Abstract
Background
Chordoma is a rare bony malignancy known to have a high rate of local recurrence after surgery. The best treatment paradigm is still being evaluated. We report our experience and review the literature. We emphasize on the difference between endoscopic and open craniotomy in regard to the anatomical compartment harboring the tumor, the limitations of the approaches and the rate of surgical resection.
Method
We retrospectively collected all patients with skull-base chordomas operated on between 2004 and 2014. Detailed radiological description of the compartments being occupied by the tumor and the degree of surgical resection is discussed.
Results
Eighteen patients were operated on in our facility for skull-base chordoma. Seventeen endoscopic surgeries were done in 15 patients, and 7 craniotomies were done in 5 patients. The mean age was 48.9 years (±19.8 years). When reviewing the anatomical compartments, we found that the most common were the upper clivus (95.6%) and lower clivus (58.3%), left cavernous sinus (66.7%) and petrous apex (∼60%). Most of the patients had intradural tumor involvement (70.8%). In all craniotomy cases, there was residual tumor in multiple compartments. In the endoscopic cases, the most difficult compartments for total resection were the lower clivus, and lateral extensions to the petrous apex or cavernous sinus.
Conclusions
Our experience shows that the endoscopic approach is a good option for midline tumors without significant lateral extension. In cases with very lateral or lower extensions, additional approaches should be added trying to achieve complete resection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chordomas are rare malignant bony tumors that arise from the notochord remnant along the cerebrospinal axis [37]. The overall incidence of chordomas is 0.08 per 100,000 [16, 29, 37]. They are aggressive, slow-growing, invasive, and locally destructive lesions [29]. Chordomas occur at extreme ends of the vertebral axis, with 35–40% of them located at the clivus or upper cervical spine and ∼60% in the sacrococcygeal junction of the spine [23, 29]. Skull-base chordomas represent 0.1–0.2% of primary intracranial tumors [13, 14, 30, 34, 35]. Since chordomas have a high rate of metastases, a very high rate of local recurrence and a high rate of seeding along the surgical tracts when operated on [3], many surgeons do not perform a biopsy before performing the definitive surgery for tumor resection when the radiologic diagnosis of skull-base chordoma is of high certainty.
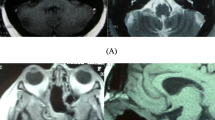
The treatment paradigm for chordomas is complete surgical resection and adjuvant radiotherapy [1, 6, 7]. Although chordomas are generally slow growing and histologically considered as being low-grade tumors, their high recurrence rate, even after postoperative radiation, renders them difficult to treat. Moreover, the recurrence rate is very high when total removal is not possible [6, 11, 23]. For spinal and sacral chordomas, the aim of surgery is en-bloc resection if possible [15, 17, 31]. However, in skull-base cases, this goal is more challenging due to the neighboring structures, which include the brainstem, the major arteries and several cranial nerves (Fig. 1) [7, 34]. The occurrence of clival chordoma is higher than that of upper spinal chordoma, with the former sometimes involving the C1-2 level (Fig. 1c) [23].
Chordomas of the skull base can invade different anatomical compartments. a MRI sagittal T1 with gadolinium showing big chordoma harboring mostly upper and lower clivus. b MRI axial T2 showing tumor invading occipital condyles and compressing the brain stem. c MRI sagittal T2; the tumor is invading lower compartments of cranio-cervical junction including C2. d MRI coronal T2; very large tumor invading extra cranial compartments
The surgical approach for resecting skull-base chordoma is still evolving. Until the recent development of extended endoscopic endonasal surgery, craniotomy in different variations was the preferred approach [9, 19, 27]. The reported rate of total removal via craniotomy ranged between ∼50 and 70%, thus exposing the patients to a high recurrence rate and to the need for adjuvant therapy, mainly radiotherapy [6, 8, 16, 19, 32, 33, 37]. In recent years, several works described the use of a transnasal endoscopic approach for resecting skull-base chordomas, particularly clival chordomas [6, 9, 23, 27]. However, given the relative novelty of the endoscopic approach, there are limited cases and clinical outcome data in the literature.
