Abstract
Background
The maxillary artery (MA) has gained attention in neurosurgery particularly in cerebral revascularization techniques, intracranial endonasal approaches and endovascular procedures.
Objectives
To describe and illustrate the anatomy of the MA and its neurosurgical importance in a detailed manner.
Methods
Six cadaveric heads (12 MAs) were injected with latex. The arteries and surrounding structures were dissected and studied using microsurgical techniques. The dimensions, course and branching patterns of the MA were recollected. In addition, 20 three-dimensional reconstruction CT head and neck angiograms (3D CTAs) of actual patients were correlated with the cadaveric findings.
Results
The MA can be divided in three segments: mandibular, pterygoid and pterygopalatine. Medial and lateral trunk variants regarding its course around the lateral pterygoid muscle can be found. The different branching patterns of the MA have a direct correlation with the course of its main trunk at the base of the skull. Branching and trunk variants on one side do not predict the findings on the contralateral side.
Conclusion
In this study the highly variable course, branching patterns and relations of the MA are illustrated and described in human cadaveric heads and 3D CTAs. MA 3D CTA with bone reconstruction can be useful preoperatively for the identification of the medial or lateral course variants of this artery, particularly its pterygoid segment, which should be taken into account when considering the MA as a donor vessel for an EC-IC bypass.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The maxillary artery has been gaining attention in neurosurgery, particularly in therapeutic techniques such as extracranial-intracranial (EC-IC) bypass, intracranial endonasal approaches and endovascular procedures. Although the anatomy of the maxillary artery has been well described in the literature, detailed characterizations and illustrations of its course, branching patterns and anatomic relations are scarce and under-demonstrated.
Methods
Six cadaveric heads (12 maxillary arteries) were injected with red (arteries) and blue (veins) latex. The arteries and surrounding structures were dissected and studied using microsurgical techniques. Details of our technique have been published in the past [3]. Special attention was given to the course and branching pattern of the maxillary artery. Complete exposure of the maxillary artery main trunk and branches was obtained by removing the zygomatic arch and coronoid process of the mandible as well as by widening the pterygomaxillary fissure by removing the lateral pterygoid plate and all the maxillary sinus walls. Twenty cerebral angiograms and 20 CT angiograms (3D reconstructions) were studied and correlated with the anatomic dissection findings.
Results
Proximal segment (mandibular)
This segment courses from its origin out of the external carotid artery to the lateral edge of the lateral pterygoid muscle (LPM). The length of this segment is 13.63 ± 0.9 mm in the medial variant and 15.83 ± 0.6 mm in the lateral variant (diameter: 3.67 ± 0.07 mm). Five main branches originate from this segment: These branches arise as the vessel passes between the ramus of the mandible and the sphenomandibular ligament and then run either medial or lateral to the LPM. In taking this course, the MA supplies the external auditory meatus (EAM), tympanic cavity, base of the skull and mandible through branches to all these structures.
Branches
-
(1)
The tympanic artery (diameter: 0.7 ± 0.002 mm): Either as a single branch or as a common trunk with the deep auricular artery. This artery penetrates the skull base through the petrotympanic fissure to provide vascular supply to the middle ear (Fig. 1).
Fig. 1 Cadaveric dissection of the mandibular segment of the maxillary artery (first segment). a Lateral variant (right side). Common trunk for the accessory meningeal artery and middle meningeal artery. Inferior alveolar artery and posterior temporal artery are born separately. b Medial variant (left side). Accessory meningeal artery and middle meningeal artery branching separately. Inferior alveolar artery and posterior temporal artery share a common trunk. M.m.a: middle meningeal artery; A.m.a.: accessory meningeal artery; Post. Temp. a. & m.: posterior deep temporal artery and muscle; Lat. Pteryg. m.& a.: lateral pterygoid muscle and artery; Med. Pteryg. m.: medial pterygoid muscle; Inf. alv. a.& n.: inferior alveolar artery and nerve; Aur. Temp.n.: auricular temporal nerve; Sup. Temp. a.: superficial temporal artery.; Aur. Temp. n.: auriculotemporal nerve; Ant Tymp. a.: Anterior tympanic artery; Deep. aur. a.: deep auricular artery; Sphen.mand. l.: sphenomandibular ligament; Inf. alv. a.: inferior alveolar artery; Lingual n.: Lingual nerve. P/tymp. f.: petrotympanic fissure; EAC: external auditory canal; Maxillary a; maxillary artery; Lat. pteryg. m.: lateral pterygoid muscle
-
(2)
The deep auricular artery (diameter: 1.0 ± 0.09 mm): either as a single or multiple branches and/or coming from a common trunk with the tympanic artery, it supplies the skin of the external auditory meatus, tympanic membrane and temporomandibular joint. It should be noted that in 50% of specimens a common trunk for the tympanic and the deep auricular artery or arteries is present (Fig. 1).
