Abstract
Background
The increasing number of elderly patients with traumatic brain injury (TBI) leads to specific neurointensive care (NIC) challenges. Therefore, elderly subjects with TBI need to be further studied. In this study we evaluated the demographics, management and outcome of elderly TBI patients receiving modern NIC.
Methods
Patients referred to our NIC unit between 2008 and 2010 were included. Patients were divided in two age groups, elderly (E) ≥65 years and younger (Y) 64–15 years. Parameters studied were the dominant finding on CT scans, neurological motor skills and consciousness, type of monitoring, neurosurgical procedures/treatments and Glasgow Outcome Scale Extended score at 6 months after injury.
Results
Sixty-two E (22 %) and 222 Y (78 %) patients were included. Falls were more common in E (81 %) and vehicle accidents were more common in Y patients (37 %). Acute subdural hematoma was significantly more common in E (50 % of cases) compared to Y patients (18 %). Intracranial pressure was monitored in 44 % of E and 57 % of Y patients. Evacuation of significant mass lesions was performed more common in the E group. The NIC mortality was similar in both groups (4–6 %). Favorable outcome was observed in 72 % of Y and 51 % of E patients. At the time of follow-up 25 % of E and 7 % of Y patients had died.
Conclusions
The outcome of elderly patients with TBI was significantly worse than in younger patients, as expected. However, as much as 51 % of the elderly patients showed a favorable outcome after NIC. We believe that these results encourage modern NIC in elderly patients with TBI. We need to study how secondary brain injury mechanisms differ in the older patients and to identify specific outcome predictors for elderly patients with TBI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic brain injury (TBI) is a very complex condition and particularly demanding to treat. Effective specific pharmacological patient treatments of the cellular and biochemical injury mechanisms do not exist, but advancements in neurointensive care have greatly contributed to improved patient outcomes over the last 20–30 years [8, 20]. Most studies about TBI-related changes in the intracranial pathophysiology are based on patients below the age of 65. With the increased number of elderly in the population [31] with more active lifestyles, we need better mechanisms to select treatable elderly patients. We also need to improve our understanding of age-specific pathophysiological changes to give optimal neurointensive care for every patient.
Elderly patients with TBI are challenging since many older patients have a high morbidity and mortality rate after surgery due to age-related physiological changes [34]. However, depending on the severity of the brain injury and premorbid status, some elderly TBI patients may recover well if they receive appropriate rehabilitation [12]. Unfortunately, less is known about age-related pathophysiological changes in TBI, which could influence the outcome. Recently, it was shown that elderly patients with TBI are more prone to losing vascular autoregulation control and cerebrovascular pressure reactivity [4]. It is reasonable to believe that more individualized patient care, targeted to age-specific aspects of the cerebral pathophysiology and individual requirements, would further improve outcomes. Therefore, the specific aim of this study was to study elderly (E) patients (≥65 years) with TBI selected for neurointensive care in comparison with younger (Y) patients (64-15 years) regarding the clinical characteristics and outcome to provide a basis for further studies of elderly patients.
Materials and methods
Patient selection and data collection
The Department of Neurosurgery at Uppsala University Hospital, Sweden, has a catchment area of approximately 2 million people. Most trauma patients are initially managed at local hospitals according to the ATLS concept and then transferred to our unit [10]. The study included 284 patients treated at the Uppsala University Hospital NIC unit from 2008 to 2010. Data were obtained from the Uppsala TBI register (www.ucr.uu.se/tbi) [27]. The register contains admission data including demographics, e.g., the mechanism of injury and injury classification. Some specific aggravating preconditions are sought, for example, previous brain disease/injury, diabetes mellitus, cardiovascular disease, alcohol overuse or ongoing anticoagulation therapy. The data from the NIC period include surgery, monitoring data, if and how long the patient was intubated, complications and the neurological condition at discharge. The register also includes 6-month outcome follow-up using the Glasgow Outcome Scale Extended (GOSE) score [40]. Specially trained nurses interview the patients by phone using a standard questionnaire. The GOSE score is used to categorize outcomes into three categories: favorable (good recovery, higher/lower; moderate disability, higher/lower), unfavorable (severe disability, higher/lower; vegetative) and death.
