatomical features of arachnoiditis
Arachnoiditis was retained by the surgeon if under the operative microscope thickened arachnoid membranes were found focally around the trigeminal root. These had to be opaque in nature, highly resistant in such a manner that they would not tear as may be the case for normal arachnoid but rather required active and repeated cutting with microscissors. Finally, to be retained as arachnoiditis, the membranes had to be adhesive to the nerve in such a way that when pulling upon them, they would also mobilize the nerve. In each case, it was noted whether the arachnoid thickening was present all along the root or only located at a focal zone on the root. Furthermore, it was noted whether the arachnoid thickening was exclusively present around the trigeminal nerve or involved surrounding structures. Adhesions were qualified either mild or important according to their degree of stickiness to the involved neurovascular structures.
Review of complications
All complications observed at discharge and at the first outpatient visit (3–4 months) were noted. A systematic examination by an ENT physician and an ophthalmologist was carried out before discharge and at the 3–4 month outpatient visit.
Statistical analysis
Since this is a descriptive cohort, no comparison group was used; however, subgroup comparisons were made.
Outcome assessment was analysed using Kaplan–Meier statistics.
Categorical variables were expressed as number (n) and percentage and quantitative variables were expressed as mean ± standard deviation.
Categorical variables were compared using the chi-squared test or Fisher’s exact test when the conditions of application of chi-squared were not met.
Quantitative variables were compared between groups using Student’s t test after verification of equality of variances when data were normally distributed, and with the nonparametric test of Wilcoxon when the hypothesis of normality of distribution was not verified.
Pain-free survival was defined from the date of surgery to the date of pain recurrence or last news. Description of pain-free survival was estimated by the Kaplan–Meier product limit method, and the effect of different parameters was assessed using the log rank test.
The statistical tests are bilateral, and the level of significance was set to 5% (p < 0.05). Statistical analyses were conducted using R version 3.4.2, a freely available software by the R Project Foundation (https://www.r-project.org).
Results
Patient population
Operative data on 395 consecutive microvascular decompression procedures performed by the senior author for classical trigeminal neuralgia from 2001 to 2014 was reviewed. Focal arachnoiditis was noticed in 51 patients (12.9%); 32 of them (62.7%) were female. The mean age at the time of surgery was 53.5 years (± 13.3). Table 1 reviews demographic data and clinical features.
Neuralgia was considered typical in 35 patients (68.6%) and atypical in the other 16 (31.4%). Pain involved a single trigeminal division in 23 patients (V1 in 4, V2 in 8 and V3 in 11), two divisions in 24 patients (V1 and V2 in 8, V1 and V3 in 2, V2 and V3 in 14) and all divisions in 3 patients. Considering all divisions involved, 15 patients (33.3%) had pain in the V1 division (alone or together with V2 or V3), 34 patients had V2 involved (66.7%) and 31 patients had involvement of V3 (64.7%) (Table 2).
Intraoperative anatomical findings
A neurovascular conflict was found in 49 of the 51 patients (96%). In the two others (4%), the arachnoid membrane alteration was the only abnormal intraoperative finding. The neurovascular compression was due to a single vessel in 31 cases 63.3%, while multiple vessels were found in 18 (36.7%). SCA was involved in 26 cases (51%), AICA in 7 (13.7%), sSPVS in 26 (51%) and dSPVS in 9 (17.6%). When considering only the most important vascular conflict if several in the same patient, it was the SCA in 24 cases (47.1%), the AICA in 3 (5.9%), the sSPVS in 16 (31.4%) and the dSPVS in 6 (11.8%). The main conflicting vessel was arterial in 27 patients (52.9%) and venous in 22 (43.1%). The conflict was grade I in 21 interventions (41.2%), grade II in 15 (29.4%) and grade III in 13 (25.5%).
Arachnoiditis was classified—on the basis of the surgeons’ intraoperative description of the adhesions to the neurovascular structures—in mild to important. A mild arachnoiditis was encountered in 20 interventions (39.2%), an important one in 31 (60.8%).