-
(3)
The middle meningeal artery (MMA) (diameter: 2.53 ± 0.38 mm): After branching off the maxillary artery in the infra-temporal fossa, it runs through the foramen spinosum to supply the dura mater and the calvaria (Fig. 1).
-
(4)
The accessory meningeal artery (AMA) (diameter: 1.9 ± 0.22): It may arise as a single branch when the MA has a deep course (medial variant) and from a common trunk with the middle meningeal artery when the MA has a superficial course (lateral variant). This artery provides blood supply to the parasellar region, Eustachian tube and soft palate (Fig. 1).
-
(5)
The inferior alveolar artery (diameter: 1.3 ± 0.07 mm): This is found as either a single branch or a common trunk with the posterior deep temporal artery (PDTA). This artery passes inferiorly and anteriorly to enter the mandibular foramen and canal. It supplies the lower molars, premolars, canine and incisors, exiting the bony canal through the mental foramen. A lateral course of the MA when single and a medial course of the MA when sharing a trunk with the deep posterior temporal artery were consistently found. This artery branched off the MA before the middle meningeal artery either at the MA origin or right before it in 50% of cases and at the same location as the MMA or right after it in the other 50% (Fig. 1).
Second segment (pterygoid)
This runs from the lateral edge of the LPM to the sphenomaxillary fissure. The length of this segment was on average 14.96 mm in the medial variant and 19.66 mm in the lateral variant. It may run laterally to the lateral pterygoid muscle (LPM) (in 66% of cases) (Fig. 2) and medially to this structure in 33% (diameter: 3.24 ± 0.2 mm) (Fig. 3). As the maxillary artery trunk crosses the infra-temporal fossa it gives off numerous muscular branches.
Cadaveric dissection of the pterygoid segment of the maxillary artery (second segment). Lateral variant (left side): Dissection showing the second segment of the maxillary artery (notice the main trunk of the artery around the lateral Pterygoid muscle). Ant. temp. a.: anterior deep temporal artery; Post. temp. a.: posterior temporal artery; Post. Sup. alv. a.: posterior superior alveolar artery. Buccal a & n.: buccal artery and nerve; Buccinator m: Buccinator muscle; pterygoid a.: pterygoid artery; Masseteric a & nerve: Masseteric artery and nerve;. I.J.V.: internal jugular vein; Masseter m: masseter muscle; Facial a.: facial artery; Parotid g. and duct: parotid gland and duct; Lingual n: lingual nerve; Maxillary a: maxillary artery; Lat. pteryg. m.: lateral pterygoid muscle; Med. Pteryg. m.: medial pterygoid muscle
Cadaveric dissection of the pterygoid segment of the maxillary artery (second segment). Medial variant (right side). a Notice that the main branch of the maxillary is not seen because it is covered by the lateral pterygoid muscle. b The lateral pterygoid muscle has been removed and thus the maxillary trunk is now visible. Lingual n.: Lingual nerve; Masseter m: masseter muscle; Parotid d.: parotid duct; Med. Pteryg. m.: medial Pterygoid muscle; Buccinator m.: buccinator muscle; Buccal a. & n.: buccal artery and nerve; Lat. Pteryg. m.: lateral pterygoid muscle; Maxillary a.: maxillary artery; Masseteric a.: masseteric artery; Inf. alv. a.: inferior alveolar artery; Masseteric n.: masseteric nerve; Lingual n.: lingual nerve; Facial v.: facial vein; Post. Temp a.: posterior temporal artery
Branches
-
(1)
Anterior deep temporal artery (diameter: 1.45 ± 0.10 mm): Runs parallel to the muscular fibers of the deepest temporalis muscle layer and supplies the temporalis muscle.
-
(2)
Posterior deep temporal artery (diameter: 1.48 ± 0.01 mm). Runs parallel to the anterior deep temporal artery. It has a common trunk with the inferior alveolar artery when the maxillary artery runs medially to the lateral pterygoid muscle and is born separately when the maxillary artery runs laterally to it (Fig. 2).
-
(3)
Masseteric artery (diameter: 1.03 ± 0.02 mm): courses laterally through the mandibular notch to reach the masseter muscle (Fig. 2).
-
(4)
Buccal artery (diameter: 1.05 ± 0.07 mm): courses superficial to the pterygomandibular ligament and buccinator muscle to supply it. It also supplies the mucous membrane and the skin of the cheek (Fig. 2).
-
(5)
Pterygoid artery(ies): consist of five to seven branches (diameter: 0.7 ± 0.003 mm) originating either from the MA trunk (diameter: 0.34 ± 0.01 mm), the posterior deep temporal artery (diameter: 0.31 ± 0.01 mm) or the buccal artery (diameter: 0.27 ± 0.01 mm) (Fig. 2).
