Abstract
We present an extremely infrequent case of brain metastasis of a parotid tumor. To our knowledge, this is the second case reported of a brain metastasis of a malignant parotid tumor, carcinoma ex pleomorphic adenoma. Pleomorphic adenoma represents 60% of tumors of the parotid gland, and although it is a benign tumor, it can transform into carcinoma ex pleomorphic adenoma in 5% of cases, one of the most aggressive neoplasms of the salivary glands. We want to note the need for an accurate diagnostic. Thanks to aggressive surgical management, our patient survived more than 1½ years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Case report
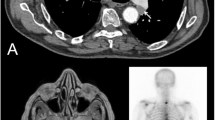
A 66-year-old female who underwent a partial left parotidectomy in 1973 for a pleomorphic adenoma consulted in December 2010 because of a left cervical adenopathy associated with a slow-growing preauricular tumor. A needle biopsy revealed carcinoma, so a complete parotidectomy (including exeresis of the facial nerve because of tumor infiltration) plus radical cervical dissection was performed. The pathologist confirmed infiltrative carcinoma ex pleomorphic adenoma (pT3pN2b), perineural and vascular invasion and ganglia metastasis. The patient was treated with adjuvant radiation therapy (66Gy), which was completed in April 2011.
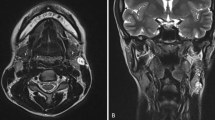
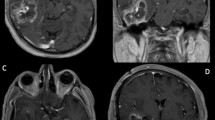
Two years later, pulmonary and mediastinal lymph node progression developed, so the patient was included in a clinical trial (ColoAd1 Evolve), obtaining a partial response of the target lesions. Nevertheless, in May 2013, she started showing neurological symptoms (headache, a decline in cognitive abilities and right hemiparesia), with a Karnofsky Index of 70. The computed tomography (CT) scan showed a subcortical left frontal lobe mass with peripheral enhancement, and the magnetic resonace imaging (MRI) study indicated a high-intensity mass on T2- (Fig. 1) and T1-weighted images (Fig. 2) with vasogenic perilesional edema and peripheral enhancement (Figs. 3 and 4), in close relation to the Sylvian fissure (Fig. 4). Metastasis was the first diagnosis suggestion, without excluding a high-grade glial tumor. Functional MR and fiber tracking showed bilateral language distribution and Broca’s area shifted above and anterior to the tumor.
The patient experienced neurological symptom improvement after corticosteroid therapy. She underwent a frontotemporal craniotomy with language mapping, achieving complete macroscopic and radiological resection (Fig. 5).
Carcinoma ex pleomorphic adenoma of salivary glands was confirmed by the pathologist (Figs. 6, 7, 8 and 9).
Postoperative radiation therapy was administered, and, 8 months after surgery, the patient scored 80 on the Karnofsky Index, with complete recovery of the hemiparesia and great improvement in her cognitive status.
HER-2, estrogen and androgen receptors were evaluated on the primary tumor, which were all negative, so no target therapy was performed. The patient underwent chemotherapy with CBDCA and 5-fluorouracil, with quick progression after two cycles. The patient finally died 1 year and 6 months after SNC surgery because of systemic progression.
Discussion
Salivary gland tumors account for 1% to 4% of all human neoplasms and 8% of all head and neck tumors [2, 7, 13], 70–80% of which are located in the parotid gland [2].
Pleomorphic adenoma represents 50% of all salivary gland tumors and 51–60% of tumors in the parotid gland [2, 13].
Although pleomorphic adenoma is a benign tumor, there are three uncommon types of malignant transformation: carcinoma ex pleomorphic adenoma, carcinosarcoma and metastasizing pleomorphic adenoma [7, 8]. Malignant parotid gland tumors usually display a painless palpable mass, and facial palsy is a malignancy sign [2].
Carcinoma ex pleomorphic adenoma is a rare malignant transformation, accounting for only 5% of all parotid tumors [2]. It is a high-grade carcinoma, and the criteria for inclusion are a major lesion in the parotid primary gland (parotid or submandibular); macroscopic elements of malignant transformation in pleomorphic adenoma, such as poorly defined and/or infiltrative tumor margins, hemorrhage, necrosis and presence of mixed (benign and malignant) elements. We can consider a biopsy that proves the presence of a previous pleomorphic adenoma in the same location as the subsequent carcinoma as a benign element. Malignant elements show multiple patterns of differentiation including undifferentiated or adenocarcinoma patterns.
Malignant transformation is associated with the age of the tumor (just 2% of tumors are present <5 years and 10% >10 years [1, 4, 6]), tumor recurrence, radiation therapy, advanced patient age (typically occurring in the 6th–8th decades of life [8]) and tumor size [1]. It is one of the most aggressive neoplasms of the salivary glands, and the 5-year survival rate is about 50% [3, 9].
Carcinoma ex pleomorphic adenoma has no specific radiological findings on MRI, so the radiological diagnosis is difficult. Hemorrhage, necrosis, irregular margins or infiltration beyond the fibrous capsule are several of the pathological findings coexisting in carcinoma ex pleomorphic adenoma. However, these features are not always present, and the diagnosis can be mistaken for pleomorphic adenoma [1]. Some studies recommend the addition of diffusion-weighted images, which allow the differentiation of benign and malignant features of salivary gland tumors.
According to Olsen et al. [6], various features are considered prognostic factors, such as pathologic stage, tumor size, grade, proportion of cancer (significant if more than 50% is malignant) and extent of invasion.
It has been shown that the survival rate is longer when the invasion is less than 15 mm, and there is no recurrence or metastasis if the invasion is less than 5 mm. Spiro et al. found a decrease in the survival rate with tumors larger than 60 mm [10].