A significant degree of global atrophy with the calibre of the nerve diminished by one-third or more was observed in 24 operations (47.1%). Out of these, in 6 patients (11.8%) the atrophy was severe, i.e. the calibre of the nerve was less than two-thirds of the normal size. In other 4 patients (7.8%), a discoloration of the nerve without reduction of its calibre was observed.
Global outcome of the series
Follow-up ranged from at least 1 year to 16 years with a 6.4-year average. All patients were followed up for a minimum of 1 year. A successful result (BNI I or II, i.e. no medication required) has been maintained to the last follow-up in 29 patients (56.9% of the population), without any pain (BNI I) in 22 of them (43.1%). The other 22 patients (43.1% of the considered population) had an unsuccessful result, marked by persistence of pain in the post-operative period in 6 patients (11.7%) or recurrence of pain after a period of more than 3 months in the remaining 16 patients (31.3%). The mean delay of recurrence was of 5.56 ± 4.46 years.
Management of recurrences
Out of the 22 patients still presenting pain after MVD, eleven benefited from intravenous tricyclic antidepressants at high doses over a period of 10 days; out of these, five still required the use of a lesioning technique—in this case radiofrequency thermocoagulation (RfTh). Two patients had RfTh without the prior use of intravenous perfusions. One patient benefited from gamma knife radiosurgery and the remaining seven patients simply continued oral medication. No patients were proposed reoperation even in early cases of recurrence.
Kaplan–Meier analysis
Overall probability of being pain free was computed to be 42.2% (27.3–65.3% 0.95 confidence interval) in patients with arachnoiditis present at surgery at 15.7 years. Figure 1 a shows this result.
Outcome according to the nature of the offending vessel
Figure 1 b compares outcome according to the main offending vessel—artery or vein. In the case of arteries, probability of being pain free was 54.5% (37.1–80.2% 0.95 CI), whereas this was 37.8% for veins (19.4–73.7% 0.95 CI). Low numbers of patients and large CI did not allow for significance testing.
Outcome according to the degree of conflict
Patients with grade III compression and arachnoiditis (n = 13) had a probability of 76.2% (55.8–100% 0.95 CI) of being pain free at over 10 years. Patients presenting with grade II compression and arachnoiditis (n = 15) had a probability of 50.0% (29.2–85.5% 0.95 CI) of being pain free at over 10 years Patients having grade I compression and arachnoiditis (n = 22) had a probability of 32.6% (15.3–69.4% 0.95 CI) of being pain free at over 10 years.
Noteworthy, no recurrences occurred after 4 years of follow-up, and two-thirds of recurrences occurred in the first year after surgery in the group with grade III NVC and arachnoiditis, whereas in the overall group, average time of recurrence was 5.56 years. For other grades, recurrences were dispersed through the follow-up period as shown in Fig. 1c.
Figure 2 shows the absolute numbers of patients with respective grades of compression as well as the nature of the main offending vessel (artery or vein). Overall, a large number of veins were present in this study (22 patients, 43.1%). Many of these (n = 10) had high grades of compression (II or III).
Large overlap was present between confidence interval of the probability of being pain free at all grades of compression. This, in addition to the low number of patients, precluded from Log rank testing for significance of the differences in KMS.
Outcome according to clinical presentation
Figure 1 d shows no difference in outcome probability which was noted between patients with typical or atypical presentation. For both, this was 46.9%; 95% confidence intervals differed little being 31.0–70.6% for typical presentation and 23.7–92.6% for atypical presentation.
Complications
In the post-operative period, the appearance of a complication was noted in 15 patients (29.4%): however, in only 7 patients (13.7%) this persisted beyond the first follow-up visit, at 3–4 months for all patients. Persistent hypoesthesia was present in 5 of these patients; this was the only neurological sign in 2 patients and was associated with other signs in another 3. Facial paralysis was persistent in one patient, associated with unilateral facial anaesthesia and hearing loss. Another two patients had persistent mild hearing loss; in one of these this was associated with facial hypoesthesia. Two patients presented a cerebellar syndrome; in one of them this syndrome was associated to diplopia and facial hypoesthesia. None of the two patients who had trochlear nerve dysfunction in the immediate post-operative period had persisting diplopia.
