Abstract
We report the case of a large osteoblastoma arising in the frontal bone of a 20-year-old female. The lesion was first noted after a fall, and grew steadily in size following further head injury during pregnancy. Initial plain radiography demonstrated an area of radiolucency, with subsequent cross-sectional imaging revealing the extent of the lesion. Following successful surgical resection, histological features were suggestive of an aggressive osteoblastoma with aneurysmal bone cyst-like changes. We consider the influence of pregnancy and trauma on osteoblastoma behavior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoblastomas account for approximately 1% of all bone tumors, with only 4% of these arising from the calvarium [24]. Osteoblastomas behave as benign lesions, although aggressive osteoblastoma is a term used to describe lesions that show atypical cytological features that may be correlated with recurrence, often making differentiation from other entities such as osteoblast-like osteosarcoma difficult. Associated aneurysmal bone cysts (ABC) are seen in up to 10% of osteoblastomas [24]. The etiopathogenesis of ABCs remains poorly understood, and our case demonstrates predisposing factors to the development of an ABC. To the best of the authors’ knowledge, this is the first report in the literature of a coexistent aggressive osteoblastoma with ABC-like changes occurring in pregnancy, with the additional precipitant of repeated head trauma. We consider the interplay between these various factors in this clinical case report.
Case report
History and presentation
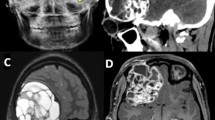
Following a fall, a 20-year-old female presented to her local emergency department, where plain skull radiography revealed a prominent lytic area in the right frontal bone with a well-defined clear margin measuring approximately 5.5 cm (Fig. 1a). The swelling remained static in size until she suffered a second episode of minor head trauma about 9 months later. At the time of the second injury, she was 7 months pregnant. The swelling subsequently increased in size to approximately 7.5 × 6 cm when she presented with headaches, nausea, and vomiting 1 month after parturition. On examination, there was tenderness on palpation of the frontal swelling, no neurological deficit or papilloedema. Laboratory investigations revealed an elevated serum alkaline phosphatase level.
Pre-operative CT and MR imaging demonstrated a large well-demarcated solitary frontal heterogeneous multi-cystic intra-osseous lesion with mass effect, causing underlying brain distortion but little surrounding reactive response (Fig. 1b, c). There was no soft tissue overlying the lesion, nor any surrounding inflammatory changes. The bone cortex was intact but thinned and there were areas of central dystrophic calcification. Differential diagnoses on initial review of imaging included hemangiopericytoma, atypical intra-osseous meningioma, calvarial osteosarcoma, fibrous dysplasia, and histiocytosis.
Operation and post-operative course
Intra-operatively, there was a protruding portion of skull bone with diameter of 100 mm, and thickness of 8 mm (Fig. 2). The outer surface had a vesicular 16-mm defect, through which herniated an internal tumor measuring 60 × 60 mm. The tumor had a fleshy appearance, and was adherent to underlying dura mater. Gross total resection was achieved with a 1-cm margin.
Histological analysis (Fig. 3) showed a bone-forming tumor composed of diffusely anastomosing trabeculae of woven bone and osteoid, lined by a row of activated osteoblasts with no evidence of cellular atypia. The intervening loose fibrovascular stroma was scattered with osteoclasts, and there were large blood-filled cystic spaces. The diploë was expanded by tumor, with a crisp cortical-periosteal reaction, but there was no evidence of host bone permeation or any other features of malignancy. The estrogen (ER) and progesterone (PR) receptor assays were negative. At 2-year post-operative follow-up, the patient remained tumor-free.
