Abstract
Introduction
Traumatic brain injury is followed by secondary neuronal degeneration, largely dependent on an inflammatory response. This response is probably gender specific, since females are better protected than males in experimental models. The reasons are not fully known. We examined aspects of the inflammatory response following experimental TBI in male and female rats to explore possible gender differences at 24 h and 72 h after trauma, times of peak histological inflammation and neuronal degeneration.
Methods
A penetrating brain injury model was used to produce penetrating focal TBI in 20 Sprague-Dawley rats, 5 males and 5 females for each time point. After 24 and 72 h the brains were removed and subjected to in situ hybridization and immunohistochemical analyses for COX-2, iNOS, osteopontin, glial fibrillary acidic protein, 3-nitrotyrosine, TUNEL and Fluoro-Jade.
Results
COX-2 mRNA and protein levels were increased in the perilesional area compared to the uninjured contralateral side and significantly higher in males at 24 h and 72 h (p < 0.05). iNOS mRNA was significantly increased in females at 24 h (p < 0.05) although protein was not. TUNEL was increased in male rats after 24 h (p < 0.05). Glial fibrillary acidic protein, osteopontin, 3-nitrotyrosine and Fluoro-Jade stained degenerating neurons were increased in the perilesional area, showing no difference between genders.
Conclusions
COX-2 regulation differed between genders after TBI. The increased COX-2 expression in male rats correlated with increased apoptotic cell death detected by increased TUNEL staining at 24 h, but not with neuronal necrosis measured by Flouro-Jade. Astrogliosis and microgliosis did not differ, confirming a comparable level of trauma. The gender-specific trait of the secondary inflammatory response may be connected to prostaglandin regulation, which may partially explain gender variances in outcome after TBI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic brain injury (TBI) is a leading global cause of death, which accounts for a major part of death and disability among young people in the industrialized world [13]. Following the initial trauma, secondary processes including inflammation have additional adverse effects on the brain. So far, no pharmacological intervention has been deemed effective to prevent secondary neuronal damage [21]. Outcome reflects the natural progress of the secondary inflammation, dependent on the initial traumatic forces and phenotype.
Autoimmune CNS inflammation differs between genders [27]. Some of these differences may be related to posttraumatic CNS inflammation, with expression of pro- and antiinflammatory enzymes with both protective and deleterious effects [44]. In experimental TBI, females are more resistant to TBI than males [23, 35]. In humans, epidemiological studies showed more contradictory results on gender-associated morbidity. Female gender correlated with reduced mortality and complication rates after TBI in some studies [15, 9, 2], while other studies found no gender association [33, 14] or even higher mortality in in females [7, 29].
The reasons for the female protection in animal models remain unclear, but may be related to female sex hormones. Edema formation and intracranial pressure after TBI vary according to the estrus cycle [18]. Progesterone reduces lipid peroxidation and suppresses neuronal hyperexcitability, leading to membrane stabilization [35]. Two important proinflammatory enzymes are COX-2 and iNOS. COX-2 catalyzes the first step of the synthesis of arachidonic acid derivatives including prostacyclin, prostaglandins and thromboxanes. COX-2 is involved in fundamental brain functions such as synaptic activity, memory consolidation and functional hyperemia, but is also rapidly induced in response to growth factors, cytokines and proinflammatory mediators [25]. Interestingly, progesterone pretreatment inhibits COX-2 expression and PGE2 synthesis in male rats [39].
In a brain ischemia-reperfusion model male mice showed increased levels of proinflammatory enzymes COX-2, NOX-2 and VCAM-1 compared to females [3]. Progesterone inhibits COX-2 expression in male rats after TBI, together with PGE2 and TNFα [39]. We therefore hypothesized that the expression of COX-2 and iNOS would differ between males and females also after penetrating focal TBI. iNOS is induced following inflammatory stimuli and accounts for a major part of the nitric oxide (NO) produced in the brain after TBI, leading to oxidative damage [28]. In addition, we analyzed markers of astrogliosis, microgliosis, oxidative stress and neuronal death to further characterize the gender-dependent response after 24 h, the peak of posttraumatic inflammation in experimental brain trauma [1, 32].