Discussion
This study focused on a selected group of patients with classical trigeminal neuralgia presenting at surgery focal arachnoiditis as an additional aggressive factor for the trigeminal root. For these patients, the probability of being pain free 15 years after microvascular decompression was shown to be 42.2%. This probability is strikingly poorer than the usually published probability of about 75% [21, 22]. Even more so, when taking into consideration the respective confidence intervals, the stronger interpretation that arachnoiditis is a poor prognostic factor should be considered even in the absence of direct comparisons of groups by statistical testing.
However, the study shows that additional factors may contribute to a poor outcome, especially those directly related to the NVC; degree of compression in particular seems to play an important role, whereas the nature of the conflicting vessel may do less so. Patients with arachnoiditis and a high grade of vascular compression had a relatively high probability of being pain free (72%) and this was relatively close to that published in our previous overall series (85%) [21]. In contrast, patients having a grade I conflict (simple contact) and arachnoiditis had a very low probability of being pain free 10 years after the surgery (32%) compared with 65% in our overall previously published series. Surprisingly, the probability of being pain free for patients with a grade II compression (50%) was strikingly lower than that for grade III, especially when compared with Grade II patients from the overall series (75%). This is particularly surprising in the fact that in our previous overall series, the difference between grade II and III was relatively small (of about 10 percentage points) with a more important difference when compared with grade I. Moreover, in the presented group with arachnoiditis, the confidence interval for grade II patients is excessively large making it hard to pinpoint whether these patients fare more like Grade III or more like Grade I patients. This suggests in our view that in high-grade patients, the role of the conflict is more determinant in the genesis of the neuralgia and therefore MVD effective. On the other hand, in lower grade patients, the role of arachnoiditis may be of more importance. Confronting this factor is the pattern of recurrence. Patients with grade III compression who recurred did so in the months or few years following surgery (up to four), whereas for both other grades, recurrences are dispersed throughout the follow-up period being possible even 10 to 15 years after the surgery.
The impact of the conflicting vessel itself is difficult to assess in the context of a relatively small population. Patients with arterial compression had a probability of being pain free at 10 years of 54.5%, whereas patients having venous compression had a probability of only 37.8%. However, confidence intervals did not allow to affirm statistically significant differences. Certainly, the small number of patients plays a role in not allowing to identify statistically significant differences; however, in previous studies patients with venous NVC, decompressions were shown to fare in a similar manner to those with arterial compression [5].
One factor that may come into play in the context of arachnoiditis is the ease of decompression. In our practice, we aim not to sacrifice the veins whenever possible. However, because of the presence of thick adhesive arachnoid membranes which may hinder decompression, it is difficult to transpose completely unless the vein is coagulated and cut. Therefore, veins may still be compressive (or become so again) and play a role in the recurrence of neuralgia over the long run.
In cases of recurrence, options include pharmacologic treatment, most notably the use of drug infusions namely tricyclic antidepressants at high doses over a prolonged period (10 days). Alternatively, or in cases of persisting pain, a lesional technique may be used with advantages and drawbacks for each of them. In this series, radiofrequency-thermolesions were the main lesional technique used.
The impact of these results is limited by some important factors. Firstly, this is a cohort of selected patients followed with a descriptive objective. For this reason, comparisons with the results of other series (even our own) can only be indirect, since the global outcome of the 395 was not studied. Obviously, this is a disadvantage but selection according to the presence of arachnoiditis was chosen by study design to best describe patients with thickened arachnoid membranes associated to neurovascular conflict in the context of trigeminal neuralgia. The group of 51 patients is thus formed by those having likely spontaneous focal arachnoiditis. This was considered distinct from inflammatory extensive arachnoiditis following infection or bleeding This was considered also distinct from patients with anatomical anomalies of arachnoid bands leading to “tethered cranial nerves”; those are mostly encountered in facial and vago-glossopharyngeal nerve compression that do not have an inflammatory nature at all. This pathology is however non-negligible representing 13% of patients operated on for trigeminal neuralgia and therefore a detailed description is warranted. Of course, studies comparing MVD in patients with and without arachnoiditis in the presence of NVC may be of interest in particular if they have a study design to allow for multivariate analysis of factors determinant to outcome.