Discussion
First described in 1932 [14], the current term osteoblastoma was independently proposed by Jaffe and Lichtenstein in 1956 [12, 16] as an osteoid tissue-forming primary neoplasm of bone sharing clinical and histological features with osteoid osteoma. Osteoblastomas most commonly affect the posterior spinal elements, but arise from the cranium in 13–21% of cases [24]; only 2–4% of all osteoblastomas arise from the calvarium, with temporal, frontal, and ethmoid bones being most commonly affected. They occur more commonly in patients under the age of 30, with a recognized male predilection, which is reportedly reversed in the case of extra-calvarial osteoblastomas [1]. Progressive painful swelling, unrelieved by non-steroidal anti-inflammatory drugs, is the most frequent clinical presentation. Other clinical features of skull osteoblastomas are signs of raised intracranial pressure, cranial nerve deficits related to location (e.g., hearing loss, tinnitus, facial palsy), seizures, and malignant transformation in less than 1% of cases [24].
Histologically, osteoblastomas are bone-forming tumors composed of anastomosing trabeculae of osteoid and woven bone lined by a layer of activated osteoblasts [4]. They mostly behave as benign tumors, but the pathologic entity of ‘aggressive osteoblastoma’ has been described [17] for variants of osteoblastoma with features encroaching on that of the malignant tumor osteosarcoma. Subsequent terms of ‘pseudomalignant osteoblastoma’ and ‘malignant osteoblastoma’ later emerged, with further suggestion that aggressive osteoblastoma is really an osteoblastoma-like osteosarcoma [18]. Such borderline tumors can be extremely difficult to differentiate [2]; the main distinguishing features being cellular atypia and permeation with osteodestructive growth into host bone.
Indeed, an osteoblastoma-like osteosarcoma was a diagnostic consideration in this case given the patient’s history of rapid tumor growth and the lesion’s radiographic appearance. Ultimately, the lack of malignant histological features (necrosis, permeation into host bone, and cellular atypia) in the sample made this diagnosis less likely. Another bony tumor that is exceedingly common in the frontal sinus is an osteoma with osteoblastoma-like features [3, 19]. Again, these tumors can be focally very difficult to differentiate from a pure osteoblastoma. They are composed primarily of dense, compact (so-called ‘ivory’) or trabecular (so-called ‘mature’) bone and a paucicellular stroma. Key differences between this entity and osteoblastoma are that there is little in the way of osteoblastic rimming (as seen in the operative sample reported here), much more mature bone formation and marked Pagetoid changes indicating intense bone remodeling.
The term aneurysmal bone cyst (ABC) was coined in 1942 [13] to describe a bone lesion with a vascular lining and a characteristic ‘soap-bubble’ roentgenographic appearance of expanded bone. More common in patients under the age of 20, with a slight female preponderance, only 3–6% of ABCs arise from the cranium [5].
ABCs are described as primary when they arise de novo in healthy bone tissue. In recent years, cytogenetic abnormalities have been implicated in the development of such lesions by a mechanism of abnormal chromosomal translocation affecting the USP6 gene [23]. ABCs are described as secondary when they arise within primary bony lesions, such as osteoblastomas. In the case presented here, exposure to trauma [25], causing hemorrhage and aberrant vascular proliferation, predisposed to the development of a secondary ABC, exhibited in the histological sample by large blood-filled cystic spaces with intervening fibrovascular stroma and scattered osteoclasts [4]. The association between head trauma and osteolytic skull lesions—creating a favorable cellular milieu for ABC formation—has been well described [8, 9, 11]. As they contain a multitude of cell types, it has been postulated that ABCs represent a restorative reaction to this insult [21]. Moreover, there are several reports of ABCs arising in pre-existing lesions during pregnancy [17], or arising de novo and growing significantly during pregnancy [10].
The trophic effects of estrogen on bone are well established [15]. This steroid hormone acts by direct and indirect binding to the estrogen receptor; both alpha and beta subtypes have been found to be expressed in osteoblasts, but are notably absent from ABCs [6]. Circulating estrogen levels rise during pregnancy, therefore the gravid state certainly has the potential to precipitate progression of bone tumor.