In this study, we divided the results into two age groups, E ≥65 years and Y 64–15 years. Neurological motor skills and consciousness were assessed according to the Reaction Level Scale (RLS) [27, 33] and Glasgow Coma Scale Motor (GCS M) scores [39] at admission and discharge from the NIC [27]. The dominant finding on the CT scans was used to categorize the TBI.
Standardized management
The patients were cared for according to standardized management protocols based on good laboratory practice (GLP) principles focusing on avoiding secondary insults [8]. Table 1 shows the target parameters used. Unconscious patients (RLS ≥ 3b or GCS-M ≤5) were intubated and initially mildly hyperventilated (pCO2 4.0–4.5 kPa). The intracranial pressure (ICP) and cerebral perfusion pressure (CPP) were continuously monitored in patients who were unconscious (RLS ≥ 3b or GCS-M ≤5) or in situations where there was great risk of developing high intracranial pressure. The ventilation was gradually changed to normoventilation under strict surveillance of the ICP. Propofol and morphine were routinely given for sedation and analgesia. Normovolemic circulation and sufficient colloid osmotic pressure were aimed for. Infusion of 20 % albumin was commonly used to treat hypovolemia/hypotension. Fever was treated with paracetamol, a cooling blanket or chlorpromazine. Lesions (contusions and extracerebral hematomas) with significant mass effect were evacuated. In situations of increased ICP despite basic NIC treatment and if no mass lesion was present, the CSF was drained. If CSF drainage was not sufficient to reduce the ICP, a thiopental infusion was started. Finally, if the ICP was still refractory, a decompressive craniectomy was performed [8].
Data analysis and statistics
Data were analyzed using Microsoft Excel 2007® commercial software (Redmond, WA, USA), Statistica® (Statsoft, Tulsa, OK, USA) and SPSS® (Armonk, NY, USA). A T-test was used to compare normally distributed values. In nonparametric values (i.e., RLS, GCS-M, GOSE), the p-value was calculated using the Mann-Whitney U test for independent data and Wilcoxon test for dependent variables. Proportional numbers were assessed with the chi2-test to test for significant differences. Parametric data are presented as means ± standard deviation. Nonparametric data are presented as median and quartile range.
Results
Demographics
Of the 284 patients included, 62 (22 %) were E and 222 (78 %) were Y. The mean age of the E group was 73 (±6) years and of the Y group 39 (±16) years (p < 0.005). In the older group 64 % were males and 36 % females. In the younger group, 84 % were males and 16 % females. The difference in the proportion of males and females in each age group was significant (p < 0.05) (Table 2). For details regarding the medical history, accident mechanism and CT findings, see Table 2. In short, diabetes mellitus, preexisting cardiovascular disease (CVD) or/and hypertension and preinjury use of anticoagulants were statistically more common in the E group (p < 0.05). Falls were more common in E (81 %, n = 50) compared to Y patients (36 %, n = 79) (p < 0.005), and vehicle accidents were significantly more common in the Y (37 %, n = 83) compared to E group (5 %, n = 3) (p < 0.05). Acute subdural hematoma (ASDH) was significantly more common in E (50 %, n = 31) compared to Y patients (18 %, n = 40) (p < 0.001). Diffuse axonal injury (DAI), epidural hematoma (EDH) and mixed type of injury were significantly more frequent in the Y group (Table 2).
In the E group, 69 % presented with other injuries compared to 88 % in the Y group (p < 0.005) (Table 2). The predominant injury in the Y patients was thoracic (including rib fractures), occurring in 61 patients. Spinal cord injury was only seen in two Y patients and in no E patients. No E patients suffered from extensive bleeding, while 13 Y patients were initially circulatory instable due to massive hemorrhage.
RLS and GCS M scores at admission
The median RLS value was 3.5 (2.0–4.0) equally in the older and younger group. The median GCS M value was 5 (5.0–6.0) in both groups (Table 3).
