Abstract
Background
While the subtemporal approach represents the surgical module milestone designed to reach the petrous apex, a novel ventral route, which is the superior eyelid endoscopic transorbital approach, has been proposed to access the skull base. Accordingly, we aimed to evaluate the feasibility of this route to the petrous apex, providing a qualitative and quantitative analysis of this relatively novel pathway.
Methods
Five human cadaveric heads were dissected at the Laboratory of Surgical NeuroAnatomy of the University of Barcelona. After proper dissection planning, anterior petrosectomy via the endoscopic transorbital route was performed. Specific quantitative analysis, as well as dedicated three-dimensional reconstruction, was done.
Results
Using the endoscopic transorbital approach, it was possible to reach the petrous apex with an average volume bone removal of 1.33 ± 0.21 cm3. Three main intradural spaces were exposed: cerebellopontine angle, middle tentorial incisura, and ventral brainstem. The first one was bounded by the origin of the trigeminal nerve medially and the facial and vestibulocochlear nerves laterally, the second extended from the origin of the oculomotor nerve to the entrance of the trochlear nerve into the tentorium free edge while the ventral brainstem area was hardly accessible through the straight, ventral endoscopic transorbital trajectory.
Conclusion
This is the first qualitative and quantitative anatomic study concerning details of the lateral aspect of the incisura and ventrolateral posterior fossa reached via the transorbital window. This manuscript is intended as a feasibility anatomic study, and further clinical contributions are mandatory to confirm the effectiveness of this approach, defining its possible role in the neurosurgical armamentarium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meningiomas [2, 4, 31, 65, 69], schwannomas [9, 33], epidermoid cysts [39, 49, 57], chordomas [61], and chondrosarcomas [13, 43, 58, 59] are common lesions located in the petrous apex and petroclival area and may extend from the middle to the posterior cranial base. Surgery of these regions is extremely challenging, because of the complex anatomy, including a large number of vessels, cranial nerves (CNs), and brainstem structures. The selection of an aggressive surgical approach may determine unnecessary morbidity whereas conservative, less invasive, routes may not guarantee adequate exposure and pathology removal [4, 8, 11, 22, 24, 27, 28, 30, 32, 34, 41, 42, 44, 52, 53, 55, 56, 68, 69, 71].
Kawase’s approach represents the milestone of the surgical modules to reach such deep anatomic targets, and various surgeons have developed several modifications of this subtemporal pathway, in a multiplicity of variations and combinations [10, 12, 37, 38, 60, 66]. Noteworthy, a so-called modified Dolenc-Kawase (MDK) approach, incorporating the surgical benefits of Dolenc’s and Kawase’s techniques, has been recently proved to achieve a larger area of exposure at the anterior petrous apex [66].
In a constant effort to develop minimally invasive neurosurgical routes, the endoscopic endonasal approach to the posterior fossa has been recently tested to provide valid surgical window to the petroclival junction. However, as a strictly midline window, this path may be unfit to properly manage lesions arising in or extending to the most lateral aspect of the skull base [26, 48, 51, 62,63,64, 70].
In this evolving neurosurgical scenario, another ventral endoscopic minimally invasive route, which is the superior eyelid endoscopic transorbital approach, has been lately proposed to access the most lateral aspect of the skull base. The latter can be considered as a ventral modification of Dolenc’s approach thus exposing in a minimally invasive interdural fashion, with very limited brain retraction or cranial nerve manipulation and, with a favorable angle of attack, the entire cavernous sinus lateral wall and the middle cranial fossa. Further, the transorbital window may overcome the inherent limits of the midline endonasal route, i.e., its extension towards the lateral skull base, thus providing a straight and valid corridor even to the petrous apex [5, 18, 25, 29, 45, 50].
To this end, our study aims to prove the feasibility of the endoscopic superior eyelid transorbital approach to the petrous apex, providing as well three-dimensional reconstruction and pertinent quantitative data. To the best of the authors’ knowledge, this is the first qualitative and quantitative anatomic study concerning details of the lateral aspect of the posterior fossa reached via the endoscopic transorbital window.
Material and methods
Pre-dissections planning
The first step before dissections was to clearly delineate the limits of the petrous apex removal, according to data presented in the pertinent literature [10, 55, 66]. The boundaries of the bone removal were defined as follows: (a) inferiorly, the greater superficial petrosal nerve (GSPN) and the petrous internal carotid artery (pICA); (b) medially, the lateral border of the mandibular division of the trigeminal nerve (V3) and the gasserian ganglion (GG) and the trigeminal pore; (c) laterally, the beginning of the inner ear, i.e., basal turn of the cochlea and superior semicircular canal; (d) superiorly, the petrous ridge and the superior petrosal sinus (SPS) (Fig. 1).
Planning of the petrous apex removal via the transorbital pathway and 3D reconstruction. The following structures were taken into account in order to appropriately define the bone removal limits: greater superficial petrosal nerve, petrous internal carotid artery (a); mandibular division of the trigeminal nerve, with gasserian ganglion and the trigeminal pore, the inner ear, i.e., basal turn of the cochlea and superior semicircular canal, and internal acoustic canal (b); the petrous ridge and the superior petrosal sinus (c). The petrous apex planned to remove via the transorbital approach is shown in blue (d). pICA petrous internal carotid artery, C cochlea, IAC internal acoustic canal, SC semicircular canal, SPS superior petrosal sinus, AE arcuate eminence, TI trigeminal impression, SOF superior orbital fissure, cICA cavernous internal carotid artery, PA petrous apex, GSW greater sphenoid wing, FZS fronto-zygomatic suture
Anatomic dissections
Anatomic dissections were performed at the Laboratory of Surgical Neuroanatomy (LSNA) of the Human Anatomy and Embryology Unit, University of Barcelona, (Barcelona, Spain) on five cadaveric heads (10 sides), whose arterial system had been injected with red latex. This study was approved by the institutional review board of the University of Barcelona.
All specimens underwent a multi-slice helical computed tomography (CT) scan (Siemens SOMATOM Sensation 64, Malvern, PA) with a 0.6-mm-thick axial spiral sections and a 0° gantry angle, before and after the dissections.
Five screws were previously implanted in the specimen’s skull as permanent bone reference markers to allow co-registration with the neuronavigation system (Medtronic, Inc. Surgical Technologies, Louisville, CO, USA). Imaging data were transferred to the laboratory navigation-planning workstation, and point registration was performed. A registration correlation tolerance of 2 mm was considered acceptable. In one specimen, an MRI study was performed in order to obtain 3D reconstruction of the main neurovascular structures.