Distal segment (pterygopalatine)
The distal part of the maxillary artery is in close relationship with the pterygopalatine fossa. The length of this segment was found to be 10.86 ± 0.4 mm in the medial variant and 10.61 ± 5 mm in the lateral variant. Each maxillary artery branch of this segment leaves the pterygopalatine fossa by passing into a bony canal or foramen. In fact, the branch and its canal or foramen share the same name. The diameter of the main trunk in this segment is 2.75 ± 0.04 mm.
Branches
-
(1)
Posterior superior alveolar artery (diameter: 1.31 ± 0.07 mm): This artery is the counterpart of the inferior alveolar artery. It supplies the upper teeth. This artery usually has a tortuous course in its descending portion. A prominent curve around the zygomatic process is usually seen (Fig. 4).
Fig. 4 Cadaveric dissection showing the three segments of the maxillary artery (right side lateral variant). (a) Inferior view and (b) lateral view. Notice that the ascending rami of the mandible including the condyle and the coronoid apophysis have been removed. The deep temporalis muscle layer has been left in place to show its relationships with the anterior and posterior deep temporal arteries. Sup. temp. a.: Superficial temporal artery; Lat. Pteryg. m.: lateral pterygoid muscle; Auriculotemp. n.: auriculotemporal nerve; Inf. alv. a.& n.: inferior alveolar artery and nerve; Post. sup. a. a.: posterior superior alveolar artery; Greater p. a.: greater palatine artery; Middle n. c.: middle nasal concha; Sphenop. a.: sphenopalatine artery; Infraorb. a.: infraorbital artery; Ant. Temp. a.: anterior deep temporal artery; Post. temp. a.: posterior deep temporal artery; Middle m. a.: middle meningeal artery; Inf. n. c.: inferior Nasal concha. Sph/mand l.: sphenomandibular ligament; Lingual n: lingual nerve; Temp. m.: temporalis muscle; Med. pteryg,. m.: medial pterygoid muscle
-
(2)
Infraorbital artery (diameter: 1.3 ± 0.08 mm): It enters the inferior orbital fissure and penetrates into the infraorbital canal at the anterior third of the orbit (Fig. 4).
-
(3)
Greater palatine artery (diameter: 0.71 mm): Usually arises close to the superior alveolar artery and descends through the greater palatine canal to exit through the greater palatine foramen (Fig. 4).
-
(4)
Sphenopalatine artery (diameter: 1.76 ± 0.48 mm): After entering the nasal cavity posterior to the turbinate, this artery gives off two sets of branches, one to supply the septum and the other one to supply the turbinates (Fig. 4).
-
(5)
Vidian artery (diameter: 0.7 ± 0.005 mm): This artery courses backward along the pterygoid canal. It supplies the upper part of the pharynx and the Eustachian tube (Table 1).
Table 1 Table showing the different segments of the maxillary artery with its branches and corresponding vascular supply
Branching patterns and variants
The average length of the maxillary artery trunk is 42.78 ± 2.7 mm. However, two main variants are encountered according to its lateral or medial course around the LPM (Fig. 5). The average lengths of the medial and lateral variant are 39.45 ± 3.48 and 46.11 mm (±4.25), respectively. Different branching patterns are correlated to these two variants.
Drawing of a left axial inferior view illustrating the medial and lateral course of the maxillary artery in regards to the lateral pterygoid muscle (Lat. Pteryg. m.). Note the medial variant (A) in red and lateral variant (B) in black. Masseter m.: Masseter muscle; Temp m.: temporalis muscle; Lat. Pteryg. m.: lateral pterygoid muscle; Med. Pteryg. m.: medial pterygoid muscle; Aud. t.: auditory tube; V CN: trigeminal nerve; I.C.A.: internal carotid artery; I.J.V.: internal jugular vein; Parotid g.: parotid gland
Medial: In this variant, the middle meningeal and accessory meningeal arteries originate directly from the maxillary artery trunk; the inferior alveolar artery and the deep posterior temporal artery arise from a common trunk (Fig. 6).
Drawing from a left lateral view of the maxillary artery illustrating the most common branching patterns. The zygomatic arch, ascending ramus of the mandible, temporalis muscle, medial and lateral pterygoid muscle and sphenomandibular ligament have been omitted in the drawing for demonstration purposes. When the maxillary artery (3) has a deep or medial course, the accessory meningeal artery (1) and the middle meningeal artery (2) are born separately, whereas the inferior alveolar artery (4) and the deep temporal artery (5) share a common origin. Conversely, when the maxillary artery has a superficial or lateral course the accessory meningeal artery (1) and the middle meningeal artery (2) have a common trunk, and the inferior alveolar artery (4) and deep temporal arteries are born apart
Lateral: In this variant the middle meningeal and accessory meningeal arteries share a single common trunk, whereas both the inferior alveolar artery the deep posterior temporal artery are born separately (Fig. 6).