To the best of the authors’ knowledge, there is only one case report of osteoblastoma presenting in pregnancy, although in this report, recurrence was not temporally associated with pregnancy itself. Gertzbein and colleagues [7] describe a recurrent osteoblastoma of the second thoracic vertebra in a 26-year-old female, who first presented while in late pregnancy. Despite complete resection of the histologically confirmed osteoblastoma, with good neurological recovery, paraparesis returned five and a half years later. Imaging confirmed recurrence of a large, partly calcified tumor in the left upper thorax, which was again completely resected. Histological features of the recurrent tumor showed increasing maturation of a benign osteoblastoma.
There is a paucity of literature regarding the association of bone tumors with pregnancy, and many reports discuss more aggressive bone tumors such as sarcomas [20, 22]. A retrospective study of 33 pregnant women studied the effect of pregnancy on the behavior of a variety of primary bone malignancies [26]. The authors concluded that pregnancy has no effect on the clinical behavior of bone sarcomas, although this assertion is tempered by the subjective, questionnaire-based methods, which yielded equivocal results. More recently, Israeli investigators retrospectively studied a cohort of women diagnosed with bone or soft tissue sarcomas, finding that even with postpartum deferral of definitive oncological treatment, the malignancy did not adversely affect the pregnancy [22]. All subjects in an earlier paper by the same group [20] reported disease progression during pregnancy, but it is not possible to ascertain if progression was more swift than in non-gestational counterparts without a direct control group.
In the case described above, the patient likely already had a slow-growing osteoblastoma at the time of the first trauma. Subsequent convergent factors of pregnancy (with attendant trophic hormonal effects on bone) and repeated trauma to the site likely caused rapid increase in the size of the lesion, most likely by a mechanism of bleeding and secondary ABC formation. There is precedent in the literature for trauma as a trigger for development of osteolytic bone lesions, including ABCs and osteoblastomas, and well-described transformations of benign osteoblastomas into more aggressive forms. However, the role and mechanism of pregnancy in perpetuating this abnormal bone growth is unclear. Pregnancy may be overlooked as a contributing factor to progression of bony tumors, which should be carefully monitored in the gravid state to avoid patients coming to emergent neurosurgical attention.
References
Aziz TZ, Neal JW, Cole G (1993) Malignant osteoblastoma of the skull. Br J Neurosurg 7(4):423–426
Dorfman HD, Weiss SW (1984) Borderline osteoblastic tumors: problems in the differential diagnosis of aggressive osteoblastoma and low-grade osteosarcoma. Semin Diagn Pathol 1(3):215–234
Eller R, Sillers M (2006) Common fibro-osseous lesions of the paranasal sinuses. Otolaryngol Clin North Am 39(3):585–600
Fletcher C, Bridge J, Hogendoorn P, Mertens F (2013) IARC WHO classification of tumours of soft tissue and bone, volume 5, 5th edn. World Health Organization, New York
Gan Y-C, Hockley AD (2007) Aneurysmal bone cysts of the cranium in children. Report of three cases and brief review of the literature. J Neurosurg 106(5 Suppl):401–406
Garber ST, Riva-Cambrin JK (2015) Occipital aneurysmal bone cyst rupture following head trauma: case report. J Neurosurg Pediatr 15(3):272–275
Gertzbein SD, Cruickshank B, Hoffman H, Taylor GA, Cooper PW (1973) Recurrent benign osteoblastoma of the second thoracic vertebra. A case report. J Bone Joint Surg (Br) 55(4):841–847