The transorbital approach was performed using a rigid endoscope 4 mm in diameter, 18 cm in length, with a 0 and 30° lens (Karl Storz, Tuttlingen, Germany). It has to be stressed that angled endoscopes were very useful during the transorbital approach. Indeed, they permitted the visualization of the most lateral aspect of the petrous apex region; further, they were useful in performing the subsequent intradural exploration. The endoscope was connected to a (300 W Xenon, Karl Storz) light source through a fiberoptic cable and to an HD camera (Endovision Telecam SL; Karl Storz). The microsurgical dissections, i.e., initial steps of the transorbital approach, were run at magnification ranging from × 3 to × 40 (OPMI; Zeiss, Oberkochen, Germany). Hence, cadaveric dissections were performed as follows.
A superior eyelid approach was performed as previously described in the literature (Fig. 2) [16,17,18]. Furthermore, a dry skull has been used to demonstrate the petrous apex visualization via the transorbital window with its surrounding key bone anatomic landmarks (Fig. 3). A temporary tarsorrhaphy or corneal protector should be used. Subsequently, skin incision was made through an eyelid wrinkle and once the orbicularis oculi muscle was reached, the undersurface of the muscle was followed up to the superolateral orbital rim and the bony orbital rim and the fronto-zygomatic suture were identified. The periosteum was cut and dissection proceeded in sub-periosteal and, then sub-periorbital, plane until the superior and inferior orbital fissures were reached. A malleable retractor was used to protect and displace the orbit contents medially and, from this point over, the procedure was completed using endoscopic visualization, alternating both 0° and 30° lenses. Of note, due to the angle of entry of the endoscope, a 30° lens was advantageous during drilling, allowing better handling of surgical instruments. The craniectomy was initially performed through the body of the zygoma to access the temporal fossa. This initial step was necessary to create adequate work room. The zygomatic body was drilled endo-orbitally, eliminating the necessity to remove the zygomatic arc as typically performed in transcranial approach, thus avoiding any cosmetic defect. Subsequently, the ventral and vertical portion of the greater sphenoid wing was drilled until the dura mater was exposed. This approach to the middle cranial fossa was bounded supero-medially by the upper and lateral portion of the superior orbital fissure; laterally by the previously exposed periosteal surface of the temporalis muscle; infero-medially by the inferior orbital fissure; and inferiorly by the floor of the middle fossa.
Exposure of the petrous apex via the transorbital approach to the middle fossa (left side) in a dry skull. After identification of the main bone anatomic landmarks (a), the craniectomy was performed at the level of the greater sphenoid wing (b) and endoscopic visualization of the petrous apex was obtained (c). Post-dissection CT-scan demonstrated the petrous apex removal via the transorbital approach (d). OC optic canal, SOF superior orbital fissure, IOF inferior orbital fissure, OR orbital rim, ZB zygomatic body, FL foramen lacerum, SPSg groove of the superior petrousal sinus, GSPNg groove of the greater superficial petrousal nerve, AE arcuate eminence, MSR mid-subtemporal ridge, MCFfl middle cranial fossa floor, ZP zygomatic process, FP frontal process; *Trigeminal impression. Dotted line limits of the transorbital middle cranial fossa craniectomy
Endoscopic superior eyelid transorbital approach to the middle fossa and petrous apex (left side). Superior eyelid access is performed (a), followed by endoscopic identification of the main intraorbital anatomic landmarks (b); drilling of the greater sphenoid wing (c) and extradural dissection of the temporal pole from the middle cranial fossa floor (d). PO periorbit, FZS fronto-zygomatic suture, TM temporalis muscle, SOF superior orbital fissure, GWS greater wing of the sphenoid, TD temporal dura, MCFfl middle cranial fossa floor
Once the dura was exposed, an interdural dissection was made between the periorbita and temporal pole in order to unlock the entire cavernous sinus lateral wall up to the trigeminal pore (Fig. 4). After that, the temporal lobe was elevated in extradural fashion and the course of the middle meningeal artery (MMA), emerging from the foramen spinosum, was shown. The MMA was cut just lateral to the mandibular trigeminal branch entrance into the foramen ovale (Fig. 5). This procedure uncovered the greater superficial petrosal nerve (GSPN) whose localization was useful as a landmark for the position of the petrous internal carotid artery (pICA). The temporal lobe was elevated superiorly up to the petrous ridge and the tentorium while up to the arcuate eminence, laterally. At this point, the trigeminal pore was opened in order to obtain space for medial mobilization of the V3-GG complex. Hence, the petrous apex, placed inferiorly to the tentorium, petrous ridge, and superior petrosal sinus, medially to the arcuate eminence, superiorly to the pICA, and laterally to the V3-GG complex, can be drilled out (Fig. 6).
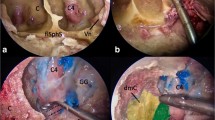
Exposure of the lateral wall of the cavernous sinus via the transorbital approach. After the identification of the meningo-orbital band (a), the cavernous sinus can be unlocked thus exposing the petrous apex region from a ventral corridor (b). PO periorbit, lwCS lateral wall of the cavernous sinus, MOB meningo-orbital band, TD temporal dura, V1 ophthalmic branch of the trigeminal nerve, V2 maxillary branch of the trigeminal nerve, V3 mandibular branch of the trigeminal nerve, MCFfl middle cranial fossa floor, MSR mid-subtemporal ridge, PA petrous apex, GG gasserian ganglion
Exposure of the petrous apex via the transorbital window. The middle meningeal artery was cut (a) and the greater superficial petrousal nerve was identified (b), thus exposing the petrous apex (c). The opening of the trigeminal pore was useful to further increase the exposure of the petrous apex (d). V2 maxillary branch of the trigeminal nerve, V3 mandibular branch of the trigeminal nerve, MCFfl middle cranial fossa floor, MSR mid-subtemporal ridge, PA petrous apex, GG gasserian ganglion, TR trigeminal root in its pore, MMA middle meningeal artery, GSPN greater superficial petrousal nerve, TD temporal dura. *Petrous internal carotid artery
Endoscopic transorbital exposure and drilling of the petrous apex (left side). The main anatomic boundaries around the petrous apex can be appreciated (a) and its drilling can be started (b). V2 maxillary branch of the trigeminal nerve, V3 mandibular branch of the trigeminal nerve, MCFfl middle cranial fossa floor, MSR mid-subtemporal ridge, PA petrous apex, GG gasserian ganglion, MMA middle meningeal artery, GSPN greater superficial petrousal nerve, TD temporal dura, SPS superior petrosal sinus. *Petrous internal carotid artery
In characterizing the exposure of intracranial neurovascular structures afforded by this transpalpebral transorbital endoscopic window, we defined three main intradural spaces that can be explored, namely, cerebellopontine, middle tentorial incisura, and ventral brainstem spaces. Such subdivision was conceived taking into account the most relevant anatomic structures herewith encountered.