Materials and methods
All experiments were approved by the Swedish ethics committee (N255/09 and N81/13). Five male and five female Sprague-Dawley rats were exposed to penetrating focal TBI as described by Plantman et al. [32] and killed at 24 h. Samples from a separate study with equally treated rats (5 males and 5 females) and animals killed at 72 h were included post hoc to corroborate the experimental findings regarding COX-2 and Fluoro-Jade. Briefly, Sprague-Dawley rats weighing between 250 and 300 g were anesthetized by a 2.4 ml/kg intra-abdominal injection of a mixture of 1 ml midazolam (5 mg/ml), 1 ml Hypnorm (VetaPharma, Leeds, UK) and 2 ml dH20. A midline incision was made through the skin and periosteum, and a 2.7-mm-diameter burr hole was drilled with its center 3 mm lateral and 3 mm posterior to the bregma. The rat was placed in a stereotactic frame and positioned with the probe directly above the dura mater. A lead pellet was accelerated by air pressure hitting a metal cylinder probe with an attached carbon fiber pin with a tip radius of 1 mm. Depth of penetration into the brain by the pin was limited to 5 mm. After the injury, the craniotomy was left open and the skin sutured. The rats were subsequently killed by an overdose of pentobarbital. The brains were snap frozen and cut into 14-μm coronal and horizontal sections using a Microm HM560 cryostat. The frozen sections were mounted onto Thermo Scientific Superfrost plus slides and stored at -70 °C.
Radioactive in situ hybridization (ISH) was done according to Dagerlind et al. [5]. The 48-mer oligonucleotides were manufactured by Cybergene AB (Huddinge Sweden) using the sequences shown in Table 1. Primary and secondary antibodies for immunohistochemistry and immunofluorescence are shown in Table 1. Detection was performed by the ABC method (Vectastain Elite ABC peroxidase kit, Vector Labs, Burlingame, CA, USA) or immunofluorescence. Sections were rehydrated in PBS followed by fixation in 4 % formaldehyde, incubation in 0.3 % H2O2, incubation for 1 h in bovine serum albumin (BSA) with 0.3 % Triton X-100, sodium azide and avidin block solution, and incubation over night at 4 ° C with the primary antibody. Sections were incubated for 1 h with a biotinylated secondary antibody and incubated with avidin-biotin complex for 1 h followed by DAB and counterstaining with HTX and Pertex mount (Histolab Products AB, Göteborg, Sweden). For immunofluorescence, sections were mounted with ProLong Gold antifade (Life Technologies, Grand Island, NY, USA). After fixation with 4 % formaldehyde, Fluoro-Jade sections were incubated for 10 min with 0.06 % KmNO4 followed by 30 min incubation with Fluoro-Jade B before being washed in dH2O and dried on a 50 °C hot plate and mounted with Pertex. TUNEL staining was done with TACS 2 TdT-Blue Label in situ Apoptosis Detection Kit (Trevigen, Gaithersburg, MD, USA) according to the manufacturer’s instructions.
The region of interest (ROI) was defined in coronal sections medially by the interhemispheric fissure and the midline, basally by the lower part of the third ventricle and laterally by the lateral border of the right hemisphere. Sections were analyzed in mid-lesion at approximately the bregma -3.86 mm level (Fig. 1). In horizontal sections, the ROI was defined medially by the interhemispheric fissure and the midline, dorsally by the dorsal cerebral border and laterally by the lateral border of the right hemisphere. Sections were analyzed in mid-lesion at approximately the bregma -1.70 mm level (Fig. 1). The central necrotic part of the contusion was omitted from the ROI. The brain region for quantification in the contralateral side correlated to the region in the ipsilateral side.