The aim of this study was to present our experience in treating a series of patients with skull-base chordomas, and to emphasize the selection criteria and limitations of the various surgical approaches. We retrospectively reviewed 18 patients who were operated on for those lesions and describe the change over time in our approach from craniotomy to an endoscopic endonasal approach, which then became the mainstay for resection of these tumors.
Methods
All patients operated on for skull-base chordomas between 2004 and 2014 were eligible for study entry. The study was approved by the local ethical committee (TLV 0585-15). Pathology was determined by a neuropathologist according to the World Health Organization criteria [20]. Records of the clinical, operative, and hospital course records were retrieved from the hospital database, and data on demographics, presenting symptoms, medical comorbidities, neuroimaging findings, and postoperative course were extracted. Information on neurological presentation, anatomical compartments occupied by the tumor, surgical resection and outcome were obtained for each operation. A few of the patients needed more than one surgical procedure via different approaches (endoscopy or craniotomy), and the data were extracted for each operation as a separate entity.
Tumor location was determined according to specific anatomical compartments that were defined as follows: upper or lower clivus (divided by the level of mid-pons), left or right cavernous sinus, left or right petrous apex, left or right occipital condyle involvement, C2 involvement, involvement of extracranial parapharyngeal spaces, intracranial lateral extension (to the middle fossa) and intradural involvement (Fig. 2). Those anatomical compartments were evaluated before and after each procedure in order to demonstrate the region of accessibility and categorized according to the extent of resection. We categorized tumor extent of resection (EOR) by volumetric analysis (described later) as: GTR (no visible residual on follow up MRI), STR (EOR ≥ 90%), PR (90% > EOR) and biopsy only (EOR < 10%). The definition of radical resection (GTR) and STR is not well established when looking at past publications. Moreover, defining skull-base chordoma’s extent of resection by direct volumetric analysis, as was done in this work, is a fairly new tool. The results of the volumetric analysis tend to be much more strict in terms of grade of resection. Hence, we have decided to use Colli and Al-Mefty’s categories of extent of resection from 2001 (radical resection—absence of residual tumor or presence of a small questionable area; STR is defined as >90% resection; PR is defined as <90% resection) [7]. For the volumetric analysis, we include every enhancement seen on the postoperative MRI as possible residual tumor. This measuring technique results in lower grade of resection rates, but we find it more reliable in terms of tumor recurrence. We specifically examined the involvement of the brainstem and the optic nerves, and evaluated whether the surgery had been successful in removing the tumor from these compartments in an attempt to create an optimal field for postoperative radiation. Postoperative complications were recorded if they occurred within 30 days after surgery, and mortality was recorded for the whole study period.
Volumetric analysis
The preoperative and postoperative MRIs were obtained and reviewed for each patient. A clinician expert in volumetric assessment who was unaware of patients’ outcome made volumetric measurements on MRI. The preoperative volume was measured using T1-weighted gadolinium-enhanced and T2-weighted MRIs (1.5– to 3-mm axial cuts) fused together using Brainlab software (Brainlab, Heimstetten, Germany), the area of contrast enhancement and relevant T2 changes were calculated for each axial section. The same was done for post operation MRI.
Statistical analysis
Since our group has a small number of included patients, we used Fisher’s exact test for statistical analysis. We also used t-test statistical analysis in order to learn about basic differences between the endoscopic group and open-surgery group.
Results
Between 2004 and 2014, 18 patients were operated on for skull-base chordomas in our department. They all had complete data on preoperative and postoperative magnetic resonance imaging (MRI) and computerized tomographic (CT) scans, preoperative and postoperative clinical assessments, and outpatient follow-up findings. Fifteen patients underwent 17 endoscopic surgeries (the ES group), and five patients underwent seven craniotomies (the OC group). One patient underwent transnasal endoscopic surgery after having undergone several craniotomies, and one patient had a second-stage craniotomy after transnasal surgery.