NIC
Length of stay, ICP monitoring and length of artificial ventilation
The mean length of stay (LOS) in the E group was 12 (±13) days and in the Y group was 11 (±10) days (n.s.) (Table 4). A total of 154 patients [44 % of E and 57 % of Y patients (p = 0.056)] received intracranial pressure monitoring (intraparenchymatous pressure monitoring and/or intraventricular drainage) (Table 4). The majority of patients in both the E and Y groups (approximately 76 %, respectively) were treated with a ventilator (Table 4). The mean duration of ventilator treatment was insignificantly higher among Y patients (mean 8 days) compared to E (mean 6 days) (Table 4).
Neurosurgery
Ninety-two of the patients underwent a craniotomy because of mass lesions: 47 % (n = 29) of E and 28 % (n = 63) of Y patients (p < 0.001) (Table 4). The type of injury leading to surgery differed between E and Y patients (Table 4). Six percent (n = 4) of E and 8 % (n = 17) of Y patients underwent acute surgery because of a life-threatening mass lesion at a local hospital before admission to the NIC unit (n.s.) (Table 4).
Thiopental treatment
None of E vs. 10 % (n = 21) of Y patients received thiopental treatment because of refractory high ICP (p < 0.005) (Table 4). The mean duration of thiopental treatment was about 6 days (Table 4).
Meningitis
In the E group, 2 % (n = 1) suffered from meningitis with a positive bacterial culture, which was similar to the Y group (2 %, n = 5).
Outcome
Neurological grade at discharge and NIC mortality
At discharge, the median RLS at discharge was 2 and median GCS M was 6 in both the E and Y groups (Table 3). The mortality rate in E patients during NIC was 6 % (n = 4); all died of circulatory arrest. The Y group had 4 % (n = 10) NIC mortality (Table 4). Four of the Y patients died because of circulatory arrest, and the six remaining patients died as a result of total brain infarction.
Change in the RLS and GCS M scores during NIC
During the NIC period, the percentage of patients who talked at admission (RLS 1–2) and later deteriorated (RLS ≥3 at discharge) was 5 % (n = 3) in the E and zero in the Y group (p < 0.05). Moreover, 29 % (n = 18) of E and 41 % (n = 92) of Y patients (p < 0.05) improved in consciousness from being in a more severe state (RLS ≥ 3) to RLS 1–2 (talkative) at NIC discharge (substantial recovery).
Six-month GOSE
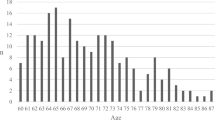
Seventy-two percent (n = 152) of Y patients had favorable 6-month outcomes vs. 51 % (n = 28) of E patients (p < 0.05) (Fig. 1). The mortality at follow-up was 25 % (n = 14) in the E and 7 % (n = 15) in the Y group (p < 0.05) (Fig. 1). The proportion of favorable outcome declined in combination with increased mortality with increasing age (Fig. 2).
Distribution of 6-month GOSE outcomes stratified into favorable (good recovery, higher/lower; moderate disability, higher/lower), unfavorable (severe disability, higher/lower; vegetative) and death. *Statistically significant difference between E and Y patients (p < 0.05). Glasgow Outcome Scale Extended (GOSE) [40]. Patients with unknown outcome (6 E and 12 Y patients) were excluded from the outcome analysis but included in the descriptive part of the study
Outcome after surgery
The clinical outcomes after surgery for different types of injury are presented in Table 5. Overall, after surgery (i.e., craniotomy because of a mass lesion) favorable outcome in the E group was 22 % (n = 6) compared to 61 % (n = 35) in the Y group (p < 0.05) (Table 5). The proportion of unfavorable outcomes and mortality was higher in the E compared to the younger group (Table 5). Subgroup analysis showed that 16 E and 36 Y patients who were awake (RLS 1–3) at admission were subsequently operated on because of a mass lesion (Table 6). Moreover, 11 E and 21 Y patients who were unconscious (RLS 4–8) at admission underwent craniotomy (Table 6). Among the E patients who were unconscious at admission and operated on, 18 % had a favorable outcome, whereas approximately 82 % had a poor outcome (Table 6). In the group with awake E patients at admission, the proportion of favorable outcomes after surgery was 25 % (Table 6). There was no significant difference in outcome after surgery in the E group between unconscious and conscious patients. It is notable that even in the worse group (RLS 4–8) some E patients had a favorable outcome after surgery (18 %, n = 2)(Table 6). In the Y group, 27 (74 %) patients who were awake at admission to the NIC had favorable outcomes, resulting in 26 % with poor outcomes in this group (Table 6). The mortality rate was 24 % in the most severe cases (RLS 4–8 at admission). The proportion of favorable outcomes was 38 % in this severe group (p < 0.05) (Table 6).