Illustrations of coronal (COX-2, iNOS, OPN, GFAP, 3-NT, TUNEL, FJ) and horizontal (COX-2, FJ) rat brain slices, with the central necrosis and perilesional area outlined. The distribution for each marker is illustrated by dots and lines (the area of staining). ISH in situ hybridization, IH, immunohistochemistry, IF immunofluorescence, FJ Fluoro-Jade
Sections were digitally photographed in 4×–20× magnification using a Nikon Eclipse E600 microscope. Quantification of the ISH was done by batch processing in ImageJ [38] by the following script: run(“8-bit”); run(“Invert”); setAutoThreshold (“Yen/default”); //run(“Threshold…”); run(“Measure”). The integrated intensity was calculated [∑ pixel intensity (corrected for background) × area], reflecting the level of staining. Quantification of COX-2 ISH was done manually by a blinded assessor because of the inability of the software to measure the response to this probe satisfactorily. The pictures were ranked according to the following system: 0 = no staining; 1 = weak staining; 2 = clearly defined staining; 3 = heavy staining. Sixteen to 80 pictures per animal were analyzed depending on resolution. Quantification of stained cells was done manually by cell counting according to our earlier experience, in 20×–40× magnification [10]. Four to 32 pictures per animal were analyzed.
Statistical analyses were done by GraphPad Prism version 6.01 for Windows (GraphPad Software, La Jolla, CA, USA). All systems (detection methods) were tested for normal distribution by the Kolmogorov-Smirnov test with Dallal-Wilkinson-Liliefors correction. The systems that passed the normality test (COX-2 24-h ISH/IF and 72-h ISH, iNOS 24-h ISH/IH, 3-nitrotyrosine, osteopontin ISH/IF, GFAP, TUNEL, FJ 24 h) were tested for statistical inference by one-way ANOVA followed by Tukey’s post-test. The systems that failed the normality test (COX-2 72-h IF, FJ 72-h) were tested for statistical inference by the non-parametric Kruskal-Wallis ANOVA followed by Dunn’s post-test. All error bars represent the standard error of the mean. P < 0.05 was considered significant.
Results
The experimental trauma produced a penetrating focal injury to the brain consisting of a central necrotic cavity surrounded by a perilesional area consisting of damaged but still viable cells (Fig. 1). All measurements were made in the ROI. Representative photomicrographs are seen in Figs. 2, 3 and 4.
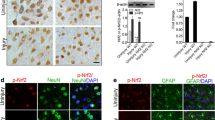
Photomicrographs of brain slices with COX-2 immunofluorescence at 24 h and 72 h. COX-2 was increased on the ipsilateral side compared to the contralateral side in both genders. Male rats had a significant ipsilateral increase compared to females at both 24 h and 72 h (p < 0.05). DAPI and COX-2/Alexa 488 pictures merged in Adobe Photoshop CS5
Photomicrographs showing brain slices with osteopontin (OPN) in situ hybridization (ISH) and immunofluorescence, GFAP in situ hybridization (ISH) and immunofluorescence (IF), 3-nitrotyrosine (3-NT) immunofluorescence (IF), TUNEL and Fluoro-Jade. TUNEL-stained apoptotic cells were increased in male rats at 24 h (p < 0.05). Due to the negative nature of the contralateral side, for illustrative purposes this is shown only in males
COX-2 mRNA increased on the ipsilateral side at 24 and 72 h following TBI, with higher expression in male rats at 24 h and 72 h (p < 0.05). The expression was found in the entire ipsilateral cortex. Weak expression was seen in the dorsal and inferior cortex on the contralateral side with no difference between genders (Figs. 1a-b,e-f, 2, 5a,c).
COX-2 protein increased in the cortex on the ipsilateral side at 24 h and 72 h following TBI, with higher expression in male rats at 24 h and 72 h (p < 0.05). The protein expression pattern matched spatially with the mRNA expression in the ipsilateral cortex with no elevated expression in the perilesional area. Weak expression was seen in the dorsal and inferior cortex on the contralateral side with no difference between genders (Figs. 1c-d,g-h, 3, 5b-d).
iNOS mRNA increased in the perilesional area at 24 h following TBI, with higher expression in female rats (p < 0.05). No upregulation was seen on the contralateral side. No correlation with COX-2 mRNA/protein was seen (Fig. 1i-j, 2, 5e).
iNOS protein increased in the cortex on the ipsilateral side at 24 h following TBI with no difference between genders. The protein expression pattern matched spatially with the mRNA expression in the perilesional area. No upregulation was seen on the contralateral side (Figs. 1k-I, 2, 5f).
Osteopontin mRNA increased specifically in the perilesional area but also diffusely on the ipsilateral side at 24 h following TBI with no difference between genders. No upregulation was seen on the contralateral side (Figs. 1m-n, 4, 6a).