The mean age at admission was 48.9 years for the whole group, 53 years for the ES group and 39 years for the OC group. The male:female ratio was 14:10. Ten patients in the ES group (58.8%) were males and four cases in the OC group (57.1%) were males. The mean follow-up time was 27.5 months for all the cases, with 19.9 months for the ES group and 46.1 months for the OC group. Table 1 summarizes the demographic parameters of all the patients.
Clinical presentation
Headache was the most common presenting symptom (66.7%: OC, 57.1%; ES, 70.6%). Other common signs at presentation were abducens palsy (62.5%: OC, 42.9%; ES, 70.6%), while complaints about actual diplopia upon admission were less common (54.1%: OC, 42.9%; ES, 58.8%). Lower cranial nerve deficits were more common in the EC group at presentation (66.7%: OC, 42.9%; ES, 76.5%). Additional signs and symptoms included orbit-related pain (25%: OC, 14.3%; ES, 29.4%), new visual deficit (12.5%: OC, none; ES, 17.6%), and nasal congestion or discharge (16.7%: OC, 14.3%; ES, 17.6%). Signs of elevated intracranial pressure (ICP), such as nausea and vomiting, were less common (16.7%: OC, 14.3%; ES, 17.6%). Interestingly, almost 17% of the patients in our group presented to their primary doctor with complaints about problems with sleeping and breathing (OC, 14.3%; ES, 17.6%). Table 2 describes the clinical presentation for the two groups.
Complications
Two patients (one in each group) needed cranio-cervical fixation after surgery for tumor removal. We used a lumbar drain in nine patients in the ES group (53%) and in one patient in the OC group (14%). None of the patients in our series had cerebrospinal fluid (CSF) leak following surgery. Four patients in the ES group (23.5%) had new diplopia after surgery, as did one patient in the OC group (14.3%). Other than new-onset diplopia, there were no other new neurologic deficits in either group. The surgical complications in both groups are summarized in Table 3.
The mean hospitalization period was 13 days for all cases, 12 days for the ES patients, and 15 days for the OC patients (p = 0.49). Two patients in the ES group and one patient in the OC group required immediate re-admission (within 1 month after surgery). Seven patients (29%) needed further surgery after their first operation in an attempt to improve the resection or due to progression of residual tumor. The mean time after the first surgery was 16 months. Four patients in the ES group needed further resection (23.5%), as did three patients in the OC group (42.9%). For one patient, a two-stage procedure was planned in advance: we started with an endoscopic resection and performed an OC for resection of a lateral extension of her tumor to the middle fossa a few months later.
MRI analysis
The volumetric analyses of the tumors before surgery revealed that the mean tumor volume for all 24 cases was 27.2 cm3, 25.3 cm3 for the ES group and 31.9 cm3 for the OC group (p = 0.38). Examinations of the anatomical compartments harboring the tumor before surgery showed that the most common were the upper clivus (96%: OC, 86%; ES, 100%), lower clivus (58.3%: OC, 43%; ES, 65%), left petrous apex (58%: OC, 57%; ES, 59% and 67%: ), right petrous apex (OC, 43%; ES, 76.5%), and cavernous sinus. Less common sites were the occipital condyles, C2 involvement, and lateral extension to the middle fossa (Table 4).
Intradural involvement was common in the re-do cases as well as in the virgin cases. There was intradural involvement in 17 out of the 24 operations (71%; 64.7% of the ES cases and 85.7% of the OC cases).