Hećimović I, Dmitrović B, Rubin O, Rukovanjski M, Vranković D (1999) Skull osteolysis after mild head trauma: case report. Surg Neurol 51(1):43–46
Hermann EJ, Hong B, Brandis A, Krauss JK (2011) Progressive osteolytic calvarial lesions in children after minor head injury. Pediatr Neurosurg 47(2):133–137
Hnenny L, Roundy N, Zherebitskiy V, Grafe M, Mansoor A, Dogan A (2015) Giant aneurysmal bone cyst of the anterior cranial fossa and paranasal sinuses presenting in pregnancy: case report and literature review. J Neurol Surg reports 76(2):e216–e221
Hornig GW, Beatty RM (1990) Osteolytic skull lesions secondary to trauma. Report of two cases. J Neurosurg 72(3):506–508
Jaffe HL (1956) Benign osteoblastoma. Bull Hosp Joint Dis 17(2):141–151
Jaffe H, Lichtenstein L (1942) Solitary unicameral bone cyst with emphasis on the roentgen picture, the pathologic appearance, and the pathogenesis. Arch Surg 44:1004–1025
Jaffe H, Mayer L (1932) An osteoblastic osteoid tissue-forming tumor of a metacarpal bone. Arch Surg 24(4):550–564
Krassas GE, Papadopoulou P (2001) Oestrogen action on bone cells. J Musculoskelet Neuronal Interact 2(2):143–151
Lichtenstein L (1956) Benign osteoblastoma; a category of osteoid-and bone-forming tumors other than classical osteoid osteoma, which may be mistaken for giant-cell tumor or osteogenic sarcoma. Cancer 9(5):1044–1052
Lucas DR, Unni KK, McLeod RA, O’Connor MI, Sim FH (1994) Osteoblastoma: clinicopathologic study of 306 cases. Hum Pathol 25(2):117–134
Lypka MA, Goos RR, Yamashita D-DR, Melrose R (2008) Aggressive osteoblastoma of the mandible. Int J Oral Maxillofac Surg 37(7):675–678
McHugh JB, Mukherji SK, Lucas DR (2009) Sino-orbital osteoma: a clinicopathologic study of 45 surgically treated cases with emphasis on tumors with osteoblastoma-like features. Arch Pathol Lab Med 133(10):1587–1593
Merimsky O, Inbar M, Issakov J, Kollender Y, Flusser G, Meller I, Bickels J (2003) Gestation-related malignant musculoskeletal tumors. Isr Med Assoc J 5(4):264–267
Mirra J, Picci P, Gold R (1989) Cysts and cyst-like lesions of bone. Bone tumors Clin. Radiol. Pathol. Correl. pp 1233–1334
Molho RB, Kollender Y, Issakov J, Bickels J, Flusser G, Azem F, Alon A, Inbar MJ, Meller I, Merimsky O (2008) The complexity of management of pregnancy-associated malignant soft tissue and bone tumors. Gynecol Obstet Invest 65(2):89–95
Oliveira AM, Hsi B-L, Weremowicz S, Rosenberg AE, Dal Cin P, Joseph N, Bridge JA, Perez-Atayde AR, Fletcher JA (2004) USP6 (Tre2) fusion oncogenes in aneurysmal bone cyst. Cancer Res 64(6):1920–1923
Pelargos PE, Nagasawa DT, Ung N, Chung LK, Thill K, Tenn S, Gopen Q, Yang I (2015) Clinical characteristics and diagnostic imaging of cranial osteoblastoma. J Clin Neurosci 22(3):445–449
Rapp TB, Ward JP, Alaia MJ (2012) Aneurysmal bone cyst. J Am Acad Orthop Surg 20(4):233–241
Simon MA, Phillips WA, Bonfiglio M (1984) Pregnancy and aggressive or malignant primary bone tumors. Cancer 53(11):2564–2569
Acknowledgements
The authors thank Dr. Harry Delaney for his assistance in preparation of the histological material.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent
Informed consent was obtained from the participant included in the study.
Conflict of interest
The authors disclose no conflicts of interest in submitting this manuscript.
Additional information
Sebastian M. Toescu and Andrew F. Alalade contributed equally to this work.
Rights and permissions
About this article
Cite this article
Toescu, S.M., Alalade, A.F., Steele, L. et al. Frontal skull osteoblastoma with aneurysmal bone cyst-like changes associated with trauma during pregnancy: a case report. Acta Neurochir 159, 393–396 (2017). https://doi.org/10.1007/s00701-016-3024-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-016-3024-9