Correlations of cadaveric findings with CTA
Six cadaveric heads (12 sides) were analyzed. On two of the heads (33%) (4 cases), the MA ran medially to the LPM, and in four heads (66%) (8 cases) it ran laterally. Congruency was found in all the anatomic dissections.
Twenty head CT angiograms (CTA) (40 sides) of actual patients were analyzed as well. The course of the maxillary artery (MA) was found to be medial to the LPM in 21 CTA sides (52.5%) and lateral to the LPM in 19 sides (47.5%). Two variants of MA can be seen clearly as shown in Figs. 7 and 8, respectively. The medial type is clearly in close relationship with the F.O., whereas the lateral type is more distant from it, and closer to the infratemporal crest. After evaluating the side congruency, nine cases (45%) were found to have the medial type and ten (50%) the lateral configuration as a mirror image along the contralateral side. Interestingly, one case (5%) had a lateral configuration on one side and a medial configuration on the other.
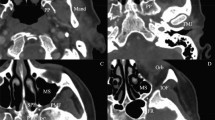
Medial Variant. The 3D CTA reconstruction including the base of the skull (inferior view) showing the pterygoid segment of the maxillary artery (Maxillary a.) and its relation to the bony structures. Note the relationship of the artery with the foramen ovale (FO) and with the most lateral edge of the middle cranial fossa (infratemporal crest). Infratemporal crest: Infratemporal c.
Lateral Variant. The 3D CTA reconstruction including the base of the skull (inferior view) demonstrating the pterygoid segment of the maxillary artery (Maxillary a.) and its relation to the bony structures. Note the relationship of the artery with the foramen ovale (FO) and with the most lateral edge of the middle cranial fossa (infratemporal crest). Infratemporal crest: Infratemporal c.
Discussion
Maxillary artery course: superficial vs. deep
Similar findings to our results were encountered by Uysal et al. [30]. They found that the MA had a superficial course in 57.1% of cases and deep course in 42.9% according to the relation of the artery with the lateral pterygoid muscle. Descriptions of the MA trunk variants including its branching patterns have been described by Lasjaunias et al. [14–16]. Racial differences in terms of the superficial or deep course of the MA have been reported by different authors; a study performed by Pretterklieber et al. reported a 55.4% superficial course in individuals born on the west side of Austria [24]. Hussain et al. reported a superficial course in 68.2% of whites in Canada [10], whereas Sashi et al. found 93% of superficial type maxillary arteries in 100 Japanese angiographies [26]. Similar results as to the higher prevalence of the superficial type in Orientals have been found in other studies [12, 20].
Maxillary artery branches
Several variants have been found, for instance, the inferior alveolar artery is reported to originate directly from the external carotid artery [28]. In all our dissections this artery originated as a direct branch or as a branch of a common trunk from the maxillary artery. Overall, in 58% of the cases the inferior alveolar artery branched off either at the origin of the MA or right before the middle meningeal artery; in 42% of the cases it was found to branch off at the same level as the middle meningeal artery or right after it.
The middle meningeal artery was present in all our specimens and was found to enter the skull through the foramen spinosum consistently; however, this artery entering the skull through the foramen ovale when the foramen spinosum is absent has been reported [24].
As to the accesory middle meningeal artery, our findings reflect those of Lasjaunias [14–16] in which this artery arises from the middle meningeal artery when the MA has a superficial course and originates directly from the MA when it has a deep course. We did not find a duplicated accessory middle meningeal artery as reported by others [24].
Vascular anastomoses between MA and ICA branches
The MA has proximal and distal anastomotic branches with the ICA. The more common are the proximal ones through the middle meningeal artery (MMA) and the accessory meningeal artery (AMA). One of the most frequently found is the meningoophtalmic artery. This artery is the embryologic remnant of the stapedial artery, which takes over the supply from the primitive ophthalmic artery resulting in supply to the distal ophthalmic artery including the ciliary arteries and the central retinal artery. The MMA and AMA may as well anastomose with the inferolateral trunk of the cavernous segment of the ICA. When these anastomoses enlarge, they may result in arterial feeders for cavernous dural arteriovenous fistulas [7].
The AMA also anastomoses with the marginal artery of the tentorium, which can arise from the ophthalmic artery, or from the inferolateral trunk or the meningohypophyseal trunk of the ICA.