For postoperative MRI analysis, we used the first postoperative MRI performed 1–3 months after surgery and checked for residual disease in the same anatomical compartments. Table 5 summarizes the results of the MRI analyses of the different anatomical compartments harboring the tumor after surgery and the extent of resection (EOR) calculated by volumetric analysis. The volumetric analyses of the tumors after surgery revealed that the mean residual tumor volume for all 24 cases was 13.1 cm3, 10.6 cm3 for the ES group and 19 cm3 for the OC group (p = 0.19). The mean EOR was 55.12% for all the cases, 61.2% for ES group and 40.3% for OC group (p = 0.15). When categorized according to tumor resection and residual tumor by the conclusion of the operating team’s re-sults, eight cases (33.3%) had gross total removal (GTR). There was no significant group difference in GTR: 35.3%% of the ES cases and 28.6% of the OC cases (p = 1). We achieved subtotal resection in six cases (25%) all of them in the ES (35.3%) and none for the OC group (p = 0.13). We had nine cases (37.5%) with partial resection, four (23.5%) in the ES group and five (71.4%) in the OC group (p = 0.06). As noted earlier, we did not perform intended tumor biopsies in this series, but had one case (4.2%) from the ES group that was categorized as tumor biopsy by volumetric analysis. We then categorized extent of resection by direct volumetric analysis done using Brainlab software. By volumetric analysis, none of the patients had complete GTR. Three patients (12.5%) had sub-total resection (≥90% resection); all of those patients are from the endoscopic surgery group (17.6% vs 0%, p = 0.5). In 20 cases, partial resection (90% > EOR > 10% tumor volume resection) was achieved, with 13 cases in the ES group and 7 for the OS group (76.5% vs 100%, p = 0.3). As for the operating team assessment, we had only one case of tumor biopsy (10% ≥ EOR tumor volume resection) and it was from the ES group. Table 6 summarizes the rates of resection in both groups by the volumetric assessment.
We compared the various anatomical compartments for complete surgical resection as seen on the postoperative scans by calculating the ratio of the number of cases that were clear of tumor to the original number of cases with tumor for each compartment. The results showed that more radical surgical resection was achieved more successfully in the ES group for the following compartments: upper clivus (ES, 70.6%; OC, 28.6%), lower clivus (ES, 45.5%; OC, no reduction), left petrous apex (ES, 40%; OC, 50%), right petrous apex (ES, 61.5%; OC, 50%), right occipital condyle (ES, 100%; OC, no reduction), and C1-2 involvement (ES, 75%; OC, 33.3%). Table 7 summarizes the complete resection rates for different compartments achieved by each of the surgical approaches.
Compression of the brain stem or optic apparatus by residual tumor occurred in nine cases (37.5%) in which some tumor was left adjacent to the brainstem. There was a trend towards better resection in the ES group, in which 29.4% of the cases had residual tumor versus 57.1% of the OC cases (p = 0.36). We achieved a total reduction in 62.5% of the cases in which the tumor was adjacent to the optic nerves before surgery. We had better results in the OC group in relieving the optic apparatus from tumor burden but without statistical significant difference (75% had reduction in tumor involvement versus 58.3% for the ES group, p = 1).
Discussion
We report our results for treating skull-base chordomas with two surgical approaches, endoscopic resection and open craniotomy. Complete surgical resection of these tumors is difficult because of the involvement of surrounding neurovascular structures [19, 23, 28]. In addition, the need for large reconstruction of the skull base after resection could be a limitation for maximal tumor removal. It is therefore crucial that the neurosurgeon and the otolaryngologist involved in treating skull-base chordomas are experienced in both the resection of the tumor as well as in the reconstruction of the skull base.
Our approach has changed during the last few years, from being strictly craniotomy to understanding that the surgical goal in skull-base chordomas can sometimes be achieved only through multiple surgeries and, preferably, using different surgical approaches for the different anatomical compartments harboring the tumor.
Fernandez–Miranda et al. [16] recently published an algorithm for a proposed approach to treat skull-base chordomas. They stated that the endoscopic endonasal approach allows a more direct anatomical route for clival lesions located predominantly in the midline, and that it offers a safer way to resect the tumor by avoiding injuries to neurovascular structures. Cavallo et al. [4, 5] illustrated the extensive view of the ventral brainstem that is obtainable through an endoscopic endonasal transclival approach by allowing a better resection anterior to the brainstem, which is a prerequisite before proton beam treatment. We also found that the tumors involving the midline from the sella above to C1 below are best approached through the sphenoid sinus using an endoscope, thereby allowing a safer way to remove the tumor from the anterior brainstem. Of the anatomical compartments at the midline, which were more accessible by the endoscopic approach, better results were achieved in the upper clivus. In a recent review, Jahangiri et al. [23] noted that the lower third of the clivus is a problematic location for surgical resection regardless of the surgical approach. Koutourousiou et al. [27] reported that the lower third of the clivus was one of the weak points for surgical intervention. In our current series, we also found that the lower clivus is a difficult area for achieving radical or even complete resection. In our experience, however, an extensive drilling of the upper clivus or floor of the sphenoid sinus is needed in order to achieve a better resection from the lower clivus compartment. In our later cases when drilling was more extensive than in our earlier ones, we achieved a better surgical resection in the lower clivus area. In general, we consider a large bilateral opening of the sphenoid sinus to be a prerequisite for better tumor resection from all compartments.