Quantitative and morphometric analysis and three-dimensional reconstruction
Using the BrainLAB navigation system (BrainLab Curve, Feldkirchen, Germany) and/or Osirix software (Osirix software, Osirix Foundation, Geneva, Switzerland), it was possible to quantify the amount of bone removal of the petrous apex obtained with the transorbital route. All data were retrieved from pre-op and post-operative CT-scan and were then uploaded in Microsoft office Excel for further analysis.
Further, the virtual 3D model related to the petrous apex removal via the transorbital pathway was created using Amira Visage Imaging (Amira Visage Imaging Inc., San Diego, California, USA). Bony and neurovascular structures were segmented from the CT and MRI scans, and pertinent structures were then represented using advanced instruments for measurement and quantification provided by the Amira workstation (Figs. 7 and 8).
Computer-based illustrations showing the exposure of the petrous apex via the transorbital approach. Middle fossa approach is performed by partial removal of zygomatic bone and greater sphenoid wing (a). The petrous apex region is identified (b). Close-up view of the petrous apex and its surrounding structures (c). Posterior fossa structures can be seen after virtual removal of the petrous apex (d). The reconstructions have been obtained in example specimens using Amira Visage Imaging
Detailed computer-based illustrations showing the main neurovascular structures around the petrous apex region that can be seen via the transorbital approach (a, b). Axial (c) and sagittal (d) perspectives of the same structures have been provided as well. The reconstructions have been obtained in example specimens using Amira Visage Imaging
Results
Qualitative assessment of surgical approaches
Stepwise endoscopic transpalpebral transorbital route to the petrous apex
Access to the petrous apex was gained via the middle fossa approach through the superior eyelid transorbital endoscopic corridor, after interdural opening of the cavernous sinus lateral wall and extradural elevation of the temporal lobe. The limit of the petrous apex involved the GSPN and the pICA inferiorly, the trigeminal complex medially, the petrous ridge with the superior petrosal sinus superiorly, and the arcuate eminence laterally. After recognition of such key landmarks, the drilling of the petrous apex started medially and proceeded in a medial-to-lateral direction in order to early recognize the internal acoustic canal, thus avoiding injury of the inner ear; further, the neuronavigator was useful at this stage to avoid any damage of the cochlea basal turn or the semicircular canals. An artistic illustration depicting the panoramic view of the petrous apex region obtained through the endoscopic transorbital route has been provided (Fig. 9).
Artistic illustration showing the main neurovascular structures of the petrous apex that can be reached via the transorbital endoscopic superior eyelid pathway. Continuous line stands for the superior eyelid access while dotted line represents the craniectomy performed between the superior and inferior orbital fissures to reach the middle cranial fossa. V1 ophthalmic branch of the trigeminal nerve, V2 maxillary branch of the trigeminal nerve, V3 mandibular branch of the trigeminal nerve, V fifth cranial nerve, III third cranial nerve, IV trochlear nerve, VI abducens nerve, MMA middle meningeal artery, GSPN greater superficial petrousal nerve, TD temporal dura, SPS superior petrosal sinus, PA petrous apex
After anterior petrosectomy, the dura of the posterior fossa was opened to expose the pertinent intradural anatomy. Accordingly, it was possible to delineate three anatomic spaces taking into account the relevant neurovascular structures encountered.
Cerebellopontine angle space
Following anterior petrosectomy and removal of the posterior fossa dura, we exposed the cerebellopontine angle structures from the transorbital perspective. This area is bounded by the origin of the trigeminal nerve medially and the facial and vestibulocochlear nerves laterally. The anterolateral portion of the pons was identified at the center of the surgical field, and more posteriorly, the petrosal surface of the cerebellum. The superior petrosal vein, i.e., Dandy’s vein, draining to the superior petrosal sinus, was identified in close relationship with the facial and vestibulocochlear nerves, as well as the anterior-inferior cerebellar and labyrinthine arteries as they coursed through the cerebellopontine angle. This ventral route afforded space to widely open the internal acoustic canal, thus exposing the entire course of the facial and vestibulocochlear nerves as they emerged from the cerebellopontine angle and entered the inner ear (Fig. 10).
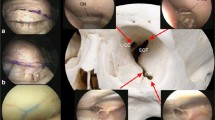
Posterior fossa structures of the cerebellopontine angle space exposed via the transorbital approach (left side). The ponto-cerebellar angle can be showed after the petrous apex removal and the internal acoustic canal can be drilled (a). Detail of the internal acoustic canal is shown by means of 30° angle endoscope turned towards the internal acoustic canal (b). The pons area between the fifth and VII-VIII origin can be appreciated (c). Pons pons portion of the brainstem, DV Dandy’s vein, Cer cerebellum, IAC internal acoustic canal, VII-VIII facial and acoustic nerves. *Anterior-inferior cerebellar artery
Middle tentorial incisura space
At this stage, intradural exploration was further advanced in a superior direction after ligation of the superior petrosal sinus, followed by elevation and cutting of the tentorium. Subsequently, the middle tentorial incisura space was evaluated via the ventral endoscopic transorbital pathway. This space was explored from the origin of the oculomotor nerve to the entrance of the trochlear nerve into the tentorium free edge. The anterolateral portion of the mesencephalon was visualized at the center of the surgical field, surrounded by the superior cerebellar artery as it coursed over the trigeminal pore. Continuing with the dissection medially, the oculomotor nerve was identified at its cisternal segment, arising from the ponto-mesencephalic sulcus. More laterally, the trochlear nerve was recognized in the ambiens division of its cisternal segment, as it emerged from the dorsal brainstem and turned around the mesencephalon in close relation with the superior cerebellar artery and the free edge of the tentorium (Fig. 11).