Distal branches of the MA anastomose with the ICA through the artery of the foramen rotundum with the inferolateral trunk of the cavernous portion of the ICA through the vidian artery arising from the petrous ICA. Another anastomotic branch known as the artery of the superior orbital fissure, arising from the pterygopalatine segment of the MA, either singly or from a common trunk with the artery of the foramen rotundum may anastomose with the inferolateral trunk and/or to the ophthalmic artery [11].
Anastomotic branches through the AMA to the ascending pharyngeal artery through its superior pharyngeal branch have been described as well.
Clinical applications
Cerebral revascularization: extracranial to intracranial bypass
The MA as the donor artery for a high ECA-ICA bypass was initially described by Vrionis et al [31] in 1996. Abdulrauf et al. [1] introduced the use of a radial artery graft followed by carotid artery ligation for treatment of a giant cavernous and supraclinoid carotid aneurysm through an “anteromedial” approach to the pterygopalatine segment of the MA. One of the main challenges of this approach is the apparent difficulty in isolating a suitable MA within the pterygopalatine fossa through an intracranial small “key hole” craniotomy between V2 and V3 in the temporal fossa. Additionally, the artery has to be pulled into the middle cranial fossa before having a segment isolated for the actual end-to-side anastomosis.
In a recent study, Eller et al. [6] performed a study to access the pterygopalatine fossa to localize the pterygoid segment of the MA by drilling the anterolateral wall of the middle fossa, specifically the greater wing of the sphenoid bone, all the way down to the infratemporal crest. The author claimed that the drilling of the greater wing of the sphenoid bone provides a larger exposure in to the pterygopalatine fossa as opossed to the intracranial anteromedial approach. Additionally, it exposes a longer segment of the vessel for an end-to-side anastomosis and avoids the need to pull the MA into the middle fossa.
Recenty, a more lateral subtemporal craniectomy has been recommended by Nosseck et al. [22]. This technique includes a zygomatic osteotomy (with or without an orbital osteotomy) followed by a frontotemporal craniotomy and subsequent temporal fossa craniectomy reaching its medial border medially to a virtual line connecting the foramen rotundum and the foramen ovale. With this technique the pterygoid segment of the MA is isolated, allowing the possibility of performing an end-to-side and/or end-to-lateral anastomosis; however, they suggest rather using the end to end because it is less demanding. Additionally, they recommend a brachiocephalic vein rather than a radial artery graft.. Among the benefits of this graft, they found that the brachiocephalic vein has few, if any valves, and the absence of potential upper extremity arterial complications that can be associated with the radial artery harvesting.
As to which MA segment is better for bypass purposes, in our anatomic study we found that among the three segments of the MA, the pterygoid segment is the most suitable for several reasons: it is the longest one; all the branches of this segment are muscular, allowing their sacrifice with impunity; no critical anastomoses are present with the internal carotid artery and its course is “free” in the infratemporal fossa.
Preoperative imaging for maxillary artery identification and preoperative planning
Based on our study, the course of the MA runs either medial (33% of cases) or lateral (66% of cases) to the lateral pterygoid muscle, making the foramen ovale alone an unreliable landmark to find the MA. Identification of the MA and its course either medially or laterally to the lateral pterygoid muscle can be identified on axial CTA head/neck axial cuts. However, due to the high congruency found between the anatomic dissections, particularly regarding the pterygoid segment of the MA and the 3D CTAs, we recommend a CTA with 3D reconstructions of the MA at the base of the skull. A better understanding of the course of the main trunk of the MA and its relation between the foramen ovale and the lateral edge of the greater wing of the sphenoidal bone (infratemporal crest) can be achieved with this imaging modality, allowing the surgeon to tailor the exposure of the pterygoid segment of the MA when the maxillary artery is used as a donor for an ECA-ICA bypass (Figs. 7 and 8).
Risk of IMax sacrifice
There are no symptoms of IMax sacrifice resulting from IMax end-to-end anastomosis to M1 according to Nossek et al. [22]. In his series all three patients who underwent this procedure had retrograde filling of the distal IMax territory by external carotid artery collaterals demonstrated on all postoperative angiograms. Of note, surgical sacrifice of the maxillary artery has been routinely used in the past for intractable epistaxis when endovascular techniques were not available and no major comorbidities have been reported. Studies have even compared maxillary artery embolization versus ligation, and although the major complications were not significantly different, the complications associated with embolization were more serious than those associated with IMA ligation [13].
Transforaminal ovale approach
Our prior anatomic study on six cadaveric specimens showed that in 16% (1 out of 6) of cases, the maxillary artery was directly in the needle’s trajectory at the entry of the foramen ovale [4]. A 2% incidence of fistulae between the maxillary artery and dural arteriovenous structures after balloon compression in a series of 100 patients have been reported [17, 18]. The incidence of maxillary fistula is probably higher than what is reported in the literature because of its minor symptoms (pulsatile tinnitus). The fact that the maxillary artery is not injured in more cases is likely due to the free-floating nature of this vascular structure in the pterygopalatine fossa. In our dissections, this free-floating nature helped protect these structures from laceration during needle introduction.