The endoscopic approach is not suitable for achieving complete surgical resection when the tumor extends laterally into the middle fossa (Fig. 3) or cavernous sinus. As for tumor invading the occipital condyle (Fig. 4), we found that as the technical skills develop, the ability to achieve GTR improved tremendously. Fernandez–Miranda et al. [16] showed that as the pathology starts spreading laterally, the endoscopic trans-clival approach can be augmented in order to achieve better control around the different segments of the internal carotid artery. They also stated that an open approach or a combination of endoscopic and open approaches in stages should be considered when the anatomical compartments harboring the tumor are too lateral or too inferior to be effectively resected with an endoscopic approach alone. The use of different approaches is crucial not only for achieving complete resection but also for avoiding postoperative cranial nerves deficits [19, 26]. Sen et al. [34, 36] recently reported the outcomes of 71 chordomas treated with different surgical approaches and demonstrated that anterior approaches led to significantly better postoperative cranial nerve function than lateral approaches. Those authors concluded that the surgeon often works through spaces between cranial nerves in lateral approaches, thus increasing the likelihood of functional impairment.
Several studies dealt with the extent of tumor resection using different surgical approaches for skull-base chordomas and chondrosarcomas. Since the methods for assessing tumor resection have been done differently in the various works dealing with extent of resection and tumor residual, it is hard to compare the results in those works. Moreover, direct volumetric analysis was not frequently utilized as an assessment tool for decision-making in follow-up of patients with skull-base chordomas. In our series, none of the cases had GTR by volumetric analysis, although eight of the cases (33.3%) had GTR by the operating team, which followed the patients. Six of those patients are still under strict follow-up without tumor progression with a mean follow-up time of 44 months. Three of the cases (12.5%), in our work, had STR with resection of more than 90% of tumor volume by the volumetric analysis. Again, better results in achieving STR were in the ES group, since none of the patients in the OC were with resection of more than 90% of the initial tumor volume. Yet, all these differences are with small numbers and did not reached statistical significance. Previous studies have suggested that the endoscopic endonasal approach for the resection of chordomas is as successful as open approaches in terms of obtaining a radical resection [19]. GTR was achieved in 54.5% of published cases, while one of the major differences between those works is the tumor volume at diagnosis [9, 18, 21, 24]. In the one endoscopic publication citing average tumor volumes, Stippler et al. [38] reported a mean tumor volume of 29.1 cm3, while the mean volume was slightly higher (34.9 cm3) in Frazer et al.’s [19] series (and 58 cm3 in their transcranial group). The mean tumor volume before surgery was 25.3 cm3 in our ES group and 31.9 cm3 in the OC group (p = 0.38): this is less than the average volume of transcranial series, which is on the order of 58 cm3.
The goal of surgical resection of skull-base chordomas is GTR or at least maximal resection around important structures before implementing radiation therapy. Several earlier publications advocated the use of staged or simultaneous procedures in specific cases [10, 15, 19, 22, 23, 28, 39]. They recommended that surgical approaches should be planned according to the location, size, and distribution of the tumor, with some cases more suitable for total resection with a midline approach, some more suitable for a lateral approach, and some requiring a staged procedure with several surgeries needed to achieve complete resection [23, 39]. In their work from 2015, Jahangiri et al. [23] used the staged approach in 14% of their skull-base chordoma cases and described that the staged procedures enabled GTR in 29% of them that would have otherwise undergone subtotal resection. In our work, six (25%) patients underwent two-stage procedures, and we repeated the endoscopic resection with good results in four of them. In three of those cases, the first surgery was an OC and the second one was performed endoscopically, while we began with ES and switched to OC for the second stage a few months later in the fourth patient.