The posterior fossa neurovascular structures belonging to the middle tentorial incisura space can be showed after elevating the tentorium (a, b). The mesencephalic portion of the brainstem can be appreciated (c) as well as the origin of the fourth and third cranial nerve (d). T tentorium, V trigeminal nerve, III third cranial nerve, IV trochlear nerve, M mesencephalic portion of the brainstem, sca superior cerebellar artery, MC Meckel’s cave, PA petrous apex
Ventral brainstem space
This most medial space was hardly accessible through the straight, ventral endoscopic transorbital trajectory. However, only by means of a 30° lens, the abducens nerve was appreciated as it entered the cavernous sinus from Dorello’s canal. Additionally, the basilar trunk at the midline gives origin to the anterior-inferior cerebellar artery (Fig. 12).
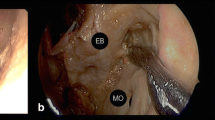
The ventral brainstem area can be showed via the transorbital approach. The abducens nerve can be seen in the cavernous sinus (a) entering from the Dorello’s canal (b, 30° angle endoscope turned medially). The anterior-inferior cerebellar artery coming from the basilar artery trunk can be appreciated by means of a 30° endoscope (c). csICA cavernous internal carotid artery, VI abducens nerve, V trigeminal nerve, GG gasserian ganglion, BA basilar artery, aica anterior-inferior cerebellar artery, Cl clivus, Pons pons portion of the brainstem, PSL petrosphenoid ligament. *Dorello’s canal
Quantitative analysis of bone removal
When quantifying the anterior petrosectomy volumes, the transorbital approach allowed a bone removal average of 1.33 cm3 (range 0.89–1.52 cm3). These volumes were similar to those recently stated in the literature (up to 1.35 cm3) reporting on alternative approaches [3]. These data appear to support, at least in the cadaveric setting, the feasibility of petrous apicectomy from a transorbital endoscopic superior eyelid approach (see Table 1).
Discussion
This study supports the hypothesis that, using a purely endoscopic, transorbital approach, anterior petrosectomy and exposure of the lateral incisural and anterolateral cerebellopontine angle exposure are feasible, at least in anatomic settings. Whereas the eyelid transorbital anterior petrosectomy affords a deep, narrow corridor to the petrous apex region, the volumes of bone removal are comparable, thus presenting a potential alternative as a target approach for select pathology of the anterior cerebellopontine angle, and potentially eliminating the need for a larger, more invasive approach with significant exposure of anatomic structures that are irrelevant to the region of interest (i.e., temporal lobe, vein of Labbe, etc.).
No-man’s land has been the designation of this intricate anatomical region, where few dared to venture given the high surgical morbi-mortality, anatomical complexity, and frustrating surgical exposure. The complex anatomy and difficult surgical exposure of the petrous apex and petroclival regions have spurred the development of multiple surgical approaches designed to minimize perioperative morbidity and maximize safe treatment of pathologies in the region [10].
Over the years, different transcranial approaches have been used, alone or in combination, to manage pathologies in these intricate anatomic spaces, with Kawase’s subtemporal, transpetrosal [35, 67], retro- and trans-labyrinthine [47], transcochlear [20], and retrosigmoid [14, 15, 21] approaches, as the most salient endeavors [40].
In most recent times, new trends in minimally invasive surgery have reshaped cranial base surgery, and based on the experience of endoscopic endonasal skull base surgery, the demand of minimal access but effective cranial base resection continues to grow.
In this line, ventral minimally invasive neurosurgical routes have been recently described, but anatomic limitations given by nasal osteology and the medial border of the cranial nerve foramina and the internal carotid arteries have set a boundary to this route, with the major corridor lying between the basilar artery and internal carotid artery. Hence, endoscopic endonasal approaches for paramedian lesions are considered the most complex and technically demanding among the endonasal approaches and require a multidisciplinary team with significant experience, advanced equipment, and pertinent anatomic knowledge [23, 36].
In this context, the transpalpebral endoscopic transorbital approach has been very lately proposed to access the most lateral aspect of the skull base. This concept, presented as “transorbital neuroendoscopic surgery (TONES),” described a group of endoscopic surgical pathways, a system of orbitotomies that could be indicated for various pathologies that affect the cranial base according to their relative location [50, 54]. One TONES corridor is the superior eyelid route, proven in the clinical setting as a means to manage select, mainly laterally placed skull base targets.
The upper eyelid approach, with the aid of the microscope and/or the endoscope and with or without the removal of the upper or lateral orbital rim, has been proposed lately, and it has been extensively reported in surgical series of both extradural pathologies, like spheno-orbital meningiomas [19], as well as intradural lesions, i.e., unruptured middle cerebral artery aneurysms [1, 6, 7, 46] and lesions of the petroclival junction extending into the anterior cerebellopontine area [44]. Particularly for meningiomas, this approach provides early assessment of periorbital invasion and the ability to remove tumor-infiltrated, hyperostotic bone from the sphenoid wing with decompression of the superior orbital fissure and optic canal. The purely endoscopic transorbital approach provides a further, less invasive access to the anterior and medial cranial fossa. Anatomical studies and early clinical reports have documented the feasibility of this approach and its efficacy in select pathology. However, this route has several drawbacks, the main being unfamiliarity with the anatomy and instrumentation. This approach includes risk to the eye during retraction, and limited literature exists on the effects of prolonged retraction of orbital contents. However, in the most recent surgical series, no orbital morbidity has been reported, even in cases of periorbital violation [19]. A plausible explanation supporting the limited damage to orbital contents may be that working space is gained by removing bone (i.e., zygomatic bone and greater wing of the sphenoid bone) and not by compressing the eye, therefore reducing pressure applied to the eye. Another concern with this approach is the possibly of cerebrospinal fluid (CSF) leak causing pulsatile exophthalmos. However, the most recent literature does not show such kind of complication. An additional limiting factor is the crowding of endoscopic instruments that can occur while moving posterior in the corridor. As technology improves, generating multifunctional endoscopic instruments, this limitation will be minimized. Anyway, the use of a two-surgeon, three/four-handed technique allows dynamic movement of the endoscope thus eliminating the loss of depth perception and obtaining a 3D perspective of the surgical field. Furthermore, the use of dedicated transorbital ports may partially overcome these limitations. Finally, cosmetic concerns involving the risk of enophtalmos have not been founded by the limited recent experience.