Osteopontin protein increased in the perilesional area at 24 h following TBI with no difference between genders. No upregulation was seen on the contralateral side (Figs. 1o-p, 4, 6b).
GFAP mRNA increased specifically in the perilesional area but also diffusely on the ipsilateral side at 24 h following TBI with no difference between genders. Weak expression was seen in medial parts of the contralateral side. (Fig. 1q-r, 4, 6c).
GFAP protein showed positive staining on the ipsilateral and contralateral side, showing no difference between sides and no difference between genders (Figs. 1s-t, 4, 6d).
Three-nitrotyrosine increased in the perilesional area at 24 h, corresponding to the iNOS mRNA/protein. No difference was seen between genders. No upregulation was seen on the contralateral side, corresponding to the absence of iNOS mRNA and protein (Figs. 1u-v, 4, 6e).
TUNEL staining indicative of apoptosis was increased on the ipsilateral side at 24 h following TBI with higher expression in male rats (p < 0.05). TUNEL expression was dispersed in the entire ipsilateral cortex, but predominantly in the hippocampus. No upregulation was seen on the contralateral side (Figs. 1x-y, 4, 6f).
Fluoro-Jade-stained neuronal degeneration increased in the perilesional area at 24 h and 72 h following TBI, with no difference between genders. No upregulation and no difference between genders was seen in the contralateral side at 24 or 72 h (Figs. 1z-cc, 4, 6g-h).
Discussion
In this study we found a significantly increased COX-2 response in male rats compared to females at 24 h following penetrating focal TBI. This provided for a putatively higher production of prostaglandins and a more extensive inflammatory response, which would be expected to cause increased neuronal damage unless balanced by simultaneous upregulation of protective mechanisms. The study was originally designed to measure the inflammatory response at 24 h, but a limited number of sections from animals undergoing identical trauma and killed at 72 h were available, so the COX-2 expression could be confirmed at an additional time point. The gender difference was thus a robust finding and probably generalizable to all Sprague-Dawley rats.
The penetrating model is a newly described model of focal TBI. It leads to cell death and cavity formation, hemorrhage, neurodegeneration, gliosis and a deficiency in reference memory, likely due to injuries of the cortex and the hippocampus [32]. The tissue loss and high reproducibility of the model facilitated the main objective of the study, the detection of putative differences in inflammatory activity between genders, which may have been undetected in other models of TBI. Comparisons were made between the injured and intact hemispheres, in line with our studies of identical or comparable inflammatory markers [10, 31]. Sham-treated animals were excluded as they would be irrelevant to the specific hypothesis and weaken the statistical power in the comparison of groups.
COX-2 mRNA and protein increased on the ipsilateral side, which corroborated earlier findings in diffuse TBI between 3 h and 7 days [4]. Surprisingly, the expression was located in the cortex of the entire hemisphere rather than the perilesional area, similar to the pattern of nestin expression in KCl-induced spreading depression [12]. It is probable that different mechanisms of upregulation explain hippocampal, perilesional and hemispheric-cortical patterns of expression and that spreading depression is one of these mechanisms [42].
TUNEL staining indicative of apoptotic activity was increased in male rats at 24 h. Due to the limited availability of 72-h sections, only 24-h sections were analyzed. We have previously shown that TUNEL staining peaked between 24–72 h [34] and neuronal death detected by Fluoro-Jade at 24 h after penetrating focal TBI [32], which is why we believed that the difference at 24 h was relevant. It is probable that differential cell death was mainly a difference in apoptosis, since Flouro-Jade findings, indicating necrotic or pronecrotic neurons, were similar in both genders. A long-term experiment is needed to determine the kinetics and final outcome regarding neuronal degeneration.