Radiation
In search of a better way to treat these patients, some investigators have recommended adopting an operative strategy that is not excessively aggressive toward reaching GTR, since adjunctive radiotherapy can be used to control a small residual tumor after the initial resection. Adjuvant radiation therapy has been shown in different series to improve local tumor control with a relatively low rate of complications [12, 16, 25].
There are conflicting reports regarding the best radiation treatment options, although proton beam therapy has been used in most of the recent series in order to achieve a very high radiation dose to the tumor. In their 2010 review on endoscopic resection of clival chordoma, Frazer et al. [19] concluded that although extent of resection is prognostically important, adjuvant radiotherapy using proton beams are vital in stabilizing residual disease and preventing further recurrence [5, 7, 21]. As is the practice of others, we also referred our patients to receive proton beam therapy after completing the different surgical steps for tumor resection, in accordance with our policy to collaborate with the radiation oncologists in these cases. We make a special effort to achieve a better brainstem and optic apparatus decompression, even in cases where complete tumor resection was not achieved from other compartments (Fig. 5).
Complications
We concur with others that the utilization of the endoscopic approach has reduced many of the complications associated with the treatment of skull-base chordomas. Significant rates of complications were reported in the major series that described the open approaches for clival chordomas. Sen and Triana [34] reported achieving GTR in 18 (85.7%) of 21 patients, with 24 new postoperative complications (CSF leaks, minor strokes). Sekhar et al. [33] described a series of 64 patients with chordomas or chondrosarcomas of the skull base. Those authors achieved GTR in 25 of the 42 patients with chordomas (59%). However, they also had a relatively high complication rate, including 21% who developed new abducens palsies and 26% who had CSF leaks. In 2008, Al-Mefty et al. [2] reported a series of 43 patients with clival pathology resected via a different technique of an open midline anterior clivectomy approach. GTR was achieved in 29 of the 38 chordomas cases (76.3%). In Al-Mefty et al. work almost 8% of the patients with chordoma developed postoperative new abducens palsies (most of them were not permanent) and 16% of the patients developed CSF leaks. In comparison, the rates of new abducens palsies and CSF leak in the endoscopic literature ranged from 0 to 33.3% [19]. Overall, there was no difference in postoperative complications between our OC and ES groups, with no patients experiencing postoperative CSF leak and five patients (21%) with new abducens palsy (23.5% in the ES group and 14.3% in the OC group, p = 1, most of which were temporary and resolved by 3–6 months after surgery). The mean length of hospital stay was essentially similar for both groups, with a slight trend for a shorter stay in the ES group.
Team approach
In our opinion, the endoscopic approach requires collaboration between the neurosurgeon and the otolaryngologist specialist in skull-base surgery. At our institution, a joint team of the two specialties does all surgeries [19].
Conclusions
The endoscopic endonasal approach for resection of skull-base chordomas provides a good view and pathway for midline lesions. This approach is limited by extensive lateral involvement of the skull-base compartments by the tumor and significant vascular involvement. Better preparation, including a wide and deep opening of the sphenoid sinus, enables good surgical resection in the difficult lower areas in the lower clivus and below. Multiple different surgical approaches are indicated in selected cases when a complete as possible resection of the tumor is attempted. The surgical plan should enable postoperative radiation treatment after optimal brainstem and optic apparatus decompression. A team approach will ensure better tumor resection and skull-base reconstruction and minimize surgical complications.