In this study, we demonstrated that the ventral endoscopic pathway affords, in an interdural fashion, exposure of the entire lateral wall of the cavernous sinus up to the petrous apex with very limited brain retraction or cranial nerve manipulation. This route provides coplanar, path-to-target dissection to the aforementioned structures. In this contribution, we demonstrate the use of this route in cadaveric specimens to complete a comparable volume of petrous apicectomy when considering traditional transpetrosal approaches (1.33 cm3 compared to about 1.35 cm3) [66]. Using our approach, the main anatomic landmarks classically used for the Kawase’s approach were exposed. As for the transcranial approach to the middle fossa, the greater superficial petrosal nerve (GSPN), originating from the geniculate ganglion and running in its groove towards the pterygoid canal, represents the most reliable superficial landmark on the middle cranial fossa for drilling of the petrous apex. The GSPN should be clearly recognized before drilling at Kawase’s triangle to avoid the risk of injury to the internal carotid artery (ICA), located immediately in an inferior position as seen via the transorbital pathway. Comparatively, the GSPN is considered the superficial inferior border of the anterior petrosectomy on the middle fossa to avoid risk of the ICA injury. Moreover, the superior petrosal sinus, draining the petrosal vein, is the superior border of the petrosectomy and it could be ligated to expose the middle tentorial incisura space. On the other hand, the gasserian ganglion, together with the mandibular branch of the trigeminal nerve, is considered the medial border of the petrosectomy; the latter can be medially mobilized after opening the trigeminal pore in order to expose a larger portion of the anterior petrous apex surface. Finally, the most lateral boundary is represented by the arcuate eminence, which must be preserved in order to avoid hearing loss. However, in some cases, using such ventral trajectory, it may be difficult to clearly expose and identify that landmark and dedicated neuronavigation is required. Moreover, the ventral brainstem area may be difficult to access being the transorbital approach a ventral, anterior-to-posterior path; on the contrary, the traditional subtemporal Kawase approach, lateral-to-medial route and worldwide used for large sphenopetroclival meningiomas and basilar trunk aneurysms, permits an optimal management of the ventral brainstem area. This specific working angle difference between the two approaches should be taken into account when dealing with appropriate patient selection. However, comparison between the two approaches is far beyond the aim of the present paper whose primary goal is to provide a thorough, solid anatomic basis to better quantify, in laboratory setting only, the role of the relatively novel endoscopic superior eyelid transorbital corridor. Anatomic comparative studies between the transorbital approach and the Kawase one are currently ongoing in order to obtain proper quantitative analysis when using the two routes to reach the petrous apex.
This cadaveric study describes technical nuances and anatomic details of the superior eyelid endoscopic transorbital approach, with specific focus on the petrous apex and its complex surrounding anatomy. Advantages of the transorbital route include exposure of petrous apex with limited brain retraction, relatively simple and fast craniectomy, and a direct parallel view of the target. It affords a straight route to petrous apex, without disrupting the cavernous sinus, manipulation of the temporal lobe, or a large dural opening. Drawbacks include ocular complications, unfamiliarity with anatomical perspective and instrumentation, deeper exposure, and limited surgical maneuverability, thus potentially reducing its indications to very select pathology. Furthermore, it has to be stressed that control of the venous bleedings while dissecting the cavernous sinus lateral wall will be demanding but the possibility to work in this area may depend, at least in part, on the space created by the lesion. However, the discussion related to advantages and limitations of the clinical utility of the endoscopic transorbital corridor to the petrous apex will deserve additional study based on direct surgical experiences.
Finally, this study aims to address the continuous need of thoughtfully exploring less invasive corridors to assist with the surgical management of skull base pathology. Potential clinical applications reside on the treatment of lesions located in the petrous apex and petroclival area that are suboptimally managed via traditional transcranial routes.
Study limitations
In this purely anatomic study, one limitation was obviously the use of cadaveric specimens. Given that cadaveric models are useful to investigate surgical approaches but not fully replicative of the clinical environment, tissue characteristics, bleeding, and alteration of the normal anatomy due to pathology must be considered. Particular concerns for the endoscopic transorbital approach include the burden of orbital content retraction, despite recent report of clinical tolerance. Intraoperative globe tonometry and visual-evoked potentials might be useful to determine the maximal safe degree of globe retraction. Alternatively, intermittent relief from retraction could be useful to protect the globe when greater tension is necessary.
Conclusion
The purely endoscopic superior eyelid transorbital approach affords good visualization and comparable removal of the petrous apex respective of that obtained using conventional approaches. This anatomical study describes the neurovascular structures relevant to the surgical treatment of lesions in the region, as previously described in anatomical series addressing traditional approaches, as a measure of feasibility and control of the surgical theater using the transorbital approach when compared to that typically uncounted when treating lesions in the petrous apex region using conventional approaches. The transorbital pathway to the petrous apex appears limited to select surgical settings. However, rather than discourage its application, this route appears to address some of the shortcomings of conventional approaches and may constitute a valuable addition to the established array of surgical options. Surgical case series are needed to establish the clinical value of this approach to better determine its place in the armamentarium of modern skull base surgery.