Discussion
The number of elderly people in society is increasing along with more active lifestyles. The incidence of TBI in the elderly has doubled the last 18 years [29]. In our previous TBI material the proportion of elderly patients >60 years was 16 % [8] compared to 22 % in the present study (>65 years). It is known that elderly patients with TBI fare far worse than younger patients [2, 3, 5, 13, 15, 16, 18, 23–25, 32, 35, 38, 42, 43]. However, to our knowledge there are only a few recent papers describing the results after neurointensive care of the current status [13, 24, 35]. The increasing number of elderly patients with TBI leads to difficult considerations regarding optimizing and individualizing patient care as well as reflections on quality of life in elderly TBI patients. Therefore, it is very important to increase our knowledge regarding elderly patients with TBI. In this article, we aimed to evaluate the demographics, management and outcome of elderly TBI patients receiving modern NIC as a starting point for further analysis of elderly patients with TBI. Our main findings confirmed the results of previous studies showing that the outcome of elderly patients with TBI is significantly worse than in the younger population. It was however notable that 51 % of the elderly had a favorable outcome. Furthermore, elderly patients undergoing a craniotomy because of an extracerebral hematoma with mass effect generally have a poor prognosis. However, some have a favorable outcome even if they are unconscious preoperatively. We will discuss the results in detail.
Pre-NIC factors
A main question is whether the poorer results in the elderly after TBI are due to the brain injury or to an overall age-related weakness and premorbid status leading to a more complicated medical state. In our study, 20 % of the E population compared to 13 % in the Y group suffered from previous brain injury/disease (n.s.). We found a significant difference between the rates of preexisting diabetes mellitus (16 % of E and 4 % of Y patients). Diabetes mellitus leads to various systemic complications (e.g., chronic inflammation [26]), which may have adverse effects on secondary brain injury. Nearly half of the elderly suffered from CVD/hypertension or were treated with anticoagulants. This finding differed significantly from the younger group (10 % suffered from CVD/hypertension and 5 % were treated with anticoagulants). Preexisting anticoagulant treatment has been associated with worsened outcome [19, 22]. Therefore, it is clear that several unavoidable factors exist in the elderly such as preexisting brain pathology, chronic inflammation/diabetes mellitus and coagulopathy, reasonably contributing to increased secondary brain injury and poorer outcomes.
In our study the most frequent injury mechanism in the elderly was falls (in 81 %), whereas in the younger group motor vehicle accidents were the most frequent (in 37 %). These results are similar to what others have published [11, 17, 24, 36]. There are several reasons why fall accidents among the elderly are more common, such as age-related muscle weakness, inappropriate medication, insufficient physiotherapy, orthostatic hypotension, vertigo, diabetes mellitus, poor vision and unsuitable home environments, to mention a few [6, 28]. In our article, half of the elderly group suffered from ASDH, a significantly larger proportion than in the younger group where DAI, EDH and mixed type of injury occurred more commonly. This injury distribution is in line with other studies [15, 35, 41] and may be explained by the injury mechanism. The increased risk of ASDH in the elderly due to reduced brain volume cannot be influenced. However, some causes of falls in the elderly could reasonably be prevented by improved medical care and living environments for the elderly. Organized training programs for the elderly are also currently receiving much attention [7].