References
Al-Mefty O, Borba LA (1997) Skull base chordomas: a management challenge. J Neurosurg 86:182–189
Al-Mefty O, Kadri PA, Hasan DM, Isolan GR, Pravdenkova S (2008) Anterior clivectomy: surgical technique and clinical applications. J Neurosurg 109:783–793
Arnautovic KI, Al-Mefty O (2001) Surgical seeding of chordomas. J Neurosurg 95:798–803
Cavallo LM, Messina A, Gardner P, Esposito F, Kassam AB, Cappabianca P, de Divitiis E, Tschabitscher M (2005) Extended endoscopic endonasal approach to the pterygopalatine fossa: anatomical study and clinical considerations. Neurosurg Focus 19:E5
Cavallo LM, Cappabianca P, Messina A, Esposito F, Stella L, de Divitiis E, Tschabitscher M (2007) The extended endoscopic endonasal approach to the clivus and cranio-vertebral junction: anatomical study. Childs Nerv Syst 23:665–671
Chibbaro S, Cornelius JF, Froelich S, Tigan L, Kehrli P, Debry C, Romano A, Herman P, George B, Bresson D (2014) Endoscopic endonasal approach in the management of skull base chordomas—clinical experience on a large series, technique, outcome, and pitfalls. Neurosurg Rev 37:217–224, discussion 224–215
Colli BO, Al-Mefty O (2001) Chordomas of the skull base: follow-up review and prognostic factors. Neurosurg Focus 10:E1
Crumley RL, Gutin PH (1989) Surgical access for clivus chordoma. The University of California, San Francisco, experience. Arch Otolaryngol Head Neck Surg 115:295–300
Dehdashti AR, Karabatsou K, Ganna A, Witterick I, Gentili F (2008) Expanded endoscopic endonasal approach for treatment of clival chordomas: early results in 12 patients. Neurosurgery 63:299–307, discussion 307–299
DeMonte F, Diaz E Jr, Callender D, Suk I (2001) Transmandibular, circumglossal, retropharyngeal approach for chordomas of the clivus and upper cervical spine. Technical note. Neurosurg Focus 10:E10
Di Maio S, Kong E, Yip S, Rostomily R (2013) Converging paths to progress for skull base chordoma: review of current therapy and future molecular targets. Surg Neurol Int 4:72
Di Maio S, Yip S, Al Zhrani GA, Alotaibi FE, Al Turki A, Kong E, Rostomily RC (2015) Novel targeted therapies in chordoma: an update. Ther Clin Risk Manag 11:873–883
Diaz RJ, Cusimano MD (2011) The biological basis for modern treatment of chordoma. J Neurooncol 104:411–422
Diaz RJ, Maggacis N, Zhang S, Cusimano MD (2014) Determinants of quality of life in patients with skull base chordoma. J Neurosurg 120:528–537
Dubory A, Missenard G, Lambert B, Court C (2014) “En bloc” resection of sacral chordomas by combined anterior and posterior surgical approach: a monocentric retrospective review about 29 cases. Eur Spine J 23:1940–1948
Fernandez-Miranda JC, Gardner PA, Snyderman CH, Devaney KO, Mendenhall WM, Suarez C, Rinaldo A, Ferlito A (2014) Clival chordomas: a pathological, surgical, and radiotherapeutic review. Head Neck 36:892–906
Fourney DR, Gokaslan ZL (2003) Current management of sacral chordoma. Neurosurg Focus 15:E9
Frank G, Sciarretta V, Calbucci F, Farneti G, Mazzatenta D, Pasquini E (2006) The endoscopic transnasal transsphenoidal approach for the treatment of cranial base chordomas and chondrosarcomas. Neurosurgery 59:ONS50-ONS57, discussion ONS50-57
Fraser JF, Nyquist GG, Moore N, Anand VK, Schwartz TH (2010) Endoscopic endonasal transclival resection of chordomas: operative technique, clinical outcome, and review of the literature. J Neurosurg 112:1061–1069
Fuller GN, Scheithauer BW (2007) The 2007 revised World Health Organization (WHO) classification of tumours of the central nervous system: newly codified entities. Brain Pathol 17:304–307
Hong Jiang W, Ping Zhao S, Hai Xie Z, Zhang H, Zhang J, Yun Xiao J (2009) Endoscopic resection of chordomas in different clival regions. Acta Otolaryngol 129:71–83