Histologic subtype, described by Tortoledo et al. [10], is nowadays a controversial prognostic factor depending on the series [4]. On the other hand, LiVolsi and Perzin [4] and Tortoledo et al. [12] considered the extent of tumor infiltration beyond the capsule as the most reliable prognostic marker [4]. Furthermore, after any cancer progression or recurrence, the average survival rate is less than 1 year [6].
Enucleation of the adenoma has the highest recurrence rate, versus superficial or total parotidectomy [8], like in our case. According to Lin et al. [2], parotidectomy alone is an adequate treatment for tumors in the early stage and f low grade, parotidectomy plus postoperative adjuvant radiation therapy is recommended for tumors in the early stage but of high grade, and, in case of advanced stage tumors, parotidectomy plus neck dissection and postoperative adjuvant radiation therapy should be performed.
Some recent papers have shown the presence of HER2, androgen or estrogen receptor in salivary gland cancers, suggesting that endocrine function could play a role in these tumors [5, 11]. HER2 is expressed in less than 30% of these tumors, and its role in medical management has yet to be defined [5]. In carcinoma ex pleomorphic adenoma, it has been published that between 50% [11] and 100% [5] of cases have positive nuclear staining for androgen receptors, which could be significant for the prognosis and target therapy.
To our knowledge, there has been just one more case of hematogenous brain metastasis of carcinoma ex pleomorphic adenoma reported [8]. The first case report shows a young patient initially diagnosed with carcinoma ex pleomorphic adenoma with nodal and extranodal extension, treated with total parotidectomy, radical neck dissection and postoperative radiation therapy, who after 19 months suffered multiple brain and spinal cord metastasis. In this case, a biopsy confirmed the diagnosis, and the patient died 2 months later [8]. In our case, the presence of a single brain frontal metastasis and the complete resection with brain mapping surgery allowed our patient to improve neurological symptoms and her quality of life.
Our case demonstrates the importance of an aggressive treatment from the beginning even though pleomorphic adenoma is considered a benign lesion, since the possibility of becoming malignant with poor outcome always exists.
Conclusion
We report the second case of hematogenous brain metastasis of carcinoma ex pleomorphic adenoma, with an extremely rare clinical presentation of a malignant parotid tumor, in which aggressive management offered a chance of improving the prognosis with better functional and neurological status.
Patient consent
The patient guardian has consented to the submission of the case report for submission to the journal.
References
Kato H, Kanematsu M, Mizuta K, Ito Y, Hirose Y (2008) Carcinoma ex pleomorphic adenoma of the parotid gland: radiologic-pathologic correlation with MR imaging including diffusion-weighted imaging. Am J Neuroradiol 29:865–867
Lin CC, Tsai MH, Huang CC, Hua CH, Tseng HC, Huang ST (2008) Parotid tumors: a 10 year experience. American Journal of Otolaryngology-Head and Neck. Med Surg 29:94–100
Lingen M (2010) Head and neck: neoplasms of the salivary glands. In Robbins and Cotran (eds) Pathologic basis of diseases. Philadelphia, pp. 1478–79
Livolsi VA, Perzin KH (1977) Malignant mixed tumors arising in salivary glands. I. Carcinomas arising in bening mixed tumors: a clinicopathologic study. Cancer 39:2209–2230
Nasser SM, Faquin W, Dayal Y (2003) Expression of androgen, estrogen and progesterone in receptors in salivary gland tumors. Am J Clin Pathol 119:801–806
Olsen KD, Lewis JE (2001) Carcinoma ex pleomorphic adenoma: a clinicopathologic review. Head Neck 23(9):705–712
Santaliz-Ruiz L, Morales G, Santini H, Sánchez-Santiago M, Arroyo A (2012) Metastasizing pleomorphic adenoma: a fascinating enigma. Case Reports in Medicine. Volume 2012, article ID 148103, 5 pages
Sheedy SP, Welker KM, DeLone DR, Gilbertson JR (2006) CNS metastases of carcinoma ex pleomorphic adenoma of the parotid gland. Am J Neuradiol 27:1483–1485
Som PM, Brandwein MS (2003) Salivary glands: anatomy and pathology. In: Som PM, Curtin HD (eds) Head and Neck Imaging. Mosby, St Louis, pp 2067–2076
Spiro RH, Huvos AG, Strong EW (1977) Malignant mixed tumor of salivary origin: a clinicopathologic study of 146 cases. Cancer 39:388–396
Tarakji B, Kujan O (2011) An immunohistochemical study of androgen receptor in carcinoma arising in pleomorphic salivary adenoma. Medicina Oral, patología oral y cirugía buccal 16(3):e330–e334
Tortoledo ME, Luna MA, Batsakis JG (1984) Carcinomas ex pleomorphic adenoma and malignant mixed tumors. Histomorphologic indexes. Arch Otolaryngol 110:172–176
Venteicher AS, Walcott BP, Sheth SA, Snuderl M, Patel Anoop P, Curry WT, Nahed B (2013) Clinical features of brain metastasis from salivary gland tumors. J Clin Neurosci 20(11):1533–1537
Author information
Authors and Affiliations
Corresponding author
Additional information
Extremely rare case of brain metastasis of a parotid tumor.
Rights and permissions
About this article
Cite this article
Lau, R., Fernández-Coello, A., Vidal-Sarró, N. et al. Brain metastasis of carcinoma ex pleomorphic adenoma of the parotid gland: case report and review of the literature. Acta Neurochir 159, 459–463 (2017). https://doi.org/10.1007/s00701-017-3080-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-017-3080-9