Another limitation of the study was the potential subjectivity in observing the presence of arachnoiditis in these patients. To counter this, authors established the criteria of focal thickening, opaqueness, resistance and adhesion of the arachnoid surrounding the trigeminal nerve as described in the “Material and methods” section. These criteria, as well as the nature of the disease itself, will require further validation through confirmed observations, imaging studies and studies of biological markers of inflammation in the nervous system most notably the CSF. Histologic studies of the thickened arachnoid membranes are imaginable and desirable under very controlled conditions.
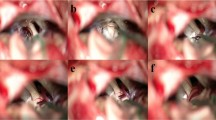
In all cases, arachnoiditis was a finding at surgery. Intraoperatively, the presence of arachnoid thickening surrounding the neurovascular structures and leading to coalescence of these was observed without prior imaging signs of such findings. In particular, no band sign (i.e. presence of non-vascular bands deviating the nerve) was seen on T2 drive and no pathological contrast uptake was observed, on T1 with gadolinium. On some MRI, a small crowdy posterior fossa could be observed; however, in the absence of a comparative study, it is difficult to affirm that this is a characteristic finding. The presence of opaque membranes sticking to the vessels and nerves of the cerebello-pontine angle is shown for illustration in Fig. 3.
a Normal arachnoid as seen in an rightside MVD surgery for TN. The arachnoid while present and touching the surrounding structures is not adhesive to them, is delicate and transparent and the structures are easily seen through it. b Intraoperative image illustrating a case of arachnoiditis. The arachnoid membranes are thick and they are particularly adhesive to the trigeminal nerve and superior cerebellar artery. -Rightside MVD for TN. IV, trochlear nerve; V, trigeminal nerve; SCA, superior cerebellar artery; *thickened coalescing arachnoiditis between the SCA and the trigeminal nerve
The fact that the origin of this type of arachnoid thickening remains unknown makes it even more puzzling. Its relatively limited nature makes it distinct from arachnoiditis after infection or subarachnoid bleeding. These factors where actively sought in the medical history of these patients without any such findings. More focal inflammation may be a possible etiological factor. This would include otitis and mastoiditis. In our practice, however, all patients were examined by an ENT physician prior to surgery and any history or clinical signs of such disease would have been pointed out.
It is to be discussed if such membranes are simply an incidental occurrence, a prognostic factor or may even be involved in the pathogenesis of the vascular conflict. It can be imagined that the presence and evolution of such arachnoid thickening may lead to progressive coalescence of the neurovascular structures up to contact and then clear cut conflict.
The presence of arachnoiditis is not necessarily associated with a different clinical presentation as the proportion of patients with atypical presentation was less than 40%. Neither does this impact on outcome since probability of being pain free at 10 years was identical for typical and atypical presentation.
It would be important to predict participation of focal arachnoiditis in the NVC the more so as complications rate seems higher than in our previously published overall series [21]. No specific clinical presentation features could be found and no direct stigma could be depicted on MRI; however, after reviewing the imaging studies for this series, the authors found that often a small and crowded posterior cranial fossa was observed leading to coalescence of cranial nerves and vessels. This is in concordance with other findings of smaller posterior fossa in specific groups of patients with TN [9].
Implications of arachnoiditis as a spontaneous surgical finding are easier to define. Firstly, this cautions the surgeon as to the difficulty of decompression but at the same time of careful inspection of the root. Indeed, since manipulating the structures of the CP angle may be more difficult, one might have the tendency to limit decompression to the first encountered vessels. However, the importance of a decompression all along the trigeminal root cannot be overstressed. Decompressing the arachnoid membranes themselves may serve a role but peeling the arachnoid from the trigeminal nerve is certainly traumatic and would lead to sensory deficit. As shown in this series, in patients with high-grade conflicts, one can reasonably expect a good result despite present arachnoiditis; but, on the other hand, in cases of low grade conflict, a good result is unlikely. It might be thus considered to perform an eventual complementary action on the root, namely internal neurolysis [12].
Conclusion
While demonstrable only at surgery, the presence of thickened arachnoid membranes leading to coalescence of neurovascular structures is not an uncommon finding in patients with classical trigeminal neuralgia. This may play a role in poor outcome especially in patients with low grade neurovascular conflicts.