Hug EB (2001) Review of skull base chordomas: prognostic factors and long-term results of proton-beam radiotherapy. Neurosurg Focus 10:E11
Jahangiri A, Chin AT, Wagner JR, Kunwar S, Ames C, Chou D, Barani I, Parsa AT, McDermott MW, Benet A, El-Sayed IH, Aghi MK (2015) Factors predicting recurrence after resection of clival chordoma using variable surgical approaches and radiation modalities. Neurosurgery 76:179–185, discussion 185–176
Jho HD, Ha HG (2004) Endoscopic endonasal skull base surgery: part 3—the clivus and posterior fossa. Minim Invasive Neurosurg 47:16–23
Kim JH, Jung HH, Chang JH, Chang JW, Park YG, Chang WS (2014) Gamma Knife surgery for intracranial chordoma and chondrosarcoma: radiosurgical perspectives and treatment outcomes. J Neurosurg 121(Suppl):188–197
Koechlin NO, Simmen D, Briner HR, Reisch R (2014) Combined transnasal and transcranial removal of a giant clival chordoma. J Neurol Surg Rep 75:e98–e102
Koutourousiou M, Gardner PA, Tormenti MJ, Henry SL, Stefko ST, Kassam AB, Fernandez-Miranda JC, Snyderman CH (2012) Endoscopic endonasal approach for resection of cranial base chordomas: outcomes and learning curve. Neurosurgery 71:614–624, discussion 624–615
Lanzino G, Dumont AS, Lopes MB, Laws ER Jr (2001) Skull base chordomas: overview of disease, management options, and outcome. Neurosurg Focus 10:E12
McMaster ML, Goldstein AM, Bromley CM, Ishibe N, Parry DM (2001) Chordoma: incidence and survival patterns in the United States, 1973–1995. Cancer Causes Control 12:1–11
Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, Wolinsky Y, Kruchko C, Barnholtz-Sloan J (2014) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol 16(Suppl 4):iv1–63
Ruggieri P, Angelini A, Ussia G, Montalti M, Mercuri M (2010) Surgical margins and local control in resection of sacral chordomas. Clin Orthop Relat Res 468:2939–2947
Saito K, Toda M, Tomita T, Ogawa K, Yoshida K (2012) Surgical results of an endoscopic endonasal approach for clival chordomas. Acta Neurochir (Wien) 154:879–886
Sekhar LN, Pranatartiharan R, Chanda A, Wright DC (2001) Chordomas and chondrosarcomas of the skull base: results and complications of surgical management. Neurosurg Focus 10:E2
Sen C, Triana A (2001) Cranial chordomas: results of radical excision. Neurosurg Focus 10:E3
Sen CN, Sekhar LN, Schramm VL, Janecka IP (1989) Chordoma and chondrosarcoma of the cranial base: an 8-year experience. Neurosurgery 25:931–940, discussion 940–931
Sen C, Triana AI, Berglind N, Godbold J, Shrivastava RK (2010) Clival chordomas: clinical management, results, and complications in 71 patients. J Neurosurg 113:1059–1071
Stacchiotti S, Sommer J, Chordoma Global Consensus G (2015) Building a global consensus approach to chordoma: a position paper from the medical and patient community. Lancet Oncol 16:e71–e83
Stippler M, Gardner PA, Snyderman CH, Carrau RL, Prevedello DM, Kassam AB (2009) Endoscopic endonasal approach for clival chordomas. Neurosurgery 64:268–277, discussion 277–268
Tamaki N, Nagashima T, Ehara K, Motooka Y, Barua KK (2001) Surgical approaches and strategies for skull base chordomas. Neurosurg Focus 10:E9
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this research.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
This is a retrospective study, for which formal consent is not required.
Disclosures
There are no prior publications or submissions with any overlapping information, including studies and patients.
Rights and permissions
About this article
Cite this article
Shimony, N., Gonen, L., Shofty, B. et al. Surgical resection of skull-base chordomas: experience in case selection for surgical approach according to anatomical compartments and review of the literature. Acta Neurochir 159, 1835–1845 (2017). https://doi.org/10.1007/s00701-016-3032-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-016-3032-9