The RLS and GCS-M at NIC admission did not differ between the older and younger group. The RLS and GSC-M were 3.5 and 5, respectively, in both age groups. These results are consistent with studies showing similar admission GCS scores between age groups [43]. However, in a recent study elderly patients had better GCS scores than younger TBI patients with similar TBI severity [30], suggesting that the brain injury could be worse in older patients with similar GCS scores as younger patients. This means that if the neurological scores at admission are equal between the age groups, the elderly could still have a more extensive brain injury. This effect is likely due to the fact that both the RLS and GCS scales are primarily consciousness scales and that elderly patients with reduced brain size can harbor a larger or more widespread injury before developing decreased consciousness due to a mass effect.
NIC
In our study, elderly and younger patients had a similar LOS in our NIC unit (approximately 11–12 days in both groups). Likewise, there were no significant differences in terms of ventilator treatment (occurring in 76 % of both groups), duration of ventilator treatment (6–8 days both groups) and NIC mortality rate (4–6 %). These findings are in line with other reports [12]. Ventilator treatment should not be restricted for older patients when an indication appears [9]. Our results confirm that we did not withhold ventilator treatment from the elderly if needed. However, artificial ventilation in the elderly is associated with a higher risk of side effects, such as the development of critical illness polyneuropathy, which could have a large effect on the outcome [14]. Further studies need to clarify the extent of this complication in our material.
The intracranial pressure was monitored in 44 % of E and 57 % of Y patients (n.s.). We have previously shown a strong compliance (79 %) with the standardized management protocols recommending that all unconscious patients not responding to commands (i.e., RLS >3a and GCS-M ≤5) should have ICP monitoring [1]. When we investigated the reasons for not monitoring the ICP in cases where it was indicated according to the protocol, reasonable explanations were found, e.g., coagulopathy [27]. Therefore, we believe we did not withhold ICP monitoring in elderly patients simply because of age. Other centers have published 47 % compliance with the Brain Trauma Foundation ICP monitoring guidelines [37]; we therefore consider our results regarding ICP monitoring guideline compliance satisfactory. Further studies need to address the differences between the number of secondary insults between elderly and younger patients during NIC.
Six-month outcome
We found that the overall mortality was significantly different between the age groups, i.e, 25 % and 7 % in the E and Y group, respectively (p < 0.05). Favorable outcome was seen in 72 % of the Y and 51 % of the E group at 6 months. We also observed a clear graphical trend of an age-related decrease in favorable outcome after 40 years of age (Fig. 2).
Even if the mortality was clearly higher in the elderly, we believe that a 51 % favorable 6-month outcome in the elderly group is a good result compared to previously published results showing favorable outcomes in the elderly in only 7.9 %, 23 % and 32.2 % [13, 16, 21]. For those aged >65 years, mortality was over 50 % and unfavorable outcome 74–90 % in other studies [2, 5, 13, 15, 16, 18, 42].
Surgery
A key question is whether older patients benefit from surgical evacuation of mass lesions. In our study, 93 of all patients underwent a craniotomy because of a significant mass lesion: 47 % (n = 29) of the elderly and 28 % (n = 63) of younger group (p < 0.001). The majority of the younger patients who underwent craniotomy had a favorable outcome (61 %). This stood in sharp contrast to the older patients, with favorable outcomes in 22 %. We found no significant difference in the outcome after surgery in the older patients when comparing whether they were awake or unconscious before surgery (25 vs. 18 % favorable outcome, respectively, Table 6). Thus, the consciousness level at admission does not solely define the functional outcome in the elderly, meaning that the elderly do not die because of the severity of the initial brain injury per se. This is opposed to what is seen in younger patients. A majority of the younger patients (74 %) who were awake at admission had favorable outcomes after surgery, and only 5 % of them died. The mortality rate in the unconscious group was significantly higher (24 %). Likewise, the rate of favorable outcome after surgery in the younger patients with RLS 4–8 at admission was significantly lower than in awake patients (38 % vs. 74 %, p < 0.05).