References
Abdel Aziz KM, Bhatia S, Tantawy MH, Sekula R, Keller JT, Froelich S, Happ E (2011) Minimally invasive transpalpebral “eyelid” approach to the anterior cranial base. Neurosurgery 69:ons195–ons206; discussion 206-197. https://doi.org/10.1227/NEU.0b013e31821c3ea3
Abdel Aziz KM, Sanan A, van Loveren HR, Tew JM, Keller JT, Pensak ML (2000) Petroclival meningiomas: predictive parameters for transpetrosal approaches. Neurosurgery 47:139–150 discussion 150-132
Ahmed O, Walther J, Theriot K, Manuel M, Guthikonda B (2016) Morphometric analysis of bone resection in anterior petrosectomies. J Neurol Surg B Skull Base 77:238–242. https://doi.org/10.1055/s-0035-1566301
Almefty R, Dunn IF, Pravdenkova S, Abolfotoh M, Al-Mefty O (2014) True petroclival meningiomas: results of surgical management. J Neurosurg 120:40–51. https://doi.org/10.3171/2013.8.JNS13535
Almeida JP, Ruiz-Treviño AS, Shetty SR, Omay SB, Anand VK, Schwartz TH (2017) Transorbital endoscopic approach for exposure of the sylvian fissure, middle cerebral artery and crural cistern: an anatomical study. Acta Neurochir. https://doi.org/10.1007/s00701-017-3296-8
Andaluz N, Romano A, Reddy LV, Zuccarello M (2008) Eyelid approach to the anterior cranial base. J Neurosurg 109:341–346. https://doi.org/10.3171/JNS/2008/109/8/0341
Andaluz N, Van Loveren HR, Keller JT, Zuccarello M (2003) Anatomic and clinical study of the orbitopterional approach to anterior communicating artery aneurysms. Neurosurgery 52:1140–1148 discussion 1148-1149
Asaoka K, Terasaka S (2014) Combined petrosal approach for resection of petroclival meningioma. Neurosurg Focus 36:1. https://doi.org/10.3171/2014.V1.FOCUS13446
Ayberk G, Ozveren MF, Uzum N, Tosun O, Akcay EK (2008) Cellular schwannoma of the greater superficial petrosal nerve presenting with abducens nerve palsy and xerophthalmia: case report. Neurosurgery 63:E813–E814; discussion E814. https://doi.org/10.1227/01.NEU.0000325501.75772.FD
Borghei-Razavi H, Tomio R, Fereshtehnejad SM, Shibao S, Schick U, Toda M, Kawase T, Yoshida K (2015) Anterior petrosal approach: the safety of Kawase triangle as an anatomical landmark for anterior petrosectomy in petroclival meningiomas. Clin Neurol Neurosurg 139:282–287. https://doi.org/10.1016/j.clineuro.2015.10.032
Borghei-Razavi H, Tomio R, Fereshtehnejad SM, Shibao S, Schick U, Toda M, Yoshida K, Kawase T (2016) Pathological location of cranial nerves in petroclival lesions: how to avoid their injury during anterior petrosal approach. J Neurol Surg B Skull Base 77:6–13. https://doi.org/10.1055/s-0035-1555137
Chang SW, Wu A, Gore P, Beres E, Porter RW, Preul MC, Spetzler RF, Bambakidis NC (2009) Quantitative comparison of Kawase’s approach versus the retrosigmoid approach: implications for tumors involving both middle and posterior fossae. Neurosurgery 64:ons44–ons51; discussion ons51-42. https://doi.org/10.1227/01.NEU.0000334410.24984.DD
Charachon R, Gratacap B, Lavieille JP (1992) Chondrosarcomas of the petrous apex. Skull Base Surg 2:171–175
Colasanti R, Tailor AR, Lamki T, Zhang J, Ammirati M (2015) Maximizing the petroclival region exposure via a suboccipital retrosigmoid approach: where is the intrapetrous internal carotid artery? Neurosurgery 11(Suppl 2):329–336; discussion 336-327. https://doi.org/10.1227/NEU.0000000000000749
Cui H, Zhou CF, Bao YH, Wang MS, Wang Y (2016) Extended suboccipital retrosigmoid surgical approach is effective for resection of petrous apex meningioma. J Craniofac Surg 27:e429–e433. https://doi.org/10.1097/SCS.0000000000002705
Dallan I, Castelnuovo P, Locatelli D, Turri-Zanoni M, AlQahtani A, Battaglia P, Hirt B, Sellari-Franceschini S (2015) Multiportal combined transorbital transnasal endoscopic approach for the management of selected skull base lesions: preliminary experience. World Neurosurg 84:97–107. https://doi.org/10.1016/j.wneu.2015.02.034
Dallan I, Castelnuovo P, Turri-Zanoni M, Fiacchini G, Locatelli D, Battaglia P, Sellari-Franceschini S (2016) Transorbital endoscopic assisted management of intraorbital lesions: lessons learned from our first 9 cases. Rhinology 54:247–253. https://doi.org/10.4193/Rhin15.237
Dallan I, Di Somma A, Prats-Galino A, Solari D, Alobid I, Turri-Zanoni M, Fiacchini G, Castelnuovo P, Catapano G, de Notaris M (2016) Endoscopic transorbital route to the cavernous sinus through the meningo-orbital band: a descriptive anatomical study. J Neurosurg 127:1–8. https://doi.org/10.3171/2016.8.JNS16465
Dallan I, Sellari-Franceschini S, Turri-Zanoni M, de Notaris M, Fiacchini G, Romana Fiorini F, Battaglia P, Locatelli D, Castelnuovo P (2017) Endoscopic transorbital superior eyelid approach for the management of selected spheno-orbital meningiomas: preliminary experience. Oper Neurosurg opx100. https://doi.org/10.1093/ons/opx100
De la Cruz A, Teufert KB (2009) Transcochlear approach to cerebellopontine angle and clivus lesions: indications, results, and complications. Otol Neurotol 30:373–380. https://doi.org/10.1097/MAO.0b013e31819a892b
de Souza DG, Ditzel Filho LF, Makonnen G, Zoli M, Naudy C, Muto J, Prevedello DM (2014) Retrosigmoid approach for resection of petrous apex meningioma. Neurosurg Focus 36:1. https://doi.org/10.3171/2014.V1.FOCUS13456
Diaz Day J (2012) The middle fossa approach and extended middle fossa approach: technique and operative nuances. Neurosurgery 70:192–201. https://doi.org/10.1227/NEU.0b013e31823583a1
Essayed WI, Singh H, Lapadula G, Almodovar-Mercado GJ, Anand VK, Schwartz TH (2017) Endoscopic endonasal approach to the ventral brainstem: anatomical feasibility and surgical limitations. J Neurosurg 127:1–8. https://doi.org/10.3171/2016.9.JNS161503
Eytan DF, Kshettry VR, Sindwani R, Woodard TD, Recinos PF (2014) Surgical outcomes after endoscopic management of cholesterol granulomas of the petrous apex: a systematic review. Neurosurg Focus 37:E14. https://doi.org/10.3171/2014.7.FOCUS14344