Endoscopic approaches
Flap harvesting for skull base reconstruction
The reconstructive options for skull base defects include vascular and avascular flaps. A dramatic improvement in the treatment of cerebrospinal fluid (CSF) fistulas has been seen after the advent of some of the vascular flaps coupled with the development of endoscopic techniques. Different vascular flaps are part of the armamentarium and are utilized based on the patient’s pathology and the endoscopic endonasal sagittal corridor chosen: transfrontal, transcribiform, transplanum, transsellar, transclival and transodontoid [9, 23].
Current vascular flaps include the pericranial transposed temporoparietal fascia, inferior turbinate, palatal and nasoseptal. Among these, the nasoseptal flap has emerged as the workhorse of the skull base surgeon because of its versatility and excellent results. Results showing 4% postoperative CSF leaks for elective procedures to 0% for anterior skull base reconstructions after trauma have been reported [33]. The first to describe using a nasoseptal flap for the repair of CSF leaks was Hirsch in 1952. Modifications of the flap, use of endoscopic techniques and better understanding of the anatomy allowed Hadad et al. to develop the nasoseptal flap that nowadays has become the standard flap for skull base reconstructions [9].
Anatomic/surgical landmarks to identify the third segment of the MA endonasally
Through a middle meatal transantral approach, the pterygopalatine artery can be easily reached. By using a 30- or 45-degree endoscope, the maxillary ostium is identified and enlarged. The infraorbital nerve is then identified in the roof of the maxillary sinus. At that point, the posterior wall of the maxillary sinus is drilled medially and inferiorly, so the pterygopalatine artery is exposed [2, 5] (Fig. 9).
Endoscopic identification of the sphenopalatine artery (left side). a Through a right nostril approach and using a 30- or a 45-degree endoscope, the middle nasal concha (middle n. c.) is identified. Medially to it lies the uncinate process (uncinate p.) and underneath it the ostium for the maxillary sinus (red dotted area). b Once the maxillary sinus ostium is entered, the posterior wall of the sinus is identified as well as the infraorbital nerve (infraorb. n.). c The sphenopalatine artery (Sphenopalatine a.) can be found inferior and medially to the infraorbital nerve once the posterior wall of the sinus has been removed
Endovascular techniques
Epistaxis
Ninety-five percent of all epistaxis episodes originate in the anterior septum and are easily controlled by anterior packing. Posterior epistaxis accounts for 5% of the cases and is more difficult to treat. Endoscopic ligation or electrocauterization of the sphenopalatine and anterior ethmoidal arteries is a routine technique for control of persistent active bleeding. Embolization is used in the treatment of refractory epistaxis. The lateral X-ray projection is the most useful to evaluate the ECA and IMA as well as their anatomic channels with the ICA or ophthalmic artery. Placing the tip of the microcatheter distal to the origin of the middle meningeal artery, usually in the pterygopalatine segment, is recommended because of the potential anastomosis with the ICA. PVA microparticles of 200–500 μm are usually selected. In addition to the medial and lateral branches of the sphenopalatine artery, it is also recommended to embolize the descending palatine artery. This artery originates distal to the infraorbital artery. Reflux of embolic particles into the infraorbital artery is usually considered safe. Penetration of microparticles into the deep temporal arteries may result in trismus. Success rates of endoscopic ligation versus embolization of the maxillary artery are comparable at 89% and 94%, respectively. The advantages of embolization include the use of local anesthesia mostly in high-risk patients with multiple comorbidities. Bilateral embolization of both maxillaries may be necessary if the side of the hemorrhage cannot be established. Embolization of the facial artery may be needed if epistaxis and the side are known. Complications including skin necrosis have been reported mainly when smaller particles are used (45–150 μm). One of the most feared complications is blindness, which has been attributed to no selective injection, vasospasm, injection at an excessive rate and failure to recognize dangerous anastomosis because of inadequate analysis [13].
Juvenile nasal angiofibroma (JNA)
Juvenile angiofibroma (JA) is a benign vascular neoplasm, which affects young males between 9 and 19 years of age and accounts for 0.05% of all head and neck tumors. In the USA, this lesion represents the most frequent head and neck tumor of adolescence with 1 new case per 5000 to 50,000 patients referred to an otolaryngologist [19, 21].
Preoperative embolization is recommended by most authors [25, 27] as a standard procedure to reduce blood loss during surgical resection. During the last decade, the availability of small particles and microcatheters has made it possible to reach collateral and terminal branches of the external carotid artery to avoid the risk of neurologic deficits. As suggested by Hackman et al. [8], when vessels from both external carotid systems vascularize the lesion, bilateral internal maxillary artery embolization is recommended.