Growth factors, tumor promoters, hormones, bacterial endotoxin and cytokines increase COX-2 levels in neurons, glial cells, endothelial cells and infiltrating blood cells [41]. Its role in CNS pathology is contradictory. COX-2 has both adverse and protective effects in multiple sclerosis, amyotrophic lateral sclerosis, Parkinson’s disease, Creutzfeldt-Jakob’s disease and Alzheimer’s disease [25]. After TBI, COX-2 inhibition improves cognition and motor function [4], and COX-2-derived prostanoids appear to be toxic in NMDA-related neurotoxicity [19], although prostaglandins also induce VEGF expression and angiogenesis after CNS trauma [40]. Our findings of increased COX-2 expression and increased levels of degenerating TUNEL-positive cells in males were compatible with these findings and also suggest a detrimental influence of COX-2-mediated inflammation. Moreover, Si and coworkers described a mechanistic relation between progesterone and COX-2 that agrees with our findings: progesterone treatment decreased COX-2 expression and levels of PGE2 and TNF-alpha in male rats [39].
iNOS mRNA and protein increased on the ipsilateral side at 24 h with no expression on the contralateral side, correlating to our earlier findings of mild to moderate TBI [10, 8]. iNOS is upregulated by transcription factors NFkB, STAT-1, IRF-1 and AP-1 and is a main producer of NO following TBI, causing oxidative stress [24, 28]. In contrast to reports of ischemic brain injury [30], iNOS mRNA was increased in females although not found in protein. Gender differences are possible, but results remain contradictory between trauma models.
COX-2 and iNOS were not spatially coexpressed in the brain. iNOS and COX-2 are upregulated following interrelated inflammatory stimuli [26], and NO also modulates cyclooxygenase activity and eicosanoid production [22]. After TBI, both iNOS and COX-2 are expressed in microglia [16], with similar relationships found in MS plaques [36] and ALS [25]. Our results do not support a broad coregulation of iNOS and COX-2 as a consequence of the inflammatory environment.
Peroxynitrite measured by surrogate marker 3-nitrotyrosine was increased in the perilesional area, although showing no difference between genders. NO reacts with superoxide to produce the highly deleterious peroxynitrite after TBI [17]. We suggest that the gender-specific inflammatory regulation did not primarily involve reactive oxygen species.
COX-2 may be affected by the general inflammatory response and influence putative gender specificity. We therefore measured osteopontin and GFAP as indicators of the general inflammatory response. Osteopontin is an extracellular glycosylated phosphoprotein synthesized by macrophages and activated microglia, which increases following TBI as an indication of microgliosis [31]. GFAP is an intermediate filament protein that increases in astrocytes following CNS damage [6]. Osteopontin mRNA and protein increased in the perilesional area with little upregulation contralaterally. No difference between genders was found. GFAP mRNA increased in the perilesional area with little upregulation contralaterally. No difference between genders was found. Protein levels did not differ between sides, which may reflect the delayed protein synthesis compared to mRNA synthesis. The inflammatory response hence included macrophage, microglia and astrocyte activation, showing no intensity difference between genders, further emphasizing the specificity of the differential COX-2 regulation observed in the study.
We aimed to describe major inflammatory markers and enzymes in male and female rats given the unknown mechanistic links in female neuroprotection, which may be unrelated to sex hormones. The 4-day estrous cycle in rats is not synchronized in animals held in cages [20, 37]. It would be experimentally difficult to control rapid hormonal cycles, and cycle staging requires a thorough histological examination of the reproductive organs [43]. Therefore, we could not correct for the estrous cycle, potentially leading to a type 2 error, failure to detect hormone-related differences. Even so, the difference in COX-2 regulation was robust at two different times so the estrous cycle was not a probable confounding factor. Sequential studies should aim for estrous cycle correction, but this lies beyond the scope of this initial study.
We did not assess potential downstream events as a result of the differential regulation of COX-2. COX-2, like many inflammatory regulators, shows alternating protective and deleterious properties. These are likely to be a result of the ever-changing dynamics of the inflammatory process. Inhibition of COX-2 after experimental TBI has produced both protective and deleterious effects [11]. COX-2 inhibitors may thus not be universally beneficial after TBI. Nevertheless, we have detected a possible mechanism that may explain different outcomes of TBI in females and males, warranting further experimental challenge.
Conclusion
COX-2 regulation and TUNEL, indicative of apoptosis, differed between male and female rats following TBI. Astrogliosis and microgliosis did not differ, confirming a comparable level of trauma. It is possible that the gender-specific trait of the secondary inflammatory response may be connected to prostaglandin regulation, which may partially explain gender-specific outcomes after TBI.