Ferrari M, Schreiber A, Mattavelli D, Belotti F, Rampinelli V, Lancini D, Doglietto F, Fontanella MM, Tschabitscher M, Rodella LF, Nicolai P (2016) The inferolateral Transorbital endoscopic approach: a preclinical anatomic study. World Neurosurg 90:403–413. https://doi.org/10.1016/j.wneu.2016.03.017
Freeman JL, Sampath R, Quattlebaum SC, Casey MA, Folzenlogen ZA, Ramakrishnan VR, Youssef AS (2017) Expanding the endoscopic transpterygoid corridor to the petroclival region: anatomical study and volumetric comparative analysis. J Neurosurg 1–10. https://doi.org/10.3171/2017.1.JNS161788
Grossi PM, Nonaka Y, Watanabe K, Fukushima T (2012) The history of the combined supra- and infratentorial approach to the petroclival region. Neurosurg Focus 33:E8. https://doi.org/10.3171/2012.6.FOCUS12141
Gupta SK, Salunke P (2012) Intradural anterior petrosectomy for petroclival meningiomas: a new surgical technique and results in 5 patients: technical note. J Neurosurg 117:1007–1012. https://doi.org/10.3171/2012.9.JNS12429
Harrison Priddy B, Nunes C, Beer-Furlan A, Carrau R, Dallan I, Prevedello D (2017) A side door to Meckel’s cave: anatomic feasibility study for the lateral transorbital approach. Oper Neurosurg 13:1–8. https://doi.org/10.1093/ons/opx042
Hegazy A, Alfiki A, Adel MF, Alsawy MF, Al-Dash MF, Zein M, Amin SM, Al-Shami H, Biswas A (2016) Role of surgery for small petrous apex meningiomas causing refractory trigeminal neuropathy in the minimally invasive era. Neurol India 64:973–979. https://doi.org/10.4103/0028-3886.190230
Hunter JB, Weaver KD, Thompson RC, Wanna GB (2015) Petroclival meningiomas. Otolaryngol Clin N Am 48:477–490. https://doi.org/10.1016/j.otc.2015.02.007
Ichimura S, Hori S, Hecht N, Czabanka M, Vajkoczy P (2016) Intradural anterior transpetrosal approach. Neurosurg Rev 39:625–631. https://doi.org/10.1007/s10143-016-0711-1
Ichimura S, Yoshida K, Sutiono AB, Horiguchi T, Sasaki H, Kawase T (2010) Greater petrosal nerve schwannomas-analysis of four cases and review of the literature. Neurosurg Rev 33:477–482. https://doi.org/10.1007/s10143-010-0277-2
Jacquesson T, Berhouma M, Tringali S, Simon E, Jouanneau E (2015) Which routes for Petroclival tumors? A comparison between the anterior expanded endoscopic endonasal approach and lateral or posterior routes. World Neurosurg 83:929–936. https://doi.org/10.1016/j.wneu.2015.02.003
Janjua MB, Caruso JP, Greenfield JP, Souweidane MM, Schwartz TH (2017) The combined transpetrosal approach: anatomic study and literature review. J Clin Neurosci. https://doi.org/10.1016/j.jocn.2017.03.015
Kasemsiri P, Carrau RL, Ditzel Filho LF, Prevedello DM, Otto BA, Old M, de Lara D, Kassam AB (2014) Advantages and limitations of endoscopic endonasal approaches to the skull base. World Neurosurg 82:S12–S21. https://doi.org/10.1016/j.wneu.2014.07.022
Kawase T, Shiobara R, Toya S (1991) Anterior transpetrosal-transtentorial approach for sphenopetroclival meningiomas: surgical method and results in 10 patients. Neurosurgery 28:869–875 discussion 875-866
Kawase T, Shiobara R, Toya S (1994) Middle fossa transpetrosal-transtentorial approaches for petroclival meningiomas. Selective pyramid resection and radicality. Acta Neurochir 129:113–120
King TT, Benjamin JC, Morrison AW (1989) Epidermoid and cholesterol cysts in the apex of the petrous bone. Br J Neurosurg 3:451–461
Komatsu F, Komatsu M, Di Ieva A, Tschabitscher M (2013) Endoscopic extradural subtemporal approach to lateral and central skull base: a cadaveric study. World Neurosurg 80:591–597. https://doi.org/10.1016/j.wneu.2012.12.018
Kusumi M, Fukushima T, Mehta AI, Aliabadi H, Nonaka Y, Friedman AH, Fujii K (2012) Tentorial detachment technique in the combined petrosal approach for petroclival meningiomas. J Neurosurg 116:566–573. https://doi.org/10.3171/2011.11.JNS11985
Kusumi M, Fukushima T, Mehta AI, Cunningham CD, Friedman AH, Fujii K (2013) Middle fossa approach for total resection of petrous apex cholesterol granulomas: use of vascularized galeofascial flap preventing recurrence. Neurosurgery 72:77–86; discussion 86. https://doi.org/10.1227/NEU.0b013e3182724354
Lau DP, Wharton SB, Antoun NM, Bottrill ID, Moffat DA (1997) Chondrosarcoma of the petrous apex. Dilemmas in diagnosis and treatment. J Laryngol Otol 111:368–371
Lim J, Cho K (2016) The modified lateral supraorbital approach for tumors of the petroclival junction extending into the anterior cerebellopontine area. J Neuro-Oncol 127:541–550. https://doi.org/10.1007/s11060-016-2061-9
Locatelli D, Pozzi F, Turri-Zanoni M, Battaglia P, Santi L, Dallan I, Castelnuovo P (2016) Transorbital endoscopic approaches to the skull base: current concepts and future perspectives. J Neurosurg Sci 60:514–525
Mandel M, Tutihashi R, Abramovicz Mandel S, Jacobsen Teixeira M, Gadelha Figueiredo E (2017) Minimally invasive transpalpebral “eyelid” approach to unruptured middle cerebral artery aneurysms. Oper Neurosurg 13:1–12. https://doi.org/10.1093/ons/opx021
Mandelli C, Porras L, López-Sánchez C, Sicuri GM, Lomonaco I, García-Martínez V (2008) The partial labyrinthectomy petrous apicectomy approach to petroclival meningiomas. A quantitative anatomic comparison with other approaches to the same region. Neurocirugia (Astur) 19:133–142
Maurer AJ, Bonney PA, Iser CR, Ali R, Sanclement JA, Sughrue ME (2015) Endoscopic endonasal infrapetrous transpterygoid approach to the Petroclival junction for petrous apex chondrosarcoma: technical report. J Neurol Surg Rep 76:e113–e116. https://doi.org/10.1055/s-0035-1549222
Miller ME, Mastrodimos B, Cueva RA (2012) Hearing preservation in management of epidermoids of the cerebellopontine angle: CPA epidermoids and hearing preservation. Otol Neurotol 33:1599–1603. https://doi.org/10.1097/MAO.0b013e31826bed8d
Moe KS, Bergeron CM, Ellenbogen RG (2010) Transorbital neuroendoscopic surgery. Neurosurgery 67:ons16–ons28. https://doi.org/10.1227/01.NEU.0000373431.08464.43