Given that the chance of favorable outcome is significantly lower in the elderly, it would be desirable to have better methods for more accurate prognostic prediction to select patients for meaningful surgical intervention.
Limitations of the study
This study obviously contains several limitations that need to be addressed in the future. To mention a few, we only studied patients admitted to our department. From clinical practice we know that patients of high age with severe TBI tend not to be transferred but instead treated conservatively at a local hospital. Thus, there may be a selection bias between younger and older patients that could have had an impact on the relatively good outcome ratio in the elderly group. Another important factor regards the characteristics decisive for each outcome category. We need to determine the common denominator for patients doing worse but also for elderly patients doing well despite an initial low GCS.
Concluding remarks
Although the elderly did far worse than younger patients after TBI, as many as 51 % had a favorable outcome with modern NIC. In the elderly, the outcome after surgery did not differ significantly between patients who were awake or unconscious preoperatively, indicating that it is not solely the primary brain injury per se that limits the outcome in the elderly. Instead, it is more likely that it is the primary and secondary brain injury in combination with other contributing factors associated with increased age, such as general weakening, use of anticoagulants and increased risk of complications, that determines the clinical outcome. We believe that age by itself should not be a reason for withholding treatment in elderly patients with TBI. To further improve the management of elderly patients with TBI we need better instruments for patient selection for active treatment and withdrawal of NIC. We also will need to advance targeted individualized NIC in the elderly and to improve the overall rehabilitative care after the NIC period. This article is an initial study preparing for such following investigations of the pathophysiology in elderly patients with TBI. This is urgent because of the quickly increasing proportion of active elderly people in the population.
References
Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R, Manley GT, Nemecek A, Newell DW, Rosenthal G, Schouten J, Shutter L, Timmons SD, Ullman JS, Videtta W, Wilberger JE, Wright DW, Foundation BT, Surgeons AAoN, Surgeons CoN, Joint Section on Neurotrauma and Critical Care AANSC (2007) Guidelines for the management of severe traumatic brain injury. VI. Indications for intracranial pressure monitoring. J Neurotrauma 24(Suppl 1):S37–44
Brazinova A, Mauritz W, Leitgeb J, Wilbacher I, Majdan M, Janciak I, Rusnak M (2010) Outcomes of patients with severe traumatic brain injury who have Glasgow Coma Scale scores of 3 or 4 and are over 65 years old. J Neurotrauma 27:1549–1555
Cagetti B, Cossu M, Pau A, Rivano C, Viale G (1992) The outcome from acute subdural and epidural intracranial haematomas in very elderly patients. Br J Neurosurg 6:227–231
Czosnyka M, Balestreri M, Steiner L, Smielewski P, Hutchinson PJ, Matta B, Pickard JD (2005) Age, intracranial pressure, autoregulation, and outcome after brain trauma. J Neurosurg 102:450–454
De Bonis P, Pompucci A, Mangiola A, Paternoster G, Festa R, Nucci CG, Maviglia R, Antonelli M, Anile C (2011) Decompressive craniectomy for elderly patients with traumatic brain injury: it's probably not worth the while. J Neurotrauma 28:2043–2048
Deandrea S, Lucenteforte E, Bravi F, Foschi R, La Vecchia C, Negri E (2010) Risk factors for falls in community-dwelling older people: a systematic review and meta-analysis. Epidemiology 21:658–668