Change history
20 November 2019
Figure 3 corrected.
References
Barker FG, Jannetta PJ, Bissonette DJ, Larkins MV, Jho HD (1996) The long-term outcome of microvascular decompression for trigeminal neuralgia. N Engl J Med 334(17):1077–1083. https://doi.org/10.1056/NEJM199604253341701
Burchiel KJ (2016) Trigeminal neuralgia: new evidence for origins and surgical treatment. Neurosurgery 63(Suppl 1):52–55. https://doi.org/10.1227/NEU.0000000000001276
Cevizci R, Dilci A, Tekin AM, Bayazıt Y (2017) Recovery of tinnitus and sensorineural hearing loss due to lysis of arachnoid adhesions in the posterior cranial fossa: is there a novel etiology in neurotological disorders? J Int Adv Otol 13(2):295–297. https://doi.org/10.5152/iao.2017.3393
Cruccu G, Finnerup NB, Jensen TS, Scholz J, Sindou M, Svensson P et al (2016) Trigeminal neuralgia: new classification and diagnostic grading for practice and research. Neurology 87(2):220–228. https://doi.org/10.1212/WNL.0000000000002840
Dumot C, Brinzeu A, Berthiller J, Sindou M (2017) Trigeminal neuralgia due to venous neurovascular conflicts: outcome after microvascular decompression in a series of 55 consecutive patients. Acta Neurochir 159(2):237–249. https://doi.org/10.1007/s00701-016-2994-y
Gardner WJ (1953) The mechanism of tic douloureux. In: Transactions of the American Neurological Association, 3(78th meeting), pp 168–171 discussion, 171–173
Gardner WJ (1962) Concerning the mechanism of trigeminal neuralgia and hemifacial spasm. J Neurosurg 19:947–958. https://doi.org/10.3171/jns.1962.19.11.0947
Gudmundsson K, Rhoton AL Jr, Rushton JG (1971) Detailed anatomy of the intracranial portion of the trigeminal nerve. J Neurosurg 35(5):592–600
Hardaway FA, Holste K, Ozturk G, Pettersson D, Pollock JM, Burchiel KJ (2019) Raslan AM sex-dependent posterior fossa anatomical differences in trigeminal neuralgia patients with and without neurovascular compression: a volumetric MRI age- and sex-matched case-control study. J Neurosurg:1–8. https://doi.org/10.3171/2018.9.JNS181768 Epub ahead of print
Jannetta PJ (1967) Arterial compression of the trigeminal nerve at the pons in patients with trigeminal neuralgia. J Neurosurg 26(1), Suppl):159–162. https://doi.org/10.3171/jns.1967.26.1part2.0159
Killeen T, Kamat A, Walsh D, Parker A, Aliashkevich A (2012) Severe adhesive arachnoiditis resulting in progressive paraplegia following obstetric spinal anaesthesia: a case report and review. Anaesthesia 67(12):1386–1394. https://doi.org/10.1111/anae.12017
Ko AL, Ozpinar A, Lee A, Raslan AM, McCartney S, Burchiel KJ (2015) Long-term efficacy and safety of internal neurolysis for trigeminal neuralgia without neurovascular compression. J Neurosurg 122(5):1048–1057. https://doi.org/10.3171/2014.12.JNS14469 Epub 2015 Feb 13
Leal PRL, Hermier M, Froment JC, Souza MA, Cristino-Filho G, Sindou M (2010) Preoperative demonstration of the neurovascular compression characteristics with special emphasis on the degree of compression, using high-resolution magnetic resonance imaging: a prospective study, with comparison to surgical findings, in 100 consecutive patients who underwent microvascular decompression for trigeminal neuralgia. Acta Neurochir 152(5):817–825. https://doi.org/10.1007/s00701-009-0588-7
Leal PRL, Hermier M, Souza MA, Cristino-Filho G, Froment JC, Sindou M (2011) Visualization of vascular compression of the trigeminal nerve with high-resolution 3T MRI: a prospective study comparing preoperative imaging analysis to surgical findings in 40 consecutive patients who underwent microvascular decompression for trigeminal neuralgia. Neurosurgery 69(1):15–25; discussion 26. https://doi.org/10.1227/NEU.0b013e318212bafa