Muto J, Prevedello DM, Ditzel Filho LF, Tang IP, Oyama K, Kerr EE, Otto BA, Kawase T, Yoshida K, Carrau RL (2016) Comparative analysis of the anterior transpetrosal approach with the endoscopic endonasal approach to the petroclival region. J Neurosurg 125:1171–1186. https://doi.org/10.3171/2015.8.JNS15302
Peyre M, Bozorg-Grayeli A, Rey A, Sterkers O, Kalamarides M (2012) Posterior petrous bone meningiomas: surgical experience in 53 patients and literature review. Neurosurg Rev 35:53–66; discussion 66. https://doi.org/10.1007/s10143-011-0333-6
Presutti L, Alicandri-Ciufelli M, Rubini A, Gioacchini FM, Marchioni D (2014) Combined lateral microscopic/endoscopic approaches to petrous apex lesions: pilot clinical experiences. Ann Otol Rhinol Laryngol 123:550–559. https://doi.org/10.1177/0003489414525342
Ramakrishna R, Kim LJ, Bly RA, Moe K, Ferreira M (2016) Transorbital neuroendoscopic surgery for the treatment of skull base lesions. J Clin Neurosci 24:99–104. https://doi.org/10.1016/j.jocn.2015.07.021
Rigante L, Herlan S, Tatagiba MS, Stanojevic M, Hirt B, Ebner FH (2016) Petrosectomy and topographical anatomy in traditional Kawase and posterior Intradural petrous apicectomy (PIPA) approach: an anatomical study. World Neurosurg 86:93–102. https://doi.org/10.1016/j.wneu.2015.08.083
Russo FY, De Seta D, Mosnier I, Sterkers O, Bernardeschi D (2016) Surgical management of petrous apex cholesterol granulomas by an infralabyrinthine approach: our experience with fourteen cases. Clin Otolaryngol. https://doi.org/10.1111/coa.12721
Sabin HI, Bordi LT, Symon L (1987) Epidermoid cysts and cholesterol granulomas centered on the posterior fossa: twenty years of diagnosis and management. Neurosurgery 21:798–805
Samii A, Gerganov V, Herold C, Gharabaghi A, Hayashi N, Samii M (2009) Surgical treatment of skull base chondrosarcomas. Neurosurg Rev 32:67–75; discussion 75. https://doi.org/10.1007/s10143-008-0170-4
Sbaihat A, Bacciu A, Pasanisi E, Sanna M (2013) Skull base chondrosarcomas: surgical treatment and results. Ann Otol Rhinol Laryngol 122:763–770. https://doi.org/10.1177/000348941312201206
Sharma M, Ambekar S, Guthikonda B, Nanda A (2014) A comparison between the Kawase and extended retrosigmoid approaches (retrosigmoid transtentorial and retrosigmoid intradural suprameatal approaches) for accessing the petroclival tumors. A cadaveric study. J Neurol Surg B Skull Base 75:171–176. https://doi.org/10.1055/s-0033-1359305
Shimony N, Gonen L, Shofty B, Abergel A, Fliss DM, Margalit N (2016) Surgical resection of skull-base chordomas: experience in case selection for surgical approach according to anatomical compartments and review of the literature. Acta Neurochir. https://doi.org/10.1007/s00701-016-3032-9
Shin M, Kondo K, Hanakita S, Hasegawa H, Yoshino M, Teranishi Y, Kin T, Saito N (2017) Endoscopic transsphenoidal anterior petrosal approach for locally aggressive tumors involving the internal auditory canal, jugular fossa, and cavernous sinus. J Neurosurg 126:212–221. https://doi.org/10.3171/2016.1.JNS151979
Simal-Julián JA, Miranda-Lloret P, Botella-Asunción C, Kassam A (2014) Full endoscopic endonasal expanded approach to the petroclival region: optimizing the carotid-clival window. Acta Neurochir 156:1627–1629. https://doi.org/10.1007/s00701-014-2125-6
Taniguchi M, Akutsu N, Mizukawa K, Kohta M, Kimura H, Kohmura E (2016) Endoscopic endonasal translacerum approach to the inferior petrous apex. J Neurosurg 124:1032–1038. https://doi.org/10.3171/2015.1.JNS142526
Tatagiba M, Acioly MA, Roser F (2013) Petroclival tumors. J Neurosurg 119:526–528. https://doi.org/10.3171/2013.2.JNS13319
Tripathi M, Deo RC, Suri A, Srivastav V, Baby B, Kumar S, Kalra P, Banerjee S, Prasad S, Paul K, Roy TS, Lalwani S (2015) Quantitative analysis of the Kawase versus the modified Dolenc-Kawase approach for middle cranial fossa lesions with variable anteroposterior extension. J Neurosurg 123:14–22. https://doi.org/10.3171/2015.2.JNS132876
Troude L, Carissimi M, Lavieille JP, Roche PH (2016) How I do it: the combined petrosectomy. Acta Neurochir 158:711–715. https://doi.org/10.1007/s00701-016-2740-5
Van Gompel JJ, Alikhani P, Youssef AS, Loveren HR, Boyev KP, Agazzi S (2015) Anterior petrosectomy: consecutive series of 46 patients with attention to approach-related complications. J Neurol Surg B Skull Base 76:379–384. https://doi.org/10.1055/s-0034-1543971
Wayhs SY, Lepski GA, Frighetto L, Isolan GR (2017) Petroclival meningiomas: remaining controversies in light of minimally invasive approaches. Clin Neurol Neurosurg 152:68–75. https://doi.org/10.1016/j.clineuro.2016.11.021
Wright E, Spetzler RF (2015) The expanded endoscopic endonasal approach to Petroclival lesions: a useful adjunct to traditional skull base approaches. World Neurosurg 84:224–225. https://doi.org/10.1016/j.wneu.2015.04.010
Xu F, Karampelas I, Megerian CA, Selman WR, Bambakidis NC (2013) Petroclival meningiomas: an update on surgical approaches, decision making, and treatment results. Neurosurg Focus 35:E11. https://doi.org/10.3171/2013.9.FOCUS13319
Acknowledgements
The authors thank Antonia Conti, Medical Illustrator, for the precious artistic drawing dedicated to this paper.
This work has been partly supported by the grant “Marató TV3 Project” [411/U/2011—title: Quantitative analysis and computer-aided simulation of minimally invasive approaches for intracranial vascular lesions].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Human and animal rights and informed consent
This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Di Somma, A., Andaluz, N., Cavallo, L.M. et al. Endoscopic transorbital route to the petrous apex: a feasibility anatomic study. Acta Neurochir 160, 707–720 (2018). https://doi.org/10.1007/s00701-017-3448-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-017-3448-x