El-Khoury F, Cassou B, Charles MA, Dargent-Molina P (2013) The effect of fall prevention exercise programmes on fall induced injuries in community dwelling older adults: systematic review and meta-analysis of randomised controlled trials. BMJ 347:f6234
Elf K, Nilsson P, Enblad P (2002) Outcome after traumatic brain injury improved by an organized secondary insult program and standardized neurointensive care. Crit Care Med 30:2129–2134
Ely EW, Evans GW, Haponik EF (1999) Mechanical ventilation in a cohort of elderly patients admitted to an intensive care unit. Ann Intern Med 131:96–104
Fischerstrom A, Nyholm L, Lewen A, Enblad P (2014) Acute neurosurgery for traumatic brain injury by general surgeons in Swedish county hospitals: a regional study. Acta Neurochir (Wien) 156:177–185
Fletcher AE, Khalid S, Mallonee S (2007) The epidemiology of severe traumatic brain injury among persons 65 years of age and older in Oklahoma, 1992-2003. Brain Inj 21:691–699
Frankel JE, Marwitz JH, Cifu DX, Kreutzer JS, Englander J, Rosenthal M (2006) A follow-up study of older adults with traumatic brain injury: taking into account decreasing length of stay. Arch Phys Med Rehabil 87:57–62
Gan BK, Lim JH, Ng IH (2004) Outcome of moderate and severe traumatic brain injury amongst the elderly in Singapore. Ann Acad Med Singap 33:63–67
Hermans G, De Jonghe B, Bruyninckx F, Van den Berghe G (2008) Clinical review: critical illness polyneuropathy and myopathy. Crit Care 12:238
Howard MA, Gross AS, Dacey RG, Winn HR (1989) Acute subdural hematomas: an age-dependent clinical entity. J Neurosurg 71:858–863
Hukkelhoven CW, Steyerberg EW, Rampen AJ, Farace E, Habbema JD, Marshall LF, Murray GD, Maas AI (2003) Patient age and outcome following severe traumatic brain injury: an analysis of 5600 patients. J Neurosurg 99:666–673
Ingebrigtsen T, Mortensen K, Romner B (1998) The epidemiology of hospital-referred head injury in northern Norway. Neuroepidemiology 17:139–146
Jennett B, Teasdale G, Braakman R, Minderhoud J, Heiden J, Kurze T (1979) Prognosis of patients with severe head injury. Neurosurgery 4:283–289
Karni A, Holtzman R, Bass T, Zorman G, Carter L, Rodriguez L, Bennett-Shipman VJ, Lottenberg L (2001) Traumatic head injury in the anticoagulated elderly patient: a lethal combination. Am Surg 67:1098–1100
Lenell S, Nyholm L, Lewen A, Enblad P (2015) Updated periodic evaluation of standardized neurointensive care shows that it is possible to maintain a high level of favorable outcome even with increasing mean age. Acta Neurochir (Wien) 157:417–425
McIntyre A, Mehta S, Janzen S, Aubut J, Teasell RW (2013) A meta-analysis of functional outcome among older adults with traumatic brain injury. NeuroRehabilitation 32:409–414
Mina AA, Knipfer JF, Park DY, Bair HA, Howells GA, Bendick PJ (2002) Intracranial complications of preinjury anticoagulation in trauma patients with head injury. J Trauma 53:668–672
Mosenthal AC, Lavery RF, Addis M, Kaul S, Ross S, Marburger R, Deitch EA, Livingston DH (2002) Isolated traumatic brain injury: age is an independent predictor of mortality and early outcome. J Trauma 52:907–911
Mosenthal AC, Livingston DH, Lavery RF, Knudson MM, Lee S, Morabito D, Manley GT, Nathens A, Jurkovich G, Hoyt DB, Coimbra R (2004) The effect of age on functional outcome in mild traumatic brain injury: 6-month report of a prospective multicenter trial. J Trauma 56:1042–1048
Mushkudiani NA, Engel DC, Steyerberg EW, Butcher I, Lu J, Marmarou A, Slieker F, McHugh GS, Murray GD, Maas AI (2007) Prognostic value of demographic characteristics in traumatic brain injury: results from the IMPACT study. J Neurotrauma 24:259–269
Nguyen DV, Shaw LC, Grant MB (2012) Inflammation in the pathogenesis of microvascular complications in diabetes. Front Endocrinol (Lausanne) 3:170