Maarbjerg S, Di Stefano G, Bendtsen L, Cruccu G (2017) Trigeminal neuralgia - diagnosis and treatment. Cephalalgia: Int J Headache 37(7):648–657. https://doi.org/10.1177/0333102416687280
Maarbjerg S, Sørensen MT, Gozalov A, Bendtsen L, Olesen J (2015) Field-testing of the ICHD-3 beta diagnostic criteria for classical trigeminal neuralgia. Cephalalgia: Int J Headache 35(4):291–300. https://doi.org/10.1177/0333102414542291
Maarbjerg S, Wolfram F, Gozalov A, Olesen J, Bendtsen L (2015) Significance of neurovascular contact in classical trigeminal neuralgia. Brain J Neurol 138(Pt 2:311–319. https://doi.org/10.1093/brain/awu349
Miller JP, Magill ST, Acar F, Burchiel KJ (2009) Predictors of long-term success after microvascular decompression for trigeminal neuralgia. J Neurosurg 110(4):620–626. https://doi.org/10.3171/2008.9.17660
Rongxun Z (1982) Chronic arachnoiditis in the posterior fossa: a study of 82 cases. J Neurol Neurosurg Psychiatry 45(7):598–602. https://doi.org/10.1136/jnnp.45.7.598
Sindou M, Howeidy T, Acevedo G (2002a) Anatomical observations during microvascular decompression for idiopathic trigeminal neuralgia (with correlations between topography of pain and site of the neurovascular conflict). Prospective study in a series of 579 patients. Acta Neurochir 144(1):1–12; discussion 12-13. https://doi.org/10.1007/s007010200000
Sindou M, Leston J, Decullier E, Chapuis F (2007) Microvascular decompression for primary trigeminal neuralgia: long-term effectiveness and prognostic factors in a series of 362 consecutive patients with clear-cut neurovascular conflicts who underwent pure decompression. J Neurosurg 107(6):1144–1153. https://doi.org/10.3171/JNS-07/12/1144
Sindou M, Leston J, Howeidy T, Decullier E, Chapuis F (2006) Micro-vascular decompression for primary trigeminal neuralgia (typical or atypical). Long-term effectiveness on pain; prospective study with survival analysis in a consecutive series of 362 patients. Acta Neurochir 148(12):1235–1245; discussion 1245. https://doi.org/10.1007/s00701-006-0809-2
Sindou M, Leston JM, Decullier E, Chapuis F (2008) Microvascular decompression for trigeminal neuralgia: the importance of a noncompressive technique—Kaplan-Meier analysis in a consecutive series of 330 patients. Neurosurgery 63(4 Suppl 2):341–350; discussion 350-351. https://doi.org/10.1227/01.NEU.0000327022.79171.D6
Xia L, Zhong J, Zhu J, Wang Y-N, Dou N-N, Liu M-X et al (2014) Effectiveness and safety of microvascular decompression surgery for treatment of trigeminal neuralgia: a systematic review. J Craniofac Surg 25(4):1413–1417. https://doi.org/10.1097/SCS.0000000000000984
Zakrzewska JM, Coakham HB (2012) Microvascular decompression for trigeminal neuralgia: update. Curr Opin Neurol 25(3):296–301. https://doi.org/10.1097/WCO.0b013e328352c465
Zakrzewska JM, Lopez BC, Kim SE, Coakham HB (2005) Patient reports of satisfaction after microvascular decompression and partial sensory rhizotomy for trigeminal neuralgia. Neurosurgery 56(6):1304–1311 discussion 1311-1312
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the ethics committee of the “Hopital Neurologique de Lyon”.
Informed consent
All patients signed an informed consent form with the surgeon prior to the procedure.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Functional Neurosurgery - Pain
Rights and permissions
About this article
Cite this article
Mazzucchi, E., Brinzeu, A. & Sindou, M. Arachnoiditis as an outcome factor for microvascular decompression in classical trigeminal neuralgia. Acta Neurochir 161, 1589–1598 (2019). https://doi.org/10.1007/s00701-019-03981-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-019-03981-7