Preoperative embolization of meningiomas
Preoperative embolization is commonly used nowadays for the treatment of meningiomas to ease the tumor bleeding during surgery. Meningiomas located at the skull base are particularly challenging and clearly benefit from this strategy [29, 32]. The blood supply of meningiomas usually arises from branches of the external carotid artery. Origin of the ophthalmic from the meningeal artery, a branch of the MA, has been described. Preoperative embolization of meningiomas supplied by branches of the ophthalmic artery, requires super-selective catheterization and the most distal placement of the microcatheter possible, aiming to protect the central retinal artery from any reflux. In cases in which retinal blush is observed from the MMA on selective angiography of the ECA, a surgical approach is recommended to avoid visual complications.
Conclusions
Two main different anatomic variants of the maxillary artery can be found in anatomic studies, seen clearly on imaging (angiogram and CTA). Each variant presents a separate organization. Understanding these anatomic variations is paramount for the successful outcome of anterior and middle fossa endovascular, skull base and functional approaches.
References
Abdulrauf S, Sweeney J, Mohan Y, Palejwala S (2011) Short segment internal maxillary artery to middle cerebral artery bypass: a novel technique for extracranial-to-intracranial bypass. Neurosurgery 68(3):804–809
Alfieri A, Hae-Dong J, Schettino R, Tschabitscher M (2003) Endoscopic endonasal approach to the pterygopalatine fossa: anatomic study. Neurosurgery 52(2):374–380
Alvernia JE, Pradilla G, Mertens P, Lanzino G, Tamargo RJ (2010) Latex injection of cadaver heads: technical note. Neurosurgery 67(2 Suppl Operative):362–367
Alvernia JE, Sindou MP, Nguyen DD, Maley JH, Mertens P (2010) Percutaneous approach to the foramen ovale: an anatomical study of the extracranial trajectory with the incorrect trajectories to be avoided. Acta Neurochir (Wien) 152(6):1043–1053
Elhadi A, Zaidi H, Yagmurlu K, Ahmed S, Rhoton A, Nakaji P, Preul M, Little A (2016) Infraorbital nerve: a surgically relevant landmark for the pterygopalatine fossa, cavernous sinus, and anterolateral skull base in endoscopic transmaxillary approaches. J Neurosurg 125:1460–1468
Eller JL, Sasaki-Adams D, Sweeney JM, Abdulrauf SI (2012) Localization of the internal maxillary artery for extracranial-to-intracranial bypass through the middle cranial fossa: a cadaveric study. J Neurol Surg B 73:48–53
Geibprasert S, Pongpech S, Armstrong D, Krings T (2009) Dangerous extracranial-intracranial anastomoses and supply to the cranial nerves: vessels the neurointerventionalist needs to know. Am J Neuroradiol 30:1459–1468
Hackman T, Snyderman CH, Carrau R, Vescan A, Kassam A (2009) Juvenile nasopharyngeal angiofibroma: the expanded endonasal approach. Am J Rhinol Allergy 23(1):95–99
Hadad G, Bassagasteguy L, Carrau RL, Mataza JC, Kassam A, Snyderman CH, Mintz A (2006) A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope 116:1882–1886
Hussain A, Binahmed A, Karim A, Sándor GK (2008) Relationship of the maxillary artery and lateral pterygoid muscle in a caucasian sample. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 105:32–36
Kiyosue H, Tanoue S, Hongo N, Sagara Y, Mori H (2015) Artery of the superior orbital fissure: an undescribed branch from the pterygopaltine segment of the maxillary artery to the orbital apex connecting with the anteromedial branch of the inferolateral trunk. Am J Neuroradiol 36:1741–1747
Kim JK, Cho JH, Lee YJ, Kim CH, Bae JH, Lee JG, Yoon JH (2010) Anatomical variability of the maxillary artery: findings from 100 Asian cadaveric dissections. Arch Otolaryngol Head Neck Surg 136:813–818
Krajina A, Chrobok V (2014) Radiological diagnosis and management of epistaxis. Cardiovasc Intervent Radiol 37:26–36
Lasjaunias P, Berenstein A, Ter Brugge KG (2004) Surgical neuroangiography. Springer, New York
Lasjaunias P, Berges O, Doyon D (1979) Collateral circulation of the internal maxillary artery. J Neuroradiol 6:197–205
Lasjaunias P, Théron J (1976) Radiographic anatomy of the accessory meningeal artery. Radiology 121:99–104
Lesley WS (2007) Endosurgical repair of an iatrogenic facial arteriovenous fistula due to percutaneous trigeminal balloon rhizotomy. J Neurosurg Sci 51(4):177–180