References
Bains M, Hall ED (2011) Antioxidant therapies in traumatic brain and spinal cord injury. Biochim Biophys Acta 1822(5):675–684
Berry C, Ley EJ, Tillou A, Cryer G, Margulies DR, Salim A (2009) The effect of gender on patients with moderate to severe head injuries. J Trauma 67:950–953
Brait VH, Jackman KA, Walduck AK, Selemidis S, Diep H, Mast AE, Guida E, Broughton BRS, Drummond GR, Sobey CG (2010) Mechanisms contributing to cerebral infarct size after stroke: gender, reperfusion, T lymphocytes, and Nox2-derived superoxide. J Cereb Blood Flow Metab 30:1306–1317
Cernak I, O’Connor C, Vink R (2002) Inhibition of cyclooxygenase 2 by nimesulide improves cognitive outcome more than motor outcome following diffuse traumatic brain injury in rats. Exp Brain Res 147:193–199
Dagerlind A, Friberg K, Bean AJ, Hökfelt T (1992) Sensitive mRNA detection using unfixed tissue: combined radioactive and non-radioactive in situ hybridization histochemistry. Histochemistry 98:39–49
Eng LF, Ghirnikar RS, Lee YL (2000) Glial fibrillary acidic protein: GFAP-thirty-one years (1969-2000). Neurochem Res 25:1439–1451
Farace E, Alves WM (2000) Do women fare worse: a metaanalysis of gender differences in traumatic brain injury outcome. J Neurosurg 93:539–545
Gahm C, Holmin S, Mathiesen T (2000) Temporal profiles and cellular sources of three nitric oxide synthase isoforms in the brain after experimental contusion. Neurosurgery 46:169–177
Groswasser Z, Cohen M, Keren O (1998) Female TBI patients recover better than males. Brain Inj 12:805–808
Günther M, Al Nimer F, Gahm C, Piehl F, Mathiesen T (2012) iNOS-mediated secondary inflammatory response differs between rat strains following experimental brain contusion. Acta Neurochir (Wien) 154:689–697
Hein AM, O’Banion MK (2009) Neuroinflammation and memory: the role of prostaglandins. Mol Neurobiol 40:15–32
Holmin S, von Gertten C, Sandberg-Nordqvist AC, Lendahl U, Mathiesen T (2001) Induction of astrocytic nestin expression by depolarization in rats. Neurosci Lett 314:151–155
Injury NCDPoRoPWTB (1999) Consensus conference. rehabilitation of persons with traumatic brain injury. JAMA 282:974–983
Leitgeb J, Mauritz W, Brazinova A, Janciak I, Majdan M, Wilbacher I, Rusnak M (2011) Effects of gender on outcomes after traumatic brain injury. J Trauma 71:1620–1626
Ley EJ, Short SS, Liou DZ, Singer MB, Mirocha J, Melo N, Bukur M, Salim A (2013) Gender impacts mortality after traumatic brain injury in teenagers. J Trauma Acute Care Surg 75:682–686
Loane DJ, Byrnes KR (2010) Role of microglia in neurotrauma. Neurotherapeutics 7:366–377
Lu J, Goh SJ, Tng PY, Deng YY, Ling EA, Moochhala S (2009) Systemic inflammatory response following acute traumatic brain injury. Front Biosci 14:3795–3813
Maghool F, Khaksari M, Khachki AS (2012) Differences in brain edema and intracranial pressure following traumatic brain injury across the estrous cycle: involvement of female sex steroid hormones. Brain Res 1497:61–72
Manabe Y, Anrather J, Kawano T, Niwa K, Zhou P, Ross ME, Iadecola C (2004) Prostanoids, not reactive oxygen species, mediate COX-2-dependent neurotoxicity. Ann Neurol 55:668–675
Marcondes FK, Bianchi FJ, Tanno AP (2002) Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol 62:609–614
Marklund N, Bakshi A, Castelbuono DJ, Conte V, McIntosh TK (2006) Evaluation of pharmacological treatment strategies in traumatic brain injury. Curr Pharm Des 12:1645–1680
Marnett LJ, Wright TL, Crews BC, Tannenbaum SR, Morrow JD (2000) Regulation of prostaglandin biosynthesis by nitric oxide is revealed by targeted deletion of inducible nitric-oxide synthase. J Biol Chem 275:13427–13430
McCullough LD, Hurn PD (2003) Estrogen and ischemic neuroprotection: an integrated view. Trends Endocrinol Metab 14:228–235