Nyholm L, Howells T, Enblad P, Lewén A (2013) Introduction of the Uppsala Traumatic Brain Injury register for regular surveillance of patient characteristics and neurointensive care management including secondary insult quantification and clinical outcome. Ups J Med Sci 118:169–180
Panel on Prevention of Falls in Older Persons AGS, British Geriatrics S (2011) Summary of the Updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc 59:148–157
Ramanathan DM, McWilliams N, Schatz P, Hillary FG (2012) Epidemiological shifts in elderly traumatic brain injury: 18-year trends in Pennsylvania. J Neurotrauma 29:1371–1378
Salottolo K, Levy AS, Slone DS, Mains CW, Bar-Or D (2014) The effect of age on Glasgow Coma Scale score in patients with traumatic brain injury. JAMA Surg 149:727–734
SCB (2009) The future population of Sweden 2009-2060. Demographic reports 2009:1 http://www.scb.se/statistik/_publikationer/BE0401_2009I60_BR_BE51BR0901ENG.pdf Accessed August 28, 2015
Sendroy-Terrill M, Whiteneck GG, Brooks CA (2010) Aging with traumatic brain injury: cross-sectional follow-up of people receiving inpatient rehabilitation over more than 3 decades. Arch Phys Med Rehabil 91:489–497
Starmark JE, Stålhammar D, Holmgren E (1988) The Reaction Level Scale (RLS85). Manual and guidelines. Acta Neurochir (Wien) 91:12–20
Stefan M, Iglesia Lino L, Fernandez G (2011) Medical consultation and best practices for preoperative evaluation of elderly patients. Hosp Pract (1995) 39:41–51
Stocchetti N, Paternò R, Citerio G, Beretta L, Colombo A (2012) Traumatic brain injury in an aging population. J Neurotrauma 29:1119–1125
Styrke J, Stålnacke BM, Sojka P, Björnstig U (2007) Traumatic brain injuries in a well-defined population: epidemiological aspects and severity. J Neurotrauma 24:1425–1436
Talving P, Karamanos E, Teixeira PG, Skiada D, Lam L, Belzberg H, Inaba K, Demetriades D (2013) Intracranial pressure monitoring in severe head injury: compliance with Brain Trauma Foundation guidelines and effect on outcomes: a prospective study. J Neurosurg 119:1248–1254
Taussky P, Widmer HR, Takala J, Fandino J (2008) Outcome after acute traumatic subdural and epidural haematoma in Switzerland: a single-centre experience. Swiss Med Wkly 138:281–285
Teasdale G, Jennett B (1974) Assessment of coma and impaired consciousness. A practical scale. Lancet 2:81–84
Teasdale GM, Pettigrew LE, Wilson JT, Murray G, Jennett B (1998) Analyzing outcome of treatment of severe head injury: a review and update on advancing the use of the Glasgow Outcome Scale. J Neurotrauma 15:587–597
Thompson HJ, McCormick WC, Kagan SH (2006) Traumatic brain injury in older adults: epidemiology, outcomes, and future implications. J Am Geriatr Soc 54:1590–1595
Tokutomi T, Miyagi T, Ogawa T, Ono J, Kawamata T, Sakamoto T, Shigemori M, Nakamura N (2008) Age-associated increases in poor outcomes after traumatic brain injury: a report from the Japan Neurotrauma Data Bank. J Neurotrauma 25:1407–1414
Vollmer DG, Torner JC, Jane JA, Sadovnic B, Charlebois D, Eisenberg HM, Foulkes MA, Marmarou A, Marshall LF (1991) Age and outcome following traumatic coma: why do older patients fare worse? J Neurosurg Spec Suppl 75:S37–S49
Acknowledgments
We would like to acknowledge Uppsala Clinical Research Center (Uppsala University) for the help with the TBI register. The study was supported by The Swedish Research Council and The Selanders Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics
The study was approved by the local ethics committee.
Conflict of interest
All authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements) or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Rights and permissions
About this article
Cite this article
Merzo, A., Lenell, S., Nyholm, L. et al. Promising clinical outcome of elderly with TBI after modern neurointensive care. Acta Neurochir 158, 125–133 (2016). https://doi.org/10.1007/s00701-015-2639-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-015-2639-6