Lichtor T, Mullan JF (1990) A 10-year follow-up review of percutaneous microcompression of the trigeminal ganglion. J Neurosurg 72(1):49–54
Lloyd D, Howard D, Lund VJ, Savy L (2000) Imaging for juvenile angiofibroma. J Laryngol Otol 114(9):727–730
Maeda S, Aizawa Y, Kumaki K, Kageyama I (2012) Variations of the maxillary artery in Japanese adults. Anat Sci Int 87:187–194
Nicolai P, Schreiber A, Bolzoni Villaret A (2012) Juvenile angiofriboma: evolution of management. Int J Pediatr 2012:412545
Nossek E, Constantino P, Eisenberg M, Dehdashti AR, Setton A, Chalif DJ, Ortiz R, Langer D (2014) Internal Maxillary artery to middle cerebral artery bypass: infratemporal approach for subcranial-Intracranial (SCI-IC) bypass. Neurosurgery 75(1):87–95
Oliver CL, Hackman TG, Carrau RL, Snyderman CH, Kassam AB, Prevedello DM, Gardner P (2008) Palatal flap modifications allow pedicled reconstruction of the skull base. Laryngoscope 118:2102–2106
Pretterklieber ML, Skopakoff C, Mayr R (1991) The human maxillary artery reinvestigated: I. Topographical relations in the infratemporal fossa. Acta Anat (Basel) 142(4):281–287
Rosen CL, Ammerman JM, Sekhar LN, Bank WO (2002) Outcome analysis of preoperative embolization in cranial base surgery. Acta Neurochir (Wien) 144:1157–1164
Sashi R, Tomura N, Hashimoto M, Kobayashi M, Watarai J (1996) Angiographic anatomy of the first and second segments of the maxillary artery. Radiat Med 14(3):133–138
Scholtz AW, Appenroth E, Kammen-Jolly K, Scholtz LU, Thumfart WF (2001) Juvenile nasopharyngeal angiofibroma: management and therapy. Laryngoscope 111(4):681–687
Standring S, Berkovitz BKB, Hackney CM, Ruskell GL, Collins P, Wigley C (2005) In: Standring S (ed) Head and neck, Gray’s anatomy. The anatomical basis of clinical practice, 39th edn. Elsevier Churchill Livingstone, Barcelona
Trivelatto F, Nakiri GS, Manisor M, Riva R, Al-Khawaldeh M, Kessler I, Mounayer C (2011) Preoperative onyx embolization of meningiomas fed by the ophthalmic artery: a case series. Am J Neuroradiol 32(9):1762–1766
Uysal II, Buyukmumcu M, Dogan NU, Seker M, Ziylan T (2011) Clinical significance of maxillary artery and its branches: a cadaver study and review of the literature. Int J Morphol 29(4):1274–1281
Vrionis F, Cano W, Hellman C (1996) Microsurgical anatomy of the infratemporal fossa as viewed laterally and superiorly. Neurosurgery 39(4):777–786
Waldron JS, Sughrue ME, Hetts SW, Wilson SP, Mills SA, McDermott, Dowd CF, Parsa AT (2011) Embolization of skull base meningiomas and feeding vessels arising from the internal carotid cisrculation. Neurosurgery 68(1):162–169
Wheless SA, Mckinney KA, Carrau RL, Snyderman CH, Kassam AB, Germanwala AV (2011) Nasoseptal flap closure of traumatic cerebrospinal fluid leaks. Skull Base 21(2):93–97
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this research.
Conflict of interest
None
Human and animal rights and informed consent
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Comments
The IMAX is becoming more familiar to us in the modern era of neurosurgery with the use of IMAX-MCA or PCA bypasses and also with the vascular supply of this artery (pterygopalatine segment) to endonasal vascularized flaps. The three segments are mandibular, pterygoid and pterygopalatine. I use the pterygoid segment for bypass purposes, and neuronavigation and Doppler are extremely helpful in identification of this vessel. The best anatomic landmark is an infratemporal fossa approach after zygomatic osteotomy, lateral to the foramen spinosum, and dissecting the lateral pterygoid muscle, whether medially or laterally. Intermittent Doppler use will help in denitrifying the artery. Then, the artery is transected; it is quite mobile in that region and can be moved toward the intracranial segment. This article helps neurosurgeons become more familiar with this initially thought ENT field artery.
Amir Dehdashti
NY, USA
Previous Presentation
Congress of Neurosurgical Surgeons Annual Meeting, New Orleans, LA, September 2015, Digital Poster presentation.
Rights and permissions
About this article
Cite this article
Alvernia, J.E., Hidalgo, J., Sindou, M.P. et al. The maxillary artery and its variants: an anatomical study with neurosurgical applications. Acta Neurochir 159, 655–664 (2017). https://doi.org/10.1007/s00701-017-3092-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-017-3092-5