Miljkovic D, Trajkovic V (2004) Inducible nitric oxide synthase activation by interleukin-17. Cytokine Growth Factor Rev 15:21–32
Minghetti L (2004) Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J Neuropathol Exp Neurol 63:901–910
Mémet S (2006) NF-kappaB functions in the nervous system: from development to disease. Biochem Pharmacol 72:1180–1195
Ngo ST, Steyn FJ, McCombe PA (2014) Gender differences in autoimmune disease. Front Neuroendocrinol 35:347–369
O’Connell KM, Littleton-Kearney MT (2013) The role of free radicals in traumatic brain injury. Biol Res Nurs 15:253–263
Ottochian M, Salim A, Berry C, Chan LS, Wilson MT, Margulies DR (2009) Severe traumatic brain injury: is there a gender difference in mortality? Am J Surg 197:155–158
Park EM, Cho S, Frys KA, Glickstein SB, Zhou P, Anrather J, Ross ME, Iadecola C (2006) Inducible nitric oxide synthase contributes to gender differences in ischemic brain injury. J Cereb Blood Flow Metab 26:392–401
Plantman S (2012) Osteopontin is upregulated after mechanical brain injury and stimulates neurite growth from hippocampal neurons through β1 integrin and CD44. Neuroreport 23:647–652
Plantman S, Ng KC, Lu J, Davidsson J, Risling M (2012) Characterization of a novel rat model of penetrating traumatic brain injury. J Neurotrauma 29:1219–1232
Renner C, Hummelsheim H, Kopczak A, Steube D, Schneider HJ, Schneider M, Kreitschmann-Andermahr I, Jordan M, Uhl E, Stalla GK (2012) The influence of gender on the injury severity, course and outcome of traumatic brain injury. Brain Inj 26:1360–1371
Risling M, Plantman S, Angeria M, Rostami E, Bellander BM, Kirkegaard M, Arborelius U, Davidsson J (2011) Mechanisms of blast induced brain injuries, experimental studies in rats. Neuroimage 54(Suppl 1):S89–S97
Roof RL, Hall ED (2000) Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J Neurotrauma 17:367–388
Rose JW, Hill KE, Watt HE, Carlson NG (2004) Inflammatory cell expression of cyclooxygenase-2 in the multiple sclerosis lesion. J Neuroimmunol 149:40–49
Schank JC (2001) Do Norway rats (Rattus norvegicus) synchronize their estrous cycles? Physiol Behav 72:129–139
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Si D, Li J, Liu J, Wang X, Wei Z, Tian Q, Wang H, Liu G (2014) Progesterone protects blood–brain barrier function and improves neurological outcome following traumatic brain injury in rats. Exp Ther Med 8:1010–1014
Sköld M, Cullheim S, Hammarberg H, Piehl F, Suneson A, Lake S, Sjögren A, Walum E, Risling M (2000) Induction of VEGF and VEGF receptors in the spinal cord after mechanical spinal injury and prostaglandin administration. Eur J Neurosci 12:3675–3686
Smith WL, Dewitt DL (1996) Prostaglandin endoperoxide H synthases-1 and -2. Adv Immunol 62:167–215
von Baumgarten L, Trabold R, Thal S, Back T, Plesnila N (2008) Role of cortical spreading depressions for secondary brain damage after traumatic brain injury in mice. J Cereb Blood Flow Metab 28:1353–1360
Westwood FR (2008) The female rat reproductive cycle: a practical histological guide to staging. Toxicol Pathol 36:375–384
Woodcock T, Morganti-Kossmann MC (2013) The role of markers of inflammation in traumatic brain injury. Front Neurol 4:18
Acknowledgments
This study was funded by ALF Stockholms Läns Landsting and The Swedish Defense.
Conflicts of interest
All authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements) or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Günther, M., Plantman, S., Davidsson, J. et al. COX-2 regulation and TUNEL-positive cell death differ between genders in the secondary inflammatory response following experimental penetrating focal brain injury in rats. Acta Neurochir 157, 649–659 (2015). https://doi.org/10.1007/s00701-014-2331-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